Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA
Abstract
:1. Introduction
2. Materials and Methods
3. Results
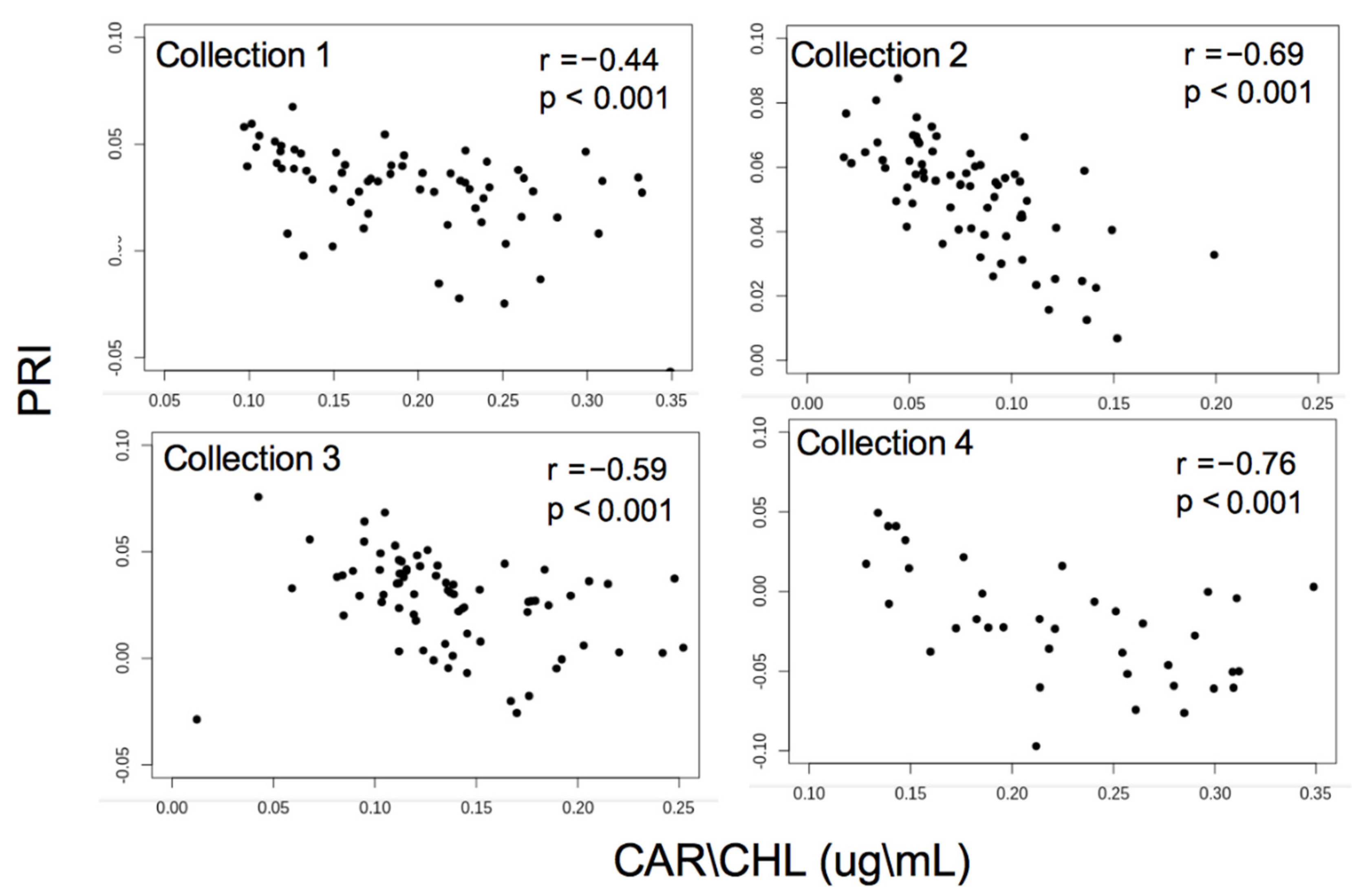
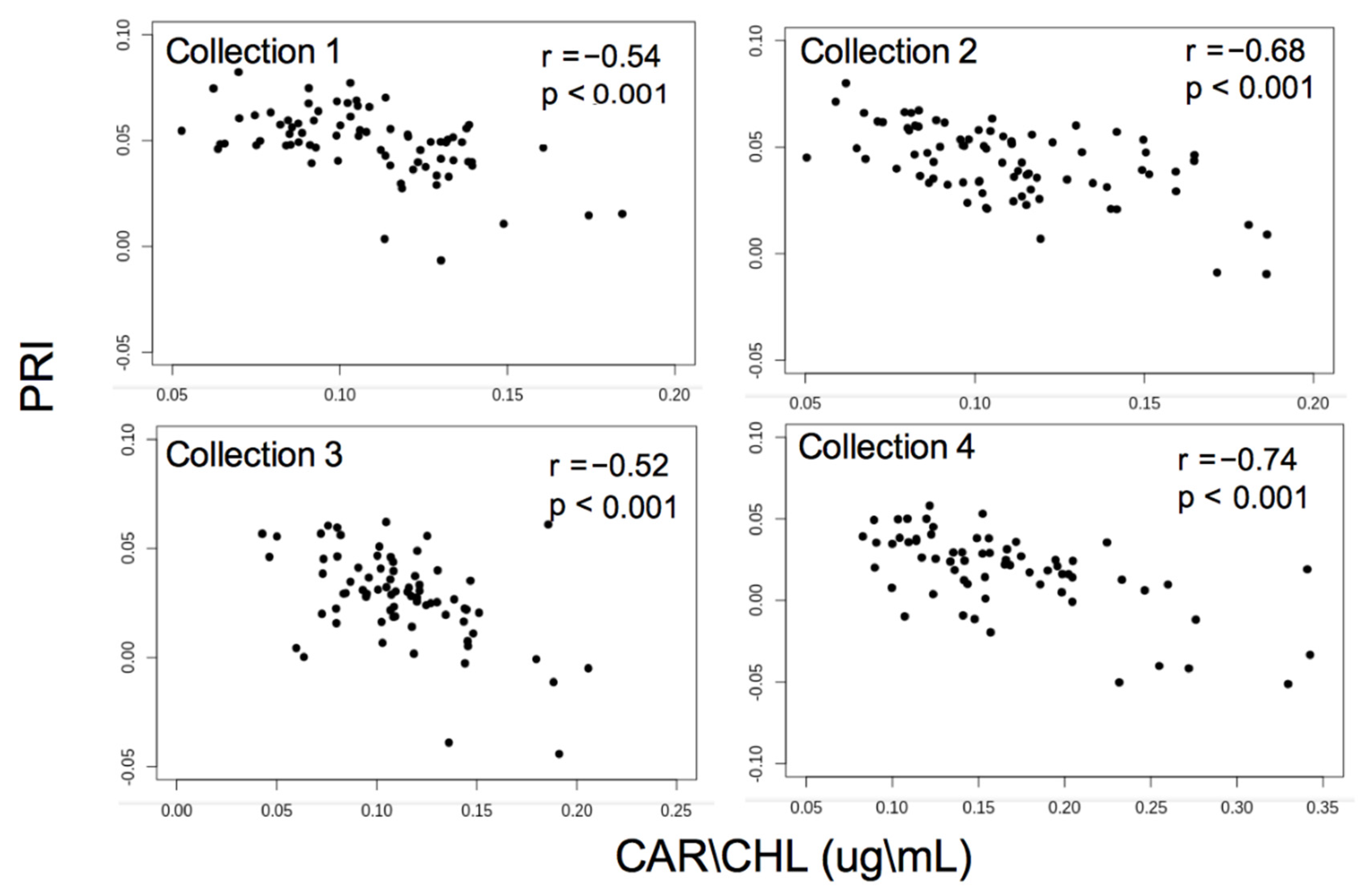
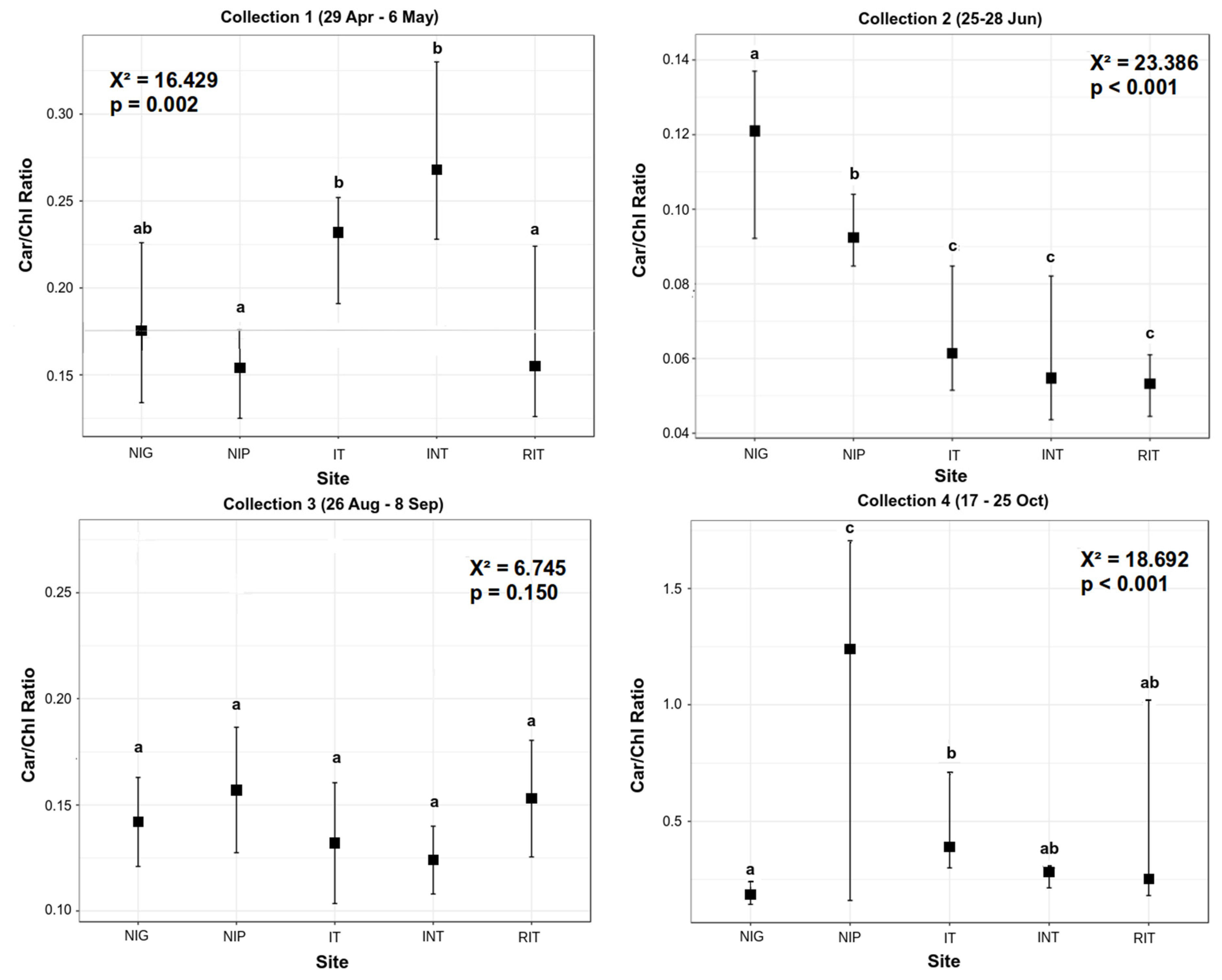
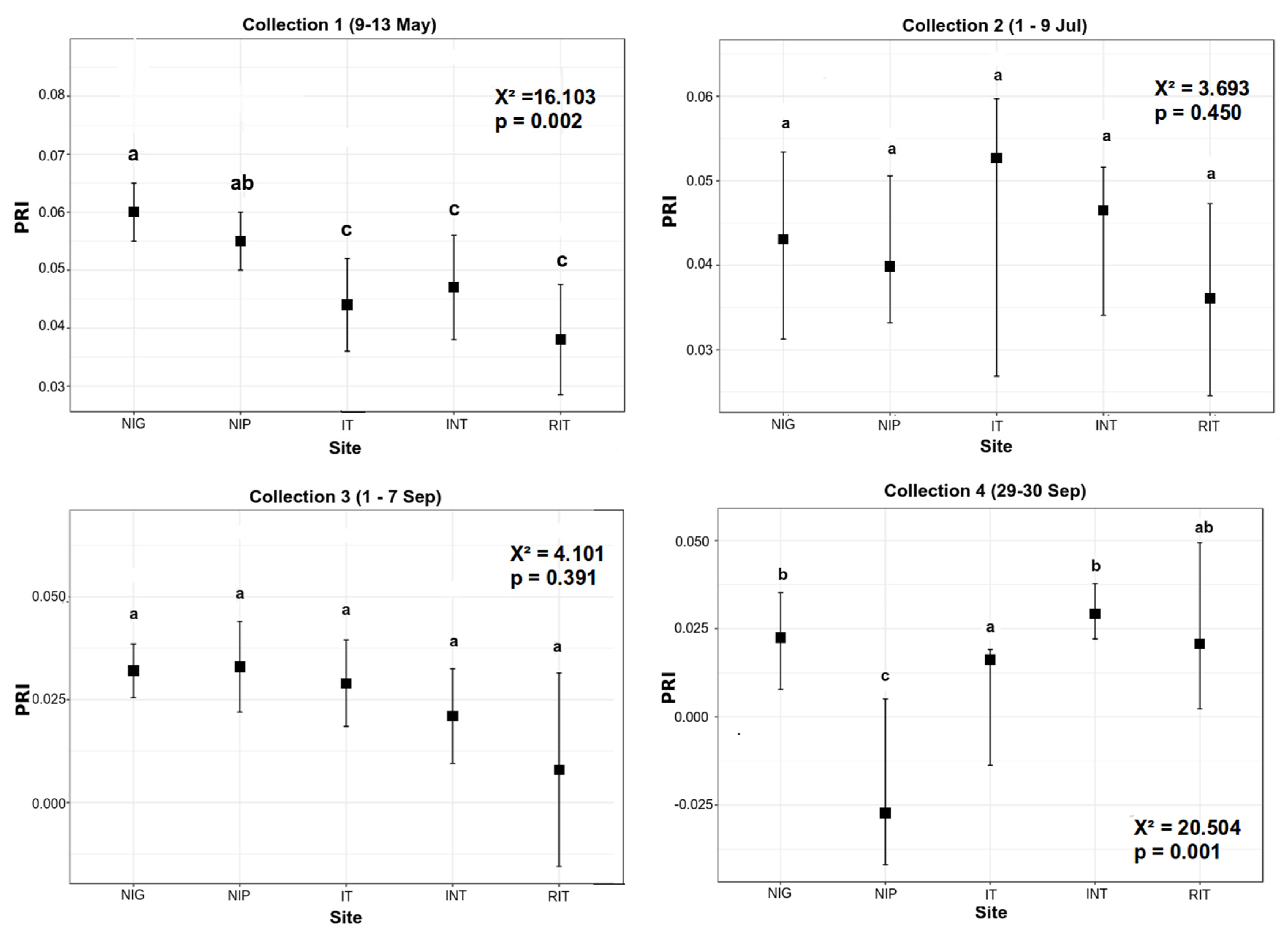
3.1. Pigment Analysis
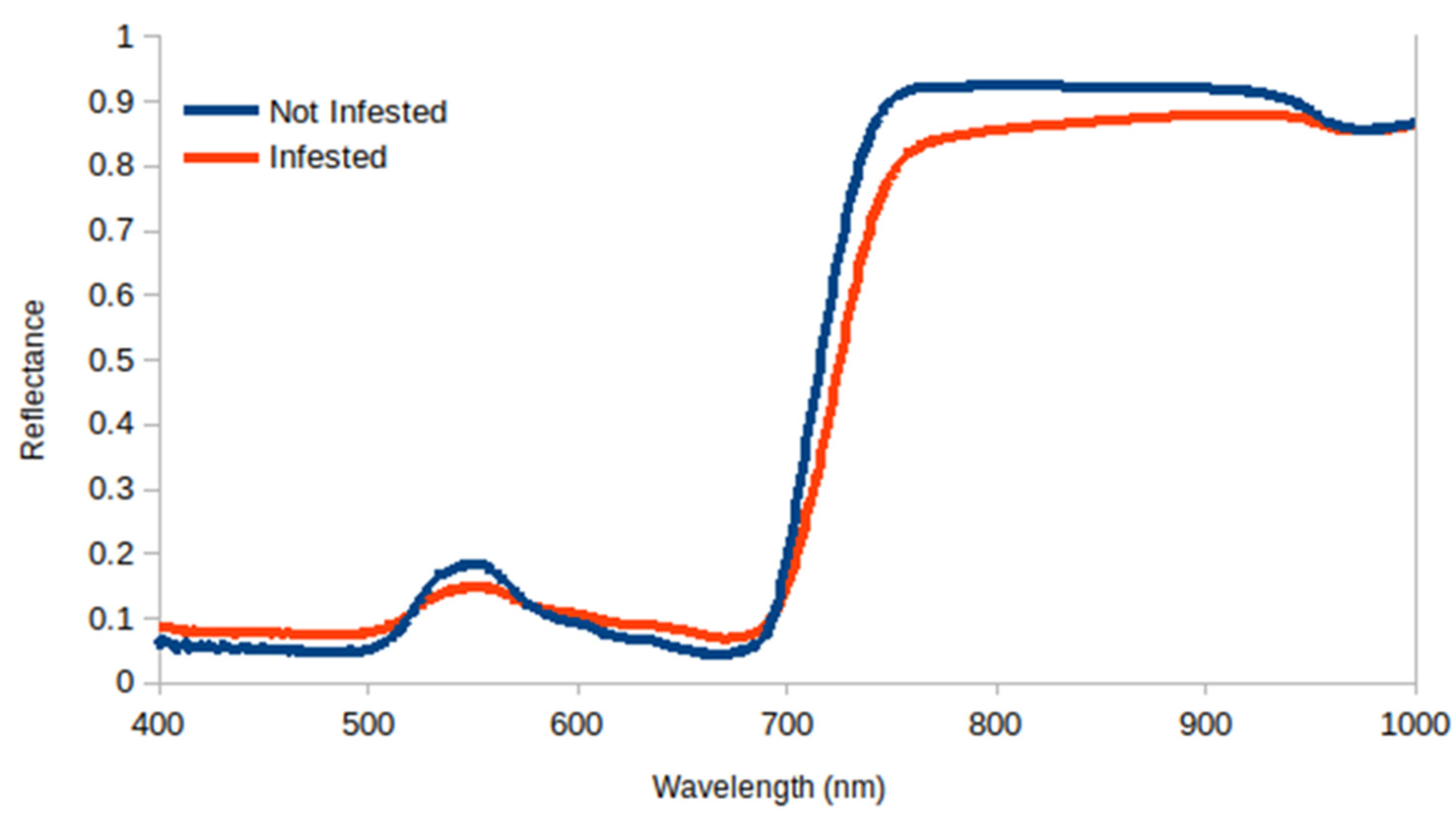
3.2. Reflectance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herms, D.A.; McCullough, D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haack, R.A.; Jendak, E.; Houping, L.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The Emerald Ash Borer: A New Exotic Pest in North America; Newsletter of the Michigan Entomological Society: Asheville, NC, USA, 2002. [Google Scholar]
- Volkovitsh, M.G.; Orlova-Bienkowskaja, M.J.; Kovalev, A.V.; Bieńkowski, A.O. An Illustrated Guide to Distinguish Emerald Ash Borer (Agrilus Planipennis) from Its Congeners in Europe. For. Int. J. For. Res. 2019, 93, 316–325. [Google Scholar] [CrossRef]
- MacFarlane, D.W.; Meyer, S.P. Characteristics and Distribution of Potential Ash Tree Hosts for Emerald Ash Borer. For. Ecol. Manag. 2005, 213, 15–24. [Google Scholar] [CrossRef]
- Poland, T.M.; McCullough, D.G. Emerald Ash Borer: Invasion of the Urban Forest and the Threat to North America’s Ash Resource. J. For. 2006, 104, 118–124. [Google Scholar] [CrossRef]
- Omernik, J.M.; Griffith, G.E. Ecoregions of the Conterminous United States: Evolution of a Hierarchical Spatial Framework. Environ. Manag. 2014, 54, 1249–1266. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, B.; Robinson, J. Early Detection of Emerald Ash Borer Infestation Using Multisourced Data: A Case Study in the Town of Oakville, Ontario, Canada. J. Appl. Remote Sens. 2014, 8, 1–19. [Google Scholar] [CrossRef]
- Pontius, J.; Martin, M.; Plourde, L.; Hallett, R. Ash Decline Assessment in Emerald Ash Borer-Infested Regions: A Test of Tree-Level, Hyperspectral Technologies. Remote Sens. Environ. 2008, 112, 2665–2676. [Google Scholar] [CrossRef]
- San Souci, J.; Hanou, I.; Puchalski, D. High-Resolution Remote Sensing Image Analysis for Early Detection and Response Planning for Emerald Ash Borer. Photogram. Eng. Remote Sens. 2009, 75, 905–909. [Google Scholar]
- Knipling, E.B. Physical and Physiological Basis for the Reflectance of Visible and Near-Infrared Radiation from Vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Williams, D.; Bartels, D.; Sawyer, A.; Mastro, V. Application of remote sensing technology to emerald ash borer survey. In Proceedings of the 16th U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and Other Invasive Species, Annapolis, MD, USA, 18–21 January 2005. [Google Scholar]
- Zhang, Q.; Li, Q.; Zhang, G. Scattering Impact Analysis and Correction for Leaf Biochemical Parameter Estimation Using Vis-NIR Spectroscopy. Spectroscopy 2011, 26. Available online: https://www.spectroscopyonline.com/view/scattering-impact-analysis-and-correction-leaf-biochemical-parameter-estimation-visnir (accessed on 7 March 2022).
- Pontius, J.; Hanavan, R.P.; Hallett, R.A.; Cook, B.D.; Corp, L.A. High Spatial Resolution Spectral Unmixing for Mapping Ash Species across a Complex Urban Environment. Remote Sens. Environ. 2017, 199, 360–369. [Google Scholar] [CrossRef]
- Murfitt, J.; He, Y.; Yang, J.; Mui, A.; De Mille, K. Ash Decline Assessment in Emerald Ash Borer Infested Natural Forests Using High Spatial Resolution Images. Remote Sens. 2016, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.A.; Hearst, J.E. Carotenoids 2: Genetics and Molecular Biology of Carotenoid Pigment Biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A.; Knapp, A.K. Leaf Optical Properties in Higher Plants: Linking Spectral Characteristics to Stress and Chlorophyll Concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A Novel Combined Spectral Index for Estimating the Ratio of Carotenoid to Chlorophyll Content to Monitor Crop Physiological and Phenological Status. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of Carotenoids: An Integrated View on Diversity, Evolution, Functional Roles and Environmental Interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing Leaf Pigment Content and Activity with a Reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral Indices for Estimating Photosynthetic Pigment Concentrations: A Test Using Senescent Tree Leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2006, 58, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Aronoff, S. The Absorption Spectra of Chlorophyll and Related Compounds. Chem. Rev. 1950, 47, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Piesik, D.; Rochat, D.; van der Pers, J.; Marion-Poll, F. Pulsed Odors from Maize or Spinach Elicit Orientation in European Corn Borer Neonate Larvae. J. Chem. Ecol. 2009, 35, 1032–1042. [Google Scholar] [CrossRef]
- Piesik, D.; Rochat, D.; Delaney, K.J.; Marion-Poll, F. Orientation of European Corn Borer First Instar Larvae to Synthetic Green Leaf Volatiles: Orientation of European Corn Borer First Instar Larvae. J. Appl. Entomol. 2013, 137, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile Organic Compounds Released by Maize Following Herbivory or Insect Extract Application and Communication between Plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Flower, C.E.; Dalton, J.E.; Knight, K.S.; Brikha, M.; Gonzalez-Meler, M.A. To Treat or Not to Treat: Diminishing Effectiveness of Emamectin Benzoate Tree Injections in Ash Trees Heavily Infested by Emerald Ash Borer. Urban For. Urban Green. 2015, 14, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Hanavan, R.; Heuss, M. Physiological Response of Ash Trees, Fraxinus spp., Infested with Emerald Ash Borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), to Emamectin Benzoate (Tree-Äge) Stem Injections. Arboric. Urban For. 2019, 45, 132–138. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of Chlorophyll a/b Ratios during Acclimation of Tropical Woody Seedlings to Nitrogen Limitation and High Light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, J.; Robinson, D.C.; Cape, J.N.; Paterson, I.S.; Francis, B.J.; Melhorn, H.; Wellburn, A.R. Use of Carotenoid Ratios, Ethylene Emissions and Buffer Capacities for the Early Diagnosis of Forest Decline. New Phytol. 1988, 109, 85–95. [Google Scholar] [CrossRef]
- Roberts, D.; Roth, K.; Perroy, R. Hyperspectral vegetation indices. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2011; pp. 309–328. ISBN 978-1-4398-4537-0. [Google Scholar]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Bendix, J. Hyperspectral Data Analysis in R: The Hsdar Package. J. Stat. Softw. 2019, 89, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Fillela, I.; Biel, C.; Serrano, L.; Save, R. The Reflectance at the 950–970 Nm Region as an Indicator of Plant Water Status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-a Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. B 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Daniel, W.W. Applied Nonparametric Statistics, 2nd ed.; PWS-Kent: Boston, MA, USA, 1990. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. 1995, 57, 289–300. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hu, B.; Li, J.; Wang, J.; Hall, B. The Early Detection of the Emerald Ash Borer (EAB) Using Advanced Geospacial Technologies. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, 40, 213. [Google Scholar]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The Relationship between the Emerald Ash Borer (Agrilus planipennis) and Ash (Fraxinus spp.) Tree Decline: Using Visual Canopy Condition Assessments and Leaf Isotope Measurements to Assess Pest Damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Ko, D.; Bristow, N.; Greenwood, D.; Weisberg, P. Canopy Cover Estimation in Semiarid Woodlands: Comparison of Field-Based and Remote Sensing Methods. For. Sci. 2009, 55, 132–141. [Google Scholar] [CrossRef]
- Riggins, J.J.; Defibaugh y Chávez, J.M.; Tullis, J.A.; Stephen, F.M. Spectral Identification of Previsual Northern Red Oak (Quercus rubra L.) Foliar Symptoms Related to Oak Decline and Red Oak Borer (Coleoptera: Cerambycidae) Attack. South. J. Appl. For. 2011, 35, 18–25. [Google Scholar] [CrossRef] [Green Version]
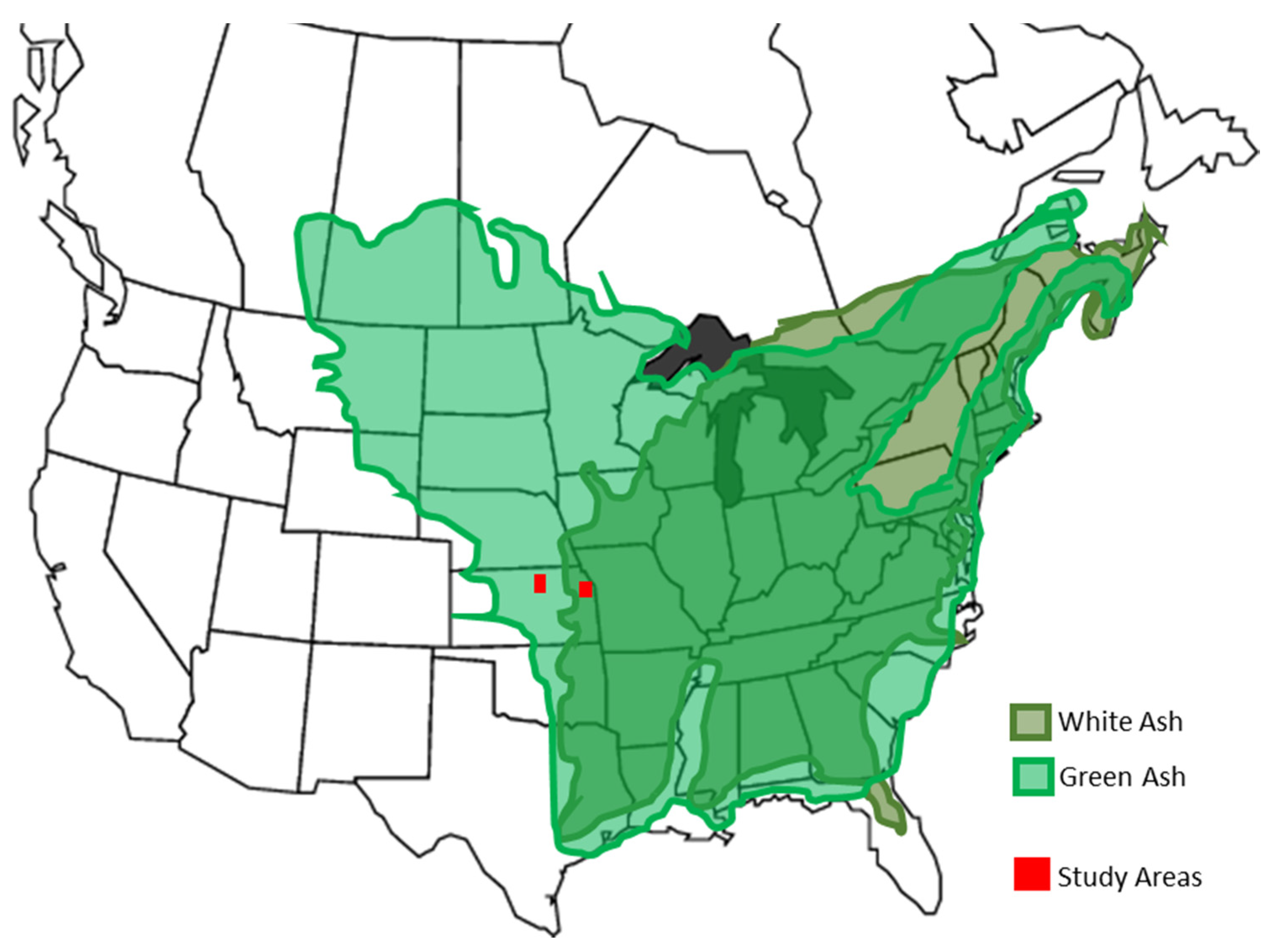
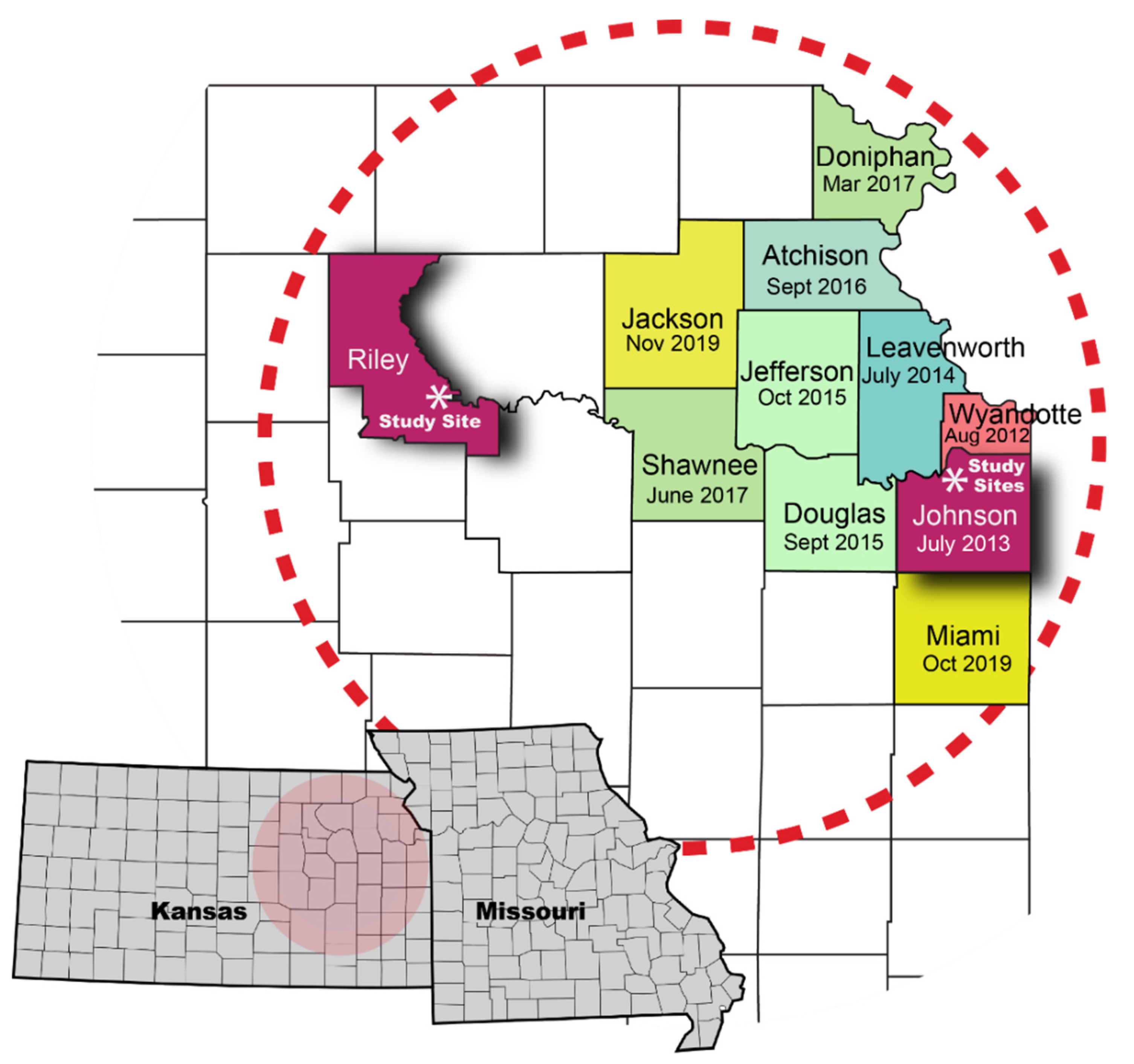


| Bonner Springs, Kansas | Ottawa, Kansas | Manhattan, Kansas | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Avg | 2017 | 2018 | Avg | 2017 | 2018 | Avg | ||||||||||
| T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | |
| April | 13 | 15.6 | 14 | 3.3 | 12 | 9.8 | 13 | 17.9 | 16 | 3.7 | 12 | 9.8 | 13 | 10.4 | 16 | 4.1 | 13 | 8.1 |
| May | 17 | 10.6 | 20 | 7.5 | 18 | 12.8 | 17 | 8.9 | 22 | 12.4 | 18 | 13.7 | 17 | 10.1 | 23 | 9.8 | 19 | 13.0 |
| June | 23 | 16.8 | 25 | 6.5 | 23 | 14.9 | 23 | 19.4 | 25 | 3.2 | 23 | 14.3 | 24 | 9.2 | 27 | 5.8 | 24 | 15.1 |
| July | 26 | 12.6 | 25 | 6.4 | 26 | 10.9 | 26 | 5.2 | 26 | 4.2 | 26 | 10.4 | 27 | 3.6 | 27 | 7.6 | 27 | 11.0 |
| August | 22 | 27.5 | 25 | 5.6 | 24 | 10.6 | 21 | 20.1 | 25 | 20.7 | 25 | 10.3 | 23 | 15.5 | 25 | 19.0 | 26 | 11.0 |
| September | 21 | 7.8 | 14 | 5.7 | 20 | 12.3 | 21 | 10.9 | 21 | 8.0 | 20 | 10.5 | 22 | 2.7 | 22 | 19.0 | 21 | 8.7 |
| October | 14 | 12.5 | 12 | 31 | 13 | 8.9 | 15 | 11.2 | 13 | 26.7 | 14 | 8.9 | 15 | 6.7 | 12 | 16.0 | 14 | 6.8 |
| Annual Precip | 115.9 | 84.1 | 103.4 | 162.7 | 101.2 | 102.4 | 96.3 | 90.4 | ||||||||||
| Sample Type | Sample Size | Collection Dates 1 | Notes 2 | ||
|---|---|---|---|---|---|
| 2017 | 2018 | 2017 | 2018 | ||
| Infested, Not Treated (INT) | 19 | 27 | 29 April–6 May 25 June–28 June 26 August–8 September 17 October–25 October | 9 May–13 May 1 July–9 July 1 September–7 September 29 September–30 September | Residential Site, Johnson County |
| Infested, Treated (IT) | 18 | 17 | 29 April–6 May 25 June–28 June 26 August–8 September 17 October–25 October | 9 May–13 May 1 July–9 July 1 September–7 September 29 September–30 September | Recreation Center Site, Johnson County |
| Recently Infested, Treated (RIT) | 9 | 9 | 29 April–6 May 25 June–28 June 26 August–8 September 17 October–25 October | 9 May–13 May 1 July–9 July 1 September–7 September 29 September–30 September | Park Site, Johnson County |
| Not Infested, Good Condition (NIG) | 15 | 15 | 29 April–6 May 25 June–28 June 26 Aug–8 September 17 October–25 October | 9 May–13 May 1 July–9 July 1 September–7 September 29 September–30 September | Campus Site, Riley County |
| Not Infested, Poorer Condition (NIP) | 16 | 15 | 29 April–6 May 25 June–28 June 26 August–8 September 17 October–25 October | 9 May–13 May 1 July–9 July 1 September–7 September 29 September–30 September | Campus Site, Riley County |
| Index | Definition 1 | Descriptive Purpose of Index 2 | Reference |
|---|---|---|---|
| Water Band Index (WBI) | R970/R900 | Water stress, general organism stress | [37] |
| Gitelson–Merzlyak B Index | R750/R700 | Chlorophyll estimation, leaf senescence | [38] |
| Normalized Phaeophytization Index (NPQI) | R435 − R415/R435 + R415 | Chlorophyll breakdown and general environmental stress | [39] |
| Combined Carotenoid/Chlorophyll Ratio Index (CCRI) | ((R720 − R521)/R521)/((R750 − R705))/R705) | Combination of a carotenoid index with a red-edge chlorophyll index | [18] |
| Photochemical Reflectance Index (PRI) | (R531 − R570)/(R531 + R570) | General organism stress | [40] |
| 2017 | 2018 | |||
|---|---|---|---|---|
| Index 1 | Car/Chl | Chl a/b | Car/Chl | Chl a/b |
| WBI | 0.12 (0.07) | −0.09 (0.04) | 0.02 (0.69) | 0.03 (0.55) |
| GMb | −0.55 (<0.001) | −0.24 (<0.001) | −0.55 (<0.001) | −0.24 (<0.001) |
| NPQI | −0.28 (<0.001) | −0.03 (0.64) | −0.17 (0.05) | −0.21 (0.01) |
| CCRI | 0.66 (<0.001) | −0.07 (0.61) | 0.69 (<0.001) | 0.38 (<0.001) |
| PRI | −0.76 (<0.001) | −0.023 (0.72) | −0.72 (<0.001) | −0.30 (<0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moley, L.M.; Goodin, D.G.; Winslow, W.P. Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA. Environments 2022, 9, 42. https://doi.org/10.3390/environments9040042
Moley LM, Goodin DG, Winslow WP. Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA. Environments. 2022; 9(4):42. https://doi.org/10.3390/environments9040042
Chicago/Turabian StyleMoley, Laura M., Douglas G. Goodin, and William P. Winslow. 2022. "Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA" Environments 9, no. 4: 42. https://doi.org/10.3390/environments9040042
APA StyleMoley, L. M., Goodin, D. G., & Winslow, W. P. (2022). Leaf-Level Spectroscopy for Analysis of Invasive Pest Impact on Trees in a Stressed Environment: An Example Using Emerald Ash Borer (Agrilus planipennis Fairmaire) in Ash Trees (Fraxinus spp.), Kansas, USA. Environments, 9(4), 42. https://doi.org/10.3390/environments9040042






