Analogue Application of Behaviour and Transport of Naturally Occurring Strontium in Cold-Region Aquatic Environments to 90Sr
Abstract
:1. Introduction
2. Materials and Methods
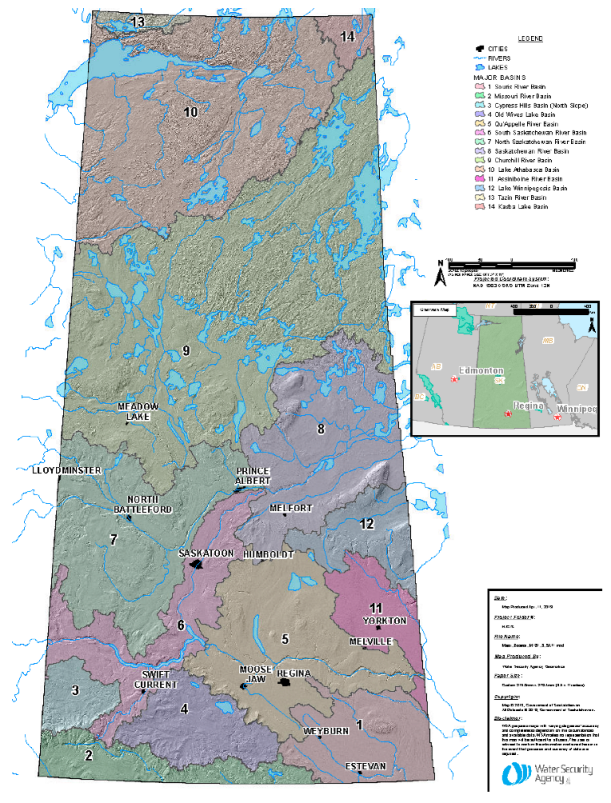
2.1. Study Region
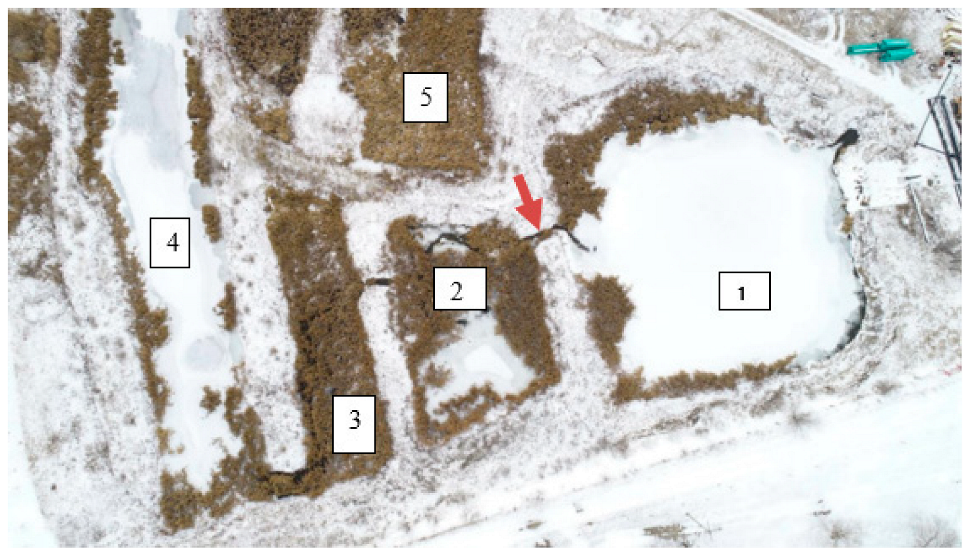
2.2. Field Samples: Water and Sediments
2.3. Laboratory Experimental Design and Analyses
3. Results and Discussion
3.1. Field Samples: Water and Sediments
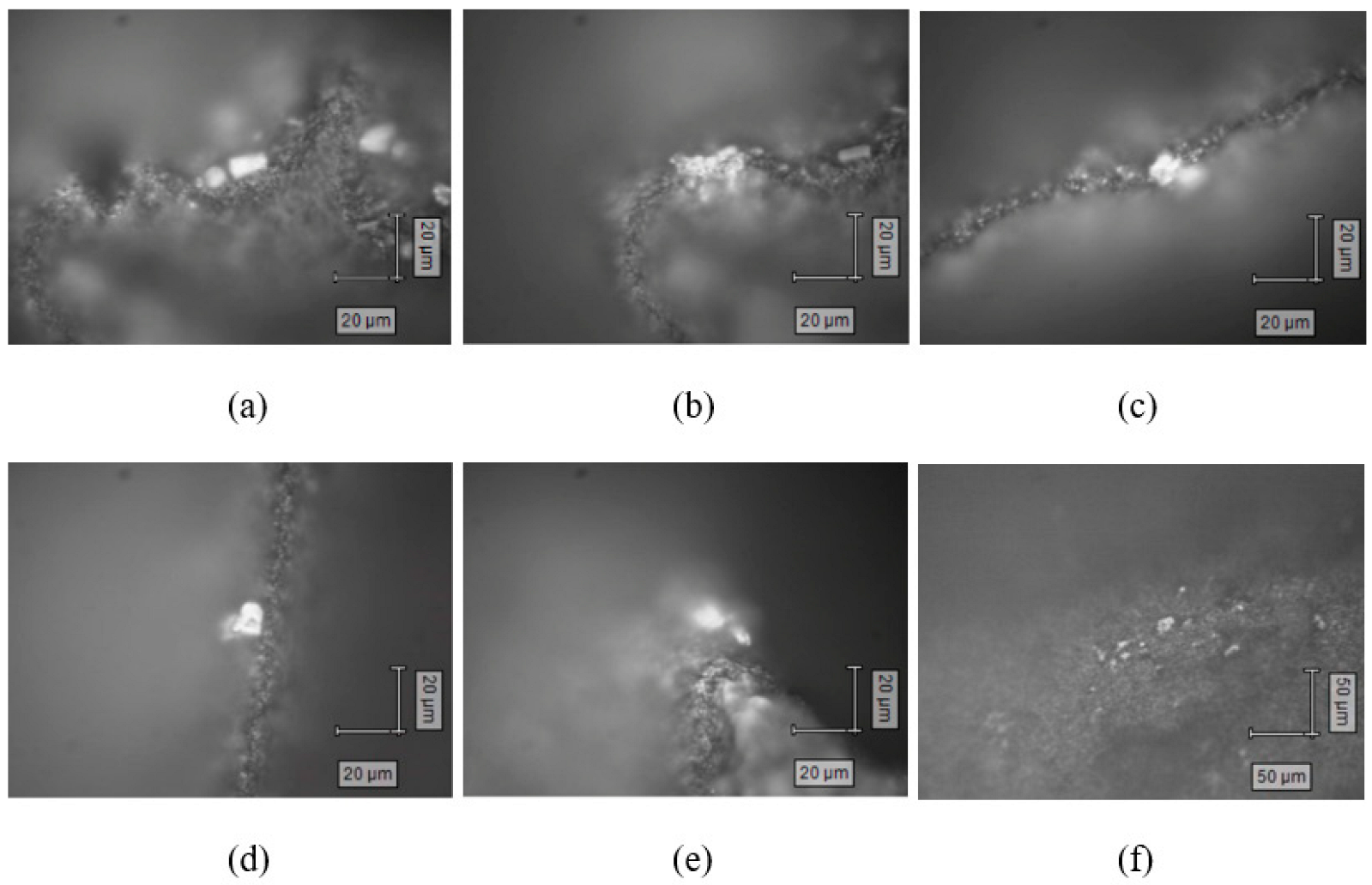
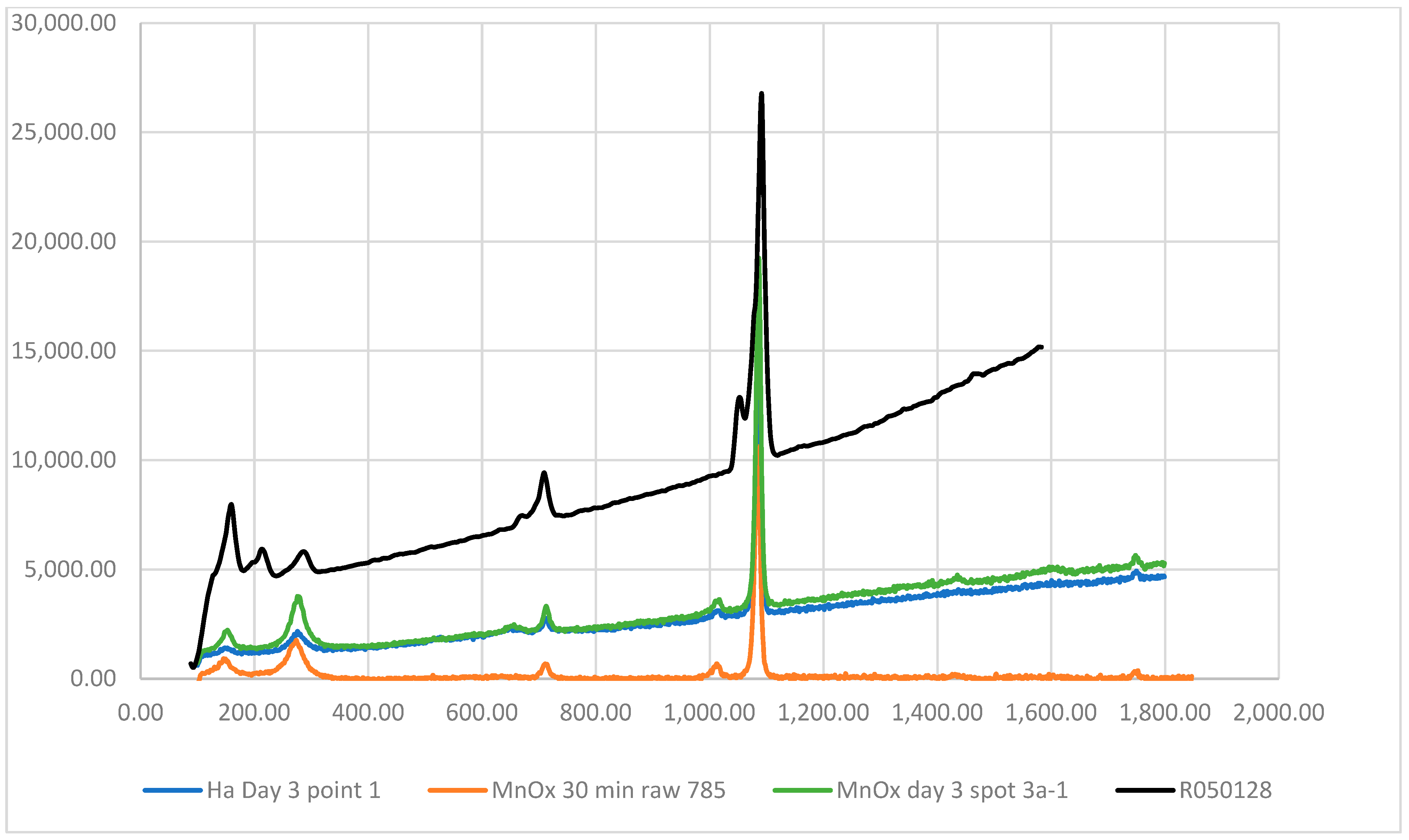
3.2. Laboratory Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salbu, B.; Skipperud, L. Nuclear Risk in Central Asia; Salbu, B., Skipperud, L., Eds.; NATO Science for Peace and Security Series; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-9317-4. [Google Scholar]
- Sunderman, D.N.; Townley, C.W. The Radiochemistry of Barium, Calcium, and Strontium; Report NAS-NS 3010; National Academy of Sciences: Washington, DC, USA; National Research Council: Washington, DC, USA, 1960. Available online: https://www.osti.gov/servlets/purl/4140481 (accessed on 15 October 2021).
- Tu, Y.-J.; You, C.-F.; Zhang, Z.; Duan, Y.; Fu, J.; Xu, D. Strontium Removal in Seawater by Means of Composite Magnetic Nanoparticles Derived from Industrial Sludge. Water 2016, 8, 357. [Google Scholar] [CrossRef]
- Carroll, S.A.; Roberts, S.K.; Criscenti, L.J. Surface complexation model for strontium sorption to amorphous silica and goethite. Geochem. Transp. 2008, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salbu, B.; Lind, O.C. Analytical techniques for charactering radioactive particles deposited in the environment. J. Environ. Radioact. 2019, 211, 106078. [Google Scholar] [CrossRef]
- Salbu, B.; Skipperud, L. Speciation of radionuclides in the environment. J. Environ. Radioact. 2009, 100, 281. [Google Scholar] [CrossRef]
- Bush, E.; Lemmen, D.S. Canada’s Changing Climate Report; Government of Canada: Ottawa, ON, Canada, 2019; ISBN 978-0-660-30222-5.
- Loeppky, A.; Anderson, W. Environmental influences on uptake kinetics and partitioning of strontium in age-0 lake sturgeon (Acipenser fulvescens): Effects of temperature and ambient calcium activities. Can. J. Fish. Aquat. Sci. 2021, 78, 612. [Google Scholar] [CrossRef]
- Mirzoeva, N.; Shadrin, N.; Arkhipova, S.; Miroshnichenko, O.; Kravchenko, N.; Anufriieva, E. Does Salinity Affect the Distribution of the Artificial Radionuclides 90Sr and 137Cs in Water of the Saline Lakes? A Case of the Crimean Peninsula. Water 2019, 12, 349. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, T.; Wallner, G. Biotransformation of Radionuclides: Trends and Challenges. In Radionuclides in the Environment; Walther, C., Gupta, D., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Salbu, B.; Kashparov, V.; Lind, O.C.; Garcia-Tenorio, R.; Johansen, M.P.; Shild, D.P.; Roos, P.; Sancho, C. Challenges associated with the behavior of radioactive particles in the environment. J. Environ. Radioact. 2017, 186, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, J.M.; Carreira, P.M. The use of environmental isotopes in groundwater studies with hydrogeoethics: Essential or dispensable? Sustain. Water Resour. Manag. 2022, 8, 74. [Google Scholar] [CrossRef]
- Barbieri, M. Isotopes in Hydrology and Hydrogeology. Water 2019, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- Zheleznyak, M.; Donchytz, G.; Hygynyak, V.; Marinetz, A.; Lyashenko, G.; Tkalich, P. RIVOTOX-one dimensional model for the simulation of the transport of radionuclides in a network of river channels. In RODOS Report Decision Support for Nuclear Emergencies; RODOS-WG4-TN(97)05; Forschungszentrum Karlsruhe: Karlsruhe, Germany, 2003. [Google Scholar]
- International Atomic Energy Agency (IAEA). Quantification of Radionuclide Transfer in Terrestrial and Freshwater Environments for Radiological Assessments; IAEA-TECDOC-1616; IAEA: Vienna, Austria, 2009; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_1616_web.pdf (accessed on 15 October 2021).
- Shoedarto, R.M.; Tada, Y.; Kashiwaya, K.; Koike, K.; Iskandar, I.; Malik, D.; Bratakusuma, B. Investigation of meteoric water and parent fluid mixing in a two-phase geothermal reservoir system using strontium isotope analysis: A case study from Southern Bandung, West Java, Indonesia. Geothermics 2021, 94, 102096. [Google Scholar] [CrossRef]
- Boschetti, T.; Awaleh, M.O.; Barbieri, M. Waters from the Djiboutian Afar: A Review of Strontium Isotopic Composition and a Comparison with Ethiopian Waters and Red Sea Brines. Water 2018, 10, 1700. [Google Scholar] [CrossRef] [Green Version]
- Jonker, G.S. The Removal of Radiostrontium by Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1976. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/07/263/7263684.pdf (accessed on 15 October 2021).
- McCauley, R.F.; Eliassen, R. Radioactive-Strontium Removal by Lime-Soda Softening. J. Am. Water Work. Assoc. 1956, 47, 494–502. Available online: https://www.jstor.org/stable/41254081 (accessed on 15 October 2021). [CrossRef]
- Cooper, C.D.; Morse, J.W. Biogeochemical controls on trace metal cycling in anoxic marine sediments. Environ. Sci. Technol. 1998, 32, 327. [Google Scholar] [CrossRef]
- Statistics Canada. Census Profile 2016: Northern [Economic Region], Saskatchewan and Saskatchewan [Province]. 2020. Available online: https://www12.statcan.gc.ca/census-recensement/index-eng.cfm (accessed on 15 October 2021).
- Health Canada. Strontium in Drinking Water—Guideline Technical Document for Public Consultation. 2018. Available online: https://www.canada.ca/en/health-canada/programs/consultation-strontium-drinking-water/document.html (accessed on 15 October 2021).
- Environment and Climate Change Canada (ECCC). Federal Environmental Quality Guidelines—Strontium. 2020. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/federal-environmental-quality-guidelines-strontium.html (accessed on 15 October 2021).
- Water Security Agency of Saskatchewan (WSASK). Surface Water Quality Data. 2013. Available online: https://www.saskatchewan.ca/residents/environment-public-health-and-safety/environmental-health/water-and-wastewater-management/water-quality-information (accessed on 15 October 2021).
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; Wiley: Hoboken, NJ, USA, 1991; ISBN 978-0-471-63731-8. [Google Scholar]
- Dandeu, A.; Humbert, B.; Carteret, C.; Muhr, H.; Plasarim, E.; Bossoutrot, J.M. Raman Spectroscopy—A Powerful Tool for the Quantitative Determination of the Composition of Polymorph Mixtures: Application to CaCO3 Polymorph Mixtures. Chem. Eng. Technol. 2006, 29, 221. [Google Scholar] [CrossRef]
- Shibano, Y.; Takahata, K.; Kawano, J.; Watanabe, T.; Enomoto, D.; Kagi, H.; Kamiya, N.; Yamamoto, J. Raman spectroscopic determination of Sr/Ca ratios of calcite samples. J. Raman Spectrosc. 2017, 48, 1755. [Google Scholar] [CrossRef]
- Kaabar, W.; Bott, S.; Devonshire, R. Raman spectroscopic study of mixed carbonate materials. Spectrochem. Acta 2011, A78, 136. [Google Scholar] [CrossRef] [PubMed]
- RRUFF. The RRUFF™ Project. 2015. Available online: https://rruff.info/ (accessed on 15 October 2021).
- Carteret, C.; Dandeu, A.; Moussaoui, S.; Humbert, B.; Plasari, E. Polymorphism studied by lattice phonon Raman Spectroscopy and Statistical Mixture Analysis Method. Application to Calcium Carbonate polymorphs during batch crystallization. Cryst. Growth Des. 2008, 9, 807. [Google Scholar] [CrossRef]
- Littlewood, J.L.; Shaw, S.; Peacock, C.L.; Bots, P.; Trivedi, D.; Burke, I.T. Mechanism of Enhanced Strontium Uptake in Calcite via an Amorphous Calcium Carbonate Crystalization Pathway. Cryst. Growth Des. 2017, 17, 1214. [Google Scholar] [CrossRef]
- Buzgar, N.; Buzatu, A.; Sanislav, I.V. The Raman Study on Certain Sulfates. Analele Ştiinţifice Ale Universităţii “AL. I. CUZA” IAŞI. Corpus ID: 67770763. 2009, Volume 55. Available online: http://geology.uaic.ro/auig/articole/2009%20no1/1_L01-Buzgar%20-%20pag%205-23.pdf (accessed on 15 October 2021).
- Liu, Y.; Wang, A.; Freeman, J.J. Raman, MIR, and NIR spectroscopic study of calcium sulfates: Gypsum, bassanite, and anhydrite. In Proceedings of the 40th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 23 March 2009; Available online: https://www.lpi.usra.edu/meetings/lpsc2009/pdf/2128.pdf (accessed on 15 October 2021).
- Krishnamurti, D. The Raman Spectra of Aragonite, Strontianite and Witherite. Proc. Indian Acad. Sci. 1960, 51, 285. [Google Scholar] [CrossRef]
- Alía, J.M.; de Mera, Y.D.; Edwards, H.G.M.; Martín, P.G.; Andrés, S.L. FT-Raman and infrared spectroscopic study of aragonite-strontianite (CaxSr-xCO3) solid solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2347. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Unit | Lowest Detection Limit | Ground Water (Summer) |
|---|---|---|---|
| Conductivity | uS/cm | 5.00 × 100 | 1.93 × 103 |
| pH | pH | 1.00 × 10−1 | 7.83 × 100 |
| Total Dissolved Solids | mg/L | 2.00 × 101 | 1.40 × 103 |
| Alkalinity, Total (as CaCO3) | mg/L | 5.00 × 100 | 4.22 × 102 |
| Ammonia, Total (as N) | mg/L | 5.00 × 10−2 | 2.40 × 100 |
| Bicarbonate (HCO3) | mg/L | 6.10 × 100 | 5.15 × 102 |
| Carbonate (CO3) | mg/L | 5.00 × 100 | <5.0 |
| Chloride (Cl) | mg/L | 2.50 × 100 | 8.60 × 101 |
| Manganese (Mn) | mg/L | 2.00 × 10−4 | 5.80 × 10−1 |
| Iron (Fe) | mg/L | 1.00 × 10−2 | 1.52 × 100 |
| Barium (Ba)-Total | mg/L | 1.00 × 10−4 | 9.70 × 10−3 |
| Calcium (Ca)-Total | mg/L | 5.00 × 10−2 | 1.19 × 102 |
| Magnesium (Mg)-Total | mg/L | 5.00 × 10−3 | 5.70 × 101 |
| Potassium (K)-Total | mg/L | 5.00 × 10−2 | 1.20 × 101 |
| Sodium (Na)-Total | mg/L | 5.00 × 10−2 | 2.34 × 102 |
| Sulfate (SO4) | mg/L | 1.50 × 100 | 5.30 × 102 |
| Uranium (U)-Total | mg/L | 1.00 × 10−5 | 1.60 × 10−3 |
| Objective | 514.5 nm Ar Laser (100% LP) LP @ Laser Head = 10.00 mW | Working Distance (mm) |
|---|---|---|
| 20×/0.40 NPLAN | 3.59 | 1.1 |
| 50×/0.75 NPLAN | 2.74 | 0.37 |
| Parameters | Unit | Inlet (Spring) | Inlet (Winter) | Outlet (Spring) | Outlet (Winter) |
|---|---|---|---|---|---|
| Conductivity | µS/cm | 5.37 × 103 | 4.79 × 103 | 3.36 × 103 | 3.87 × 103 |
| pH | pH | 7.75 × 100 | 7.89 × 100 | 7.99 × 100 | 7.91 × 100 |
| Total Dissolved Solids (TDS) | mg/L | 4.92 × 103 | 4.05 × 103 | 2.76 × 103 | 2.89 × 103 |
| Alkalinity, Total (as CaCO3) | mg/L | 1.09 × 103 | 1.09 × 103 | 6.23 × 102 | 7.55 × 102 |
| Ammonia, Total (as N) | mg/L | 6.78 × 100 | 5.30 × 100 | 6.10 × 10−1 | 2.20 × 100 |
| Chloride (Cl) | mg/L | 2.04 × 102 | 1.60 × 102 | 1.26 × 102 | 1.37 × 102 |
| Iron (Fe)-Total | mg/L | <0.05 | <0.05 | 7.30 × 10−1 | 4.51 × 10−1 |
| Calcium (Ca)-Total | mg/L | 4.80 × 102 | 4.43 × 102 | 2.68 × 102 | 3.10 × 102 |
| Magnesium (Mg)-Total | mg/L | 2.50 × 102 | 2.60 × 102 | 1.65 × 102 | 1.85 × 102 |
| Manganese (Mn)-Total | mg/L | 5.00 × 10−2 | 3.00 × 10−2 | 9.10 × 10−1 | 1.54 × 100 |
| Potassium (K)-Total | mg/L | 2.50 × 101 | 2.37 × 101 | 2.09 × 101 | 1.90 × 101 |
| Strontium (Sr)-Total | mg/L | 4.06 × 100 | 3.57 × 100 | 1.95 × 100 | 2.27 × 100 |
| Sulfur (as SO4) | mg/L | 2.26 × 103 | 2.02 × 103 | 1.34 × 103 | 1.54 × 103 |
| Sodium (Na) | mg/L | 6.26 × 102 | 4.97 × 102 | 3.69 × 102 | 3.91 × 102 |
| Saturation Index (SI) | |||||
|---|---|---|---|---|---|
| Phase | Formula | Inlet | Outlet | Control | Untreated 6 h |
| Anhydrite | CaSO4 | −5.70 × 10−1 | −8.80 × 10−1 | −7.20 × 10−1 | −7.60 × 10−1 |
| Aragonite | CaCO3 | 1.17 × 100 | 1.02 × 100 | 1.44 × 100 | 1.34 × 100 |
| Calcite | CaCO3 | 1.33 × 100 | 1.18 × 100 | 1.60 × 100 | 1.50 × 100 |
| Celestite | SrSO4 | −4.50 × 10−1 | −8.60 × 10−1 | −7.10 × 10−1 | −7.20 × 10−1 |
| Dolomite | CaMg(CO3)2 | 2.44 × 100 | 2.20 × 100 | 2.99 × 100 | 2.79 × 100 |
| Gypsum | CaSO4:H2O | −5.00 × 10−2 | −3.60 × 10−1 | −2.00 × 10−1 | −2.40 × 10−1 |
| Halite | NaCl | −5.62 × 100 | −6.00 × 100 | −5.77 × 100 | −5.85 × 100 |
| Strontianite | SrCO3 | −1.20 × 10−1 | −3.60 × 10−1 | −4.00 × 10−2 | −3.00 × 10−2 |
| Sylvianite | KCl | −6.46 × 100 | −6.71 × 100 | −6.62 × 100 | −6.72 × 100 |
| Parameter (mg/kg) | Date | Pond Cells (Saturated Paste Extractables) | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||
| Calcium | Pre-Brine | 4.52 × 102 | 4.58 × 102 | 6.74 × 102 | 3.90 × 102 | 5.15 × 102 |
| Post-Brine 1 | 1.25 × 103 | 1.55 × 103 | 2.98 × 102 | 1.46 × 103 | 4.89 × 102 | |
| Post-Brine 2 | 1.26 × 103 | 1.02 × 103 | 9.52 × 102 | 1.62 × 103 | 6.92 × 102 | |
| Magnesium | Pre-Brine | 1.08 × 102 | 2.44 × 102 | 2.54 × 102 | 2.18 × 102 | 1.87 × 102 |
| Post-Brine 1 | 6.79 × 102 | 1.15 × 103 | 1.89 × 102 | 8.88 × 102 | 3.52 × 102 | |
| Post-Brine 2 | 5.81 × 102 | 7.24 × 102 | 5.56 × 102 | 9.41 × 102 | 4.51 × 102 | |
| Sodium | Pre-Brine | 6.40 × 102 | 9.97 × 102 | 1.13 × 103 | 9.19 × 102 | 8.72 × 102 |
| Post-Brine 1 | 1.77 × 103 | 2.84 × 103 | 4.52 × 102 | 2.24 × 103 | 8.32 × 102 | |
| Post-Brine 2 | 1.87 × 103 | 1.88 × 103 | 1.43 × 103 | 2.47 × 103 | 1.13 × 103 | |
| SAR | Pre-Brine | 5.69 × 100 | 8.12 × 100 | 8.03 × 100 | 7.73 × 100 | 7.02 × 100 |
| Post-Brine 1 | 5.44 × 100 | 6.03 × 100 | 5.13 × 100 | 5.83 × 100 | 4.72 × 100 | |
| Post-Brine 2 | 6.06 × 100 | 6.00 × 100 | 4.74 × 100 | 6.08 × 100 | 5.07 × 100 | |
| Percent Average (Post-Brine) | Total Average | |||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||
| Aluminum (Al) | 54.43% | 13.68% | 28.22% | 30.07% | 14.03% | 28.09% |
| Calcium (Ca) | 14.36% | 53.80% | 38.75% | 32.11% | 53.65% | 38.53% |
| Iron (Fe) | 15.37% | 10.24% | 11.00% | 13.50% | 11.41% | 12.30% |
| Magnesium (Mg) | 1.71% | 4.85% | 8.20% | 2.07% | 4.70% | 4.31% |
| Manganese (Mn) | 5.05% | 6.54% | 6.16% | 8.77% | 4.21% | 6.15% |
| Phosphorus (P) | 2.90% | 1.35% | 2.04% | 3.41% | 1.29% | 2.20% |
| Sodium (Na) | 0.99% | 1.30% | 0.82% | 1.12% | 0.88% | 1.02% |
| Strontium (Sr) | 0.69% | 0.35% | 0.46% | 0.46% | 0.33% | 0.46% |
| Sulfur (S) | 3.92% | 7.45% | 3.74% | 8.05% | 8.87% | 6.40% |
| Total | 99.41% | 99.56% | 99.41% | 99.57% | 99.37% | 99.46% |
| Species | Mole Fraction | |
|---|---|---|
| Pond Inlet | Raw Water (Lab Experiment) | |
| Hfo_sOHCa+2 | 0.622 | 0.590 |
| Hfo_sOHSr+2 | 0.003 | 0.002 |
| Hfo_wCO3− | 0.723 | 0.651 |
| Hfo_wOHSO4−2 | 0.185 | 0.139 |
| Hfo_wOMg+ | 0.017 | 0.065 |
| Parameters (mg/L) | Untreated 0.5 h | Untreated 6 h | HA 0.5 h | HA 6 h | FA 0.5 h | FA 6 h | Control |
|---|---|---|---|---|---|---|---|
| Conductivity (µS/cm) | 4005 | 4.00 × 103 | 4.01 × 103 | 4.05 × 103 | |||
| Bicarbonate (HCO3) | 994.5 | 9.86 × 102 | 9.82 × 102 | 1.06 × 103 | |||
| Sulfate (SO4) | 1.65 × 103 | 1.63 × 103 | 1.65 × 103 | 1.65 × 103 | |||
| Calcium (Ca) | 3.33 × 102 | 3.35 × 102 | 3.43 × 102 | 3.34 × 102 | 3.42 × 102 | 3.36 × 102 | 3.92 × 102 |
| Magnesium (Mg) | 1.79 × 102 | 1.81 × 102 | 1.82 × 102 | 1.84 × 102 | 1.82 × 102 | 1.81 × 102 | 2.14 × 102 |
| Potassium (K) | 1.68 × 101 | 1.70 × 101 | 1.74 × 101 | 1.76 × 101 | 1.74 × 101 | 1.71 × 101 | 2.18 × 101 |
| Sodium (Na) | 4.15 × 102 | 4.20 × 102 | 4.19 × 102 | 4.32 × 102 | 4.28 × 102 | 4.21 × 102 | 5.17 × 102 |
| Strontium (Sr) | 2.67 × 100 | 2.63 × 100 | 2.72 × 100 | 2.64 × 100 | 2.74 × 100 | 2.63 × 100 | 2.91 × 100 |
| pH | 8.15 × 100 | 8.14 × 100 | 8.16 × 100 | 8.16 × 100 | |||
| Hardness (as CaCO3) | 1.58 × 103 | 1.59 × 103 | 1.58 × 103 | 1.86 × 103 |
| Water Quality Parameters (mg/L) | R-Untreated | R-HA | R-FA |
|---|---|---|---|
| Alkalinity, Total (as CaCO3) | 5.35 × 101 | 6.10 × 101 | 6.40 × 101 |
| Bicarbonate (HCO3) | 6.55 × 101 | 7.45 × 101 | 7.80 × 101 |
| Chloride (Cl) | 5.00 × 10−1 | 2.00 × 100 | 5.00 × 10−1 |
| Hardness (as CaCO3) | 2.80 × 102 | 2.70 × 102 | 2.80 × 102 |
| Calcium (Ca) | 5.70 × 101 | 5.80 × 101 | 5.60 × 101 |
| Magnesium (Mg) | 3.35 × 101 | 3.05 × 101 | 3.35 × 101 |
| Potassium (K) | 4.85 × 100 | 4.20 × 100 | 4.75 × 100 |
| Silicon (Si)-Dissolved | 7.00 × 10−1 | 5.50 × 10−1 | 7.50 × 10−1 |
| Sodium (Na) | 9.75 × 101 | 8.55 × 101 | 9.60 × 101 |
| Strontium (Sr)-Dissolved | 2.85 × 10−1 | 2.75 × 10−1 | 2.85 × 10−1 |
| Sulfate (SO4)-Dissolved | −5.00 × 100 | 1.00 × 101 | −5.00 × 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanacic, E.; McMartin, D.W. Analogue Application of Behaviour and Transport of Naturally Occurring Strontium in Cold-Region Aquatic Environments to 90Sr. Environments 2022, 9, 72. https://doi.org/10.3390/environments9060072
Zanacic E, McMartin DW. Analogue Application of Behaviour and Transport of Naturally Occurring Strontium in Cold-Region Aquatic Environments to 90Sr. Environments. 2022; 9(6):72. https://doi.org/10.3390/environments9060072
Chicago/Turabian StyleZanacic, Enisa, and Dena W. McMartin. 2022. "Analogue Application of Behaviour and Transport of Naturally Occurring Strontium in Cold-Region Aquatic Environments to 90Sr" Environments 9, no. 6: 72. https://doi.org/10.3390/environments9060072
APA StyleZanacic, E., & McMartin, D. W. (2022). Analogue Application of Behaviour and Transport of Naturally Occurring Strontium in Cold-Region Aquatic Environments to 90Sr. Environments, 9(6), 72. https://doi.org/10.3390/environments9060072






