Abstract
Monitoring of freshwaters allows the detection of the impacts of multiple anthropic uses and activities on aquatic ecosystems, and an eco-sustainable management of natural resources could limit these impacts. In this work, we highlighted two main issues affecting inland waters, referring to findings from the most inhabited Italian region (Lombardy, approximately 10 M inhabitants): the first issue is lake pollution by old generation pesticides, the second is river development for hydropower. In both cases, some management strategies reducing the anthropic impacts on freshwaters were discussed: organic farming and biocontrol as an alternative to diffuse pollution by agrochemicals; environmental flows and controlled sediment flushing operations to limit the hydropower impact on rivers. Although the two mentioned issues were discussed separately in this paper, the management of water resources should be carried out in a comprehensive way, accounting for the multiple impacts affecting freshwater ecosystems, including those related to the climate changes.
1. Introduction
Aside from the current climate changes [1,2], freshwaters are affected by many direct anthropic impacts (e.g., dams interrupting river continuity and altering hydrological, thermal and sediment regimes, channelization, wastewater discharges, diffuse pollution, introduction of invasive alien species) [3,4,5,6,7,8,9,10,11,12]. Up to now, some efforts have been made to restore freshwater ecosystems and adopt mitigation measures aimed at conserving their biodiversity. However, many issues persist, and new ones emerge connected to the exploitation of freshwater resources (that are renewable but finite) supporting population livelihood and other anthropic activities [13,14].
In order to support sustainable land and water management, freshwater ecosystems, including their natural components and the anthropic pressures affecting them, need to be characterized in terms of both water quantity and quality [10,15]. Accurate monitoring is essential to improve the knowledge of the structure and the functions of these ecosystems, to assess their health status, and to evaluate the impact of the mentioned anthropic pressures. Through reliable information coming from monitoring data, the management of freshwater resources can be planned and improved in order to reduce the environmental impact of multiple anthropic uses and activities [16,17].
Based on the authors’ research experiences, this work focused on two crucial issues for the management of water resources and the conservation of freshwater ecosystems. Referring to monitoring data and studies carried out in the Lombardy Region (Northern Italy) these issues are: (i) lake pollution by old generation pesticides (i.e., DDT–dichlorodiphenyltrichloroethane—and its metabolites and isomers—hereafter referred to as DDx) and related new frontiers on biocontrol, and (ii) river exploitation for hydropower and related eco-sustainable management strategies. Both issues are included in the list of the major threats (i.e., water pollution, flow modification, and habitat degradation) to freshwater biodiversity [13].
Lombardy (23,864 km2 area) is well suited for this kind of investigation due to its richness in freshwaters (Figure 1). For instance, six relatively large (up to 370 km2) lakes, mainly of glacial origin, characterize the landscape in the northern area of the region. Additionally, the hydrographic network is extremely heterogeneous, ranging from small Alpine streams to large lowland rivers draining into the Po River (i.e., the longest Italian River, marking the southern border of the region) [18,19].
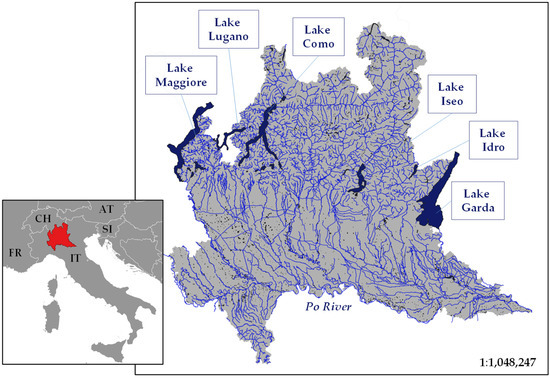
Figure 1.
Surface waters of the Lombardy Region (Northern Italy). The main six lakes along with the Po River are indicated in the map.
The monitoring of Lombardy waterbodies, carried out by the regional environmental protection agency according to the European Union (EU) Water Framework Directive requirements, indicated that, in the period 2014–2019, 13% of 54 lakes/reservoirs and 23% of 679 rivers were in poor or bad ecological status, and 35% and 34% in moderate ecological status, respectively. The chemical status, considering environmental quality standards for a list of priority substances (including heavy metals, pesticides, polycyclic aromatic hydrocarbons, and solvents) was not good for 11% of lakes/reservoirs and 28% of rivers [18,19].
Moreover, Lombardy is the most inhabited Italian region, having a total population of 9,964,993 inhabitants, and a density of 418 inhabitants/km2. It is also the largest, most industrialized, and economically important region of Northern Italy, characterized by intensive land use, widespread urbanization, large agricultural production, and increasing energy demand, i.e., all structural elements which overlap and interact with the global climate change [20].
2. Methodology
In this work, monitoring data collected by the authors in previous studies were analyzed, in order to describe and discuss the two issues mentioned in the Introduction. Particularly, for the first topic (i.e., lake pollution by DDx), the concentrations of DDx measured in pools of shads (i.e., Alosa agone, one of the fattest lake fish) from Lake Como in 1985 and from 2005 to 2014 were considered. Details on the collection and analysis of these data were reported in [21,22]. For the latter topic (i.e., river development for hydropower), the assemblage composition and community structure of benthic macroinvertebrates (i.e., widely used freshwater bioindicators) sampled in an Alpine stream, impounded by a hydropower reservoir, in 2005–2006 and 2014–2015 were compared. Details on the collection and analysis of these data were reported in [23,24]. In addition, to highlight the use of biological active substances, and the status of organic farming and hydropower in Lombardy in recent years, data from annual regional reports [25,26,27,28,29] were pooled and commented. Moreover, literature research on both subjects with specific focus on works carried out in Lombardy was performed.
3. Is DDT the Past and Biocontrol the Future?
3.1. Levels, Trends, and Sources of DDx Pollution
DDT was one of the first synthetic pesticides produced in large quantities and widely dispersed in the environment for agriculture and pest control since the Second World War. It remained the most extensively used insecticide at the global scale until the mid-1960s. The commercial product contains up to 85% of pp’DDT, and up to 20% of op’DDT, with minor proportion of pp’ and op’ DDD (dichlorodiphenyldichloroethane) and DDE (dichlorodiphenyldichloroethylene); once released into the environment, pp’DDT is mainly metabolized to pp’DDE. When DDx characteristics as persistent organic pollutants (i.e., environmentally persistent, lipophilic, semi-volatile and toxic compounds able to bioaccumulate up to the food web and move for long distances) emerged in the 1970s, DDT production and use was banned in many countries. Today DDx are included in the Stockholm Convention on persistent organic pollutants to achieve their global elimination. However, the product is still manufactured in North Korea, India, and China, and used in some Asian and African countries, mainly to control malaria, even if illegal trafficking and use of DDT in sectors other than health are still present [30,31,32].
According to Li and Macdonald [33], the global DDT production estimated since the 1940s amounts to 4.5 Mt, and the total estimated use in agriculture from 1950 to the mid-1990s is approximately 2.6 Mt. Among the 10 countries with the highest usage in agriculture, Italy ranks ninth (46 kt), and first in the EU. In Italy DDT was banned in 1978 (D.M. 11-10-1978). However, DDT production continued in a large chemical factory at Pieve Vergonte, along the Toce River (i.e., one major tributary of Lake Maggiore, Figure 1), until it was stopped in 1996. The activity of this plant from the 1940s to the mid-1990s caused intense DDx pollution of the Toce River and Lake Maggiore ecosystems: commercial fishing was banned because DDx concentrations reached values largely exceeding the limit for human consumption. After the closure of the industrial activities, the treatment of effluents and the stabilization of contaminated soils, the DDx inputs into the aquatic ecosystems dropped significantly: in fact, a decreasing trend of the DDx concentrations was observed in shad and whitefish (Coregonus lavaretus) specimens caught in Lake Maggiore between 2001 and 2015 [34], and in a sediment core from the Toce River between 2001 and 2018 [35].
Apart from this primary source of contamination, a secondary source was identified in the melting ice of Alpine glaciers. As mentioned above, before the ban, DDT was largely used for agriculture in Italy; specifically, it was also adopted for fruit-tree treatment in valleys nearby the glaciers. During this period, DDx were probably trapped in glaciers and then released into glacier-fed lakes, such as Lake Como and Lake Iseo (Figure 1), in recent years, as the consequence of glacier retreat. This secondary source of contamination determined the very high levels of DDx detected in the fauna of Lake Como and Lake Iseo in 2005, 27 years after the DDT ban [21]. After 2005, a decreasing trend of contamination was observed in Lake Como fish (Figure 2), probably related to the absence of further complete lake circulation events. In fact, DDx coming from the glaciers are likely transported into the lake mainly in association to suspended particles, and then are stored in bottom sediments until a complete lake overturn re-suspends them [36,37]. The analysis of sediment cores from Lake Como and Lake Iseo supports the hypothesis that the recent retreat of glaciers represents a secondary pollution source for old pesticides stored in the ice at the time of their widespread use: a sharp increase of DDx concentrations from the early 1990s (i.e., long after DDT agricultural use was banned in Italy) was detected in the sediment core from Lake Iseo [36], and no significant decrease over time was detected in the sediment core from Lake Como, covering a time-interval from the DDT ban to recent times [37].
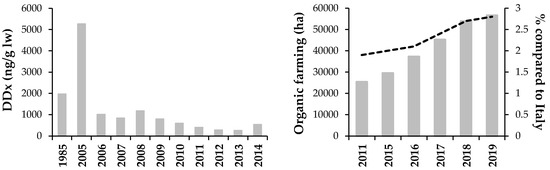
Figure 2.
Trend of DDx (concentrations are expressed on lipid weight-lw) from 1985 to 2014 in Lake Como shad Alosa agone (modified from [21,22]) (left). Total area (hectares) of organic farming in Lombardy, and the percentage compared to Italy (data from [25,26,27,28,29]) (right).
The relatively high concentrations of DDx recorded in 2008 and 2014 in Lake Como fish (Figure 2) could be related to a tertiary source of contamination, i.e., the thousands of tons of sediments (mainly composed of glacial silt) stored in the reservoirs located in the catchment of the Adda River (i.e., the main tributary of Lake Como), and released in the downstream rivers through sediment flushing operations which started in 2006 [23,38,39,40,41]. Flushed sediments partly deposit on the riverbed and then were transported to the lake by the Adda River [22].
Apart from occasional inputs related to sediment flushing from reservoirs, the current decreasing trend of DDx could be interrupted in the future by complete overturns of the lake water column during cold winters. The higher DDx concentrations detected in zooplankton compared to zooplanktivorous fish (a thermodynamic paradox in terms of bioaccumulation) from lakes Maggiore [42,43], Iseo [44], and Como [45] confirmed that the abiotic and biotic lake ecosystem components are still in unsteady condition. Although DDx are lipophilic compounds, they were detected along the pelagic water column of Lake Maggiore from 2003 to 2009, with a homogenization of the contamination starting from the end of 2006, in absence of hydrological events such as the extent of water circulation or the occurrence of significant river floods transporting polluted soils and river sediments into the lake [42].
The comparison between DDx concentrations (expressed on lipid weight-lw) in shad samples collected in 2009 in four of the major Lombardy lakes (Figure 1), showed the following ranking: Lake Maggiore (1372 ng/g lw, [34]) > Lake Como (796 ng/g lw, [22]) > Lake Iseo (539 ng/g lw, [44]) > Lake Lugano (272 ng/g lw, [34]). The level detected in Lake Maggiore fish exceeded the limit set for human consumption (D.Lgs. 172/2015), while that of Lake Como approached this limit. Thus, both ecological and sanitary issues associated to DDT pollution are still urgent.
Among DDx, it is well-known that pp’DDE is an antiadrogenic compound while op’DDT was found to determine pseudo-estrogenic effects on several organisms [46]. The high levels of DDx (29 mg/kg lw) detected in zebra mussels (Dreissena polymorpha) collected from Lake Iseo in 2005 were considered as the main responsible of histopathological damages (oocyte degeneration) [21]. In 2003, concentrations of DDx in the sediments from the Lake Maggiore outlet reached levels (4786 μg/kg) exceeding the threshold for harmful effects on aquatic organisms [35]. However, zooplankton samples collected from lakes Maggiore, Como and Iseo [42,43,44,45] did not exceed the pp’DDE concentration causing the impairment of the grazing activity of Daphnia magna (24 ng/mg dw) or the lowest observed effect concentration for fecundity reduction (109 ng/mg dw) [47]. Moreover, potential carcinogenic and non-carcinogenic effects of DDx due to the daily consumption of skinless and boneless fillet of shad from Lake Como could be excluded [22]. However, additive or synergistic effects due to cocktails of toxic pollutants may occur.
3.2. From Chemical Pesticides to Biocontrol
Many other chemical pesticides other than DDT have been applied in agriculture. In their review on the occurrence of 161 emerging organic compounds in the Italian surface water and groundwater, Meffe and de Bustamante [48] reported a serious contamination status especially by pesticides, followed by industrial chemicals and, to a lesser extent, pharmaceuticals. Several of the 139 pesticides considered in that study, 64 in surface water and 56 in groundwater, displayed concentrations higher than the environmental limits defined in the EU Directives 2006/118/EC and 2008/105/EC. Specifically, the highest concentrations in surface water have been observed for the metabolite of glyphosate AMPA, the herbicides terbuthylazine, diuron, 2,4-DB, 2,4-D, terbutryne and metolachlor, the insecticide malathion and the herbicide linuron. Other pesticides representing the most severe threat for the Italian water resources (environmental concentrations of thousands of ng/L) are dieldrin, simazine, cadusafos, endosulfan sulfate, azoxystrobin, metolachlor, pendimethalin, bentazone, and alachlor. Among them, the four most ubiquitous compounds were AMPA, glyphosate, terbuthylazine and terbuthylazine-desethyl.
One possible solution to overcome the issues related to the use of synthetic chemical pesticides in agriculture is the application of integrated pest management practices [49,50]. Specifically, the application of environmentally-friendly methods based on the use of bioinsecticides, such as predatory insects and mites, parasitoids, parasites, and microbial pathogens, should be intensified. In fact, despite the increasing amount of research on this topic [51,52,53,54], biocontrol as well as overall organic farming (i.e., the integrated farming system that strives for sustainability, the enhancement of soil fertility and biological diversity while, with rare exceptions, prohibiting synthetic pesticides, antibiotics, synthetic fertilizers, genetically modified organisms, and growth hormones - EC Regulations n. 834/2007, n. 889/2008; EU Regulation 848/2018) are still poorly applied.
In 2016, organic farming covered 58 billion of hectares at the global scale, only 1.2% of the total agricultural area, and Italy ranked sixth, with a contribution of 1.8 billion of hectares [55]. In the same year, in the Lombardy Region, organic farming covered an area of 24,462 ha, i.e., 2.6% of the utilized agricultural area. However, in recent years, an increasing trend was recorded: from 2015 to 2019, the mentioned percentage was almost doubled, from 3% to approximately 6% [25,26,27,28,29], thus increasing the contribution of the Lombardy to the Italian organic farming (Figure 2).
Focusing on the use of biological active substances in Lombardy, some discordant results are available (Table 1). The use of substances of animal and plant origin (e.g., plant oils, azadirachtin, lecithin, pyrethrins from Chrysanthemum cinerariaefolium) was always lower in the period 2014–2018 compared to 2010, while the use of microorganisms (e.g., Bacillus thuringiensis, Beauveria bassiana) was only slightly higher, and that of other substances (e.g., copper, sulfur, ethylene, ferric phosphate, paraffin oil) increased by far in recent years. However, in Italy, from 2003 to 2016 the use of biological active substances increased up to one order of magnitude (from 47 to 409 tons), while the use of fungicides (from 42,906 to 26,062 tons), insecticides and acaricides (from 8710 to 4039 tons) was approximately halved [55].

Table 1.
Use (in tons) of biological active substances for crop protection in Lombardy from 2014 to 2018 (data from [25,26,27,28,29]). The percentage respect to the Italian use is reported within brackets. Moreover, the percentage of variation in the use compared to the year 2010 is shown.
Many reasons can explain the slow take-off of both organic farming and biocontrol. Although in this sector both international and national funds, along with the market demand and other incentives (e.g., education, research), are increasing, the combination of agronomic and regulatory constraints considerably limit the success of organic farming. This kind of farming, unlike the conventional one, has fewer alternatives of choice, both in terms of techniques of crops defense and as a range of substances usable for this purpose, and requires more manpower (e.g., biological active substances require more applications than synthetic substances). Moreover, the current oligopolistic market of pesticides is still dominated by products for conventional farming: in 2017, only 4 out of the 65 billion of dollars employed in the sector pertained products for organic farming. In addition, among the latter, innovative products that can compete with those based on sulfur and copper are few [55].
In the field of products of biological origin, there is a strong development of research oriented to the use of antagonistic microorganisms of main pathogens rather than synthetic molecules. However, many studies on new biocontrol techniques have been carried out in the laboratory [56,57,58,59], still lacking the assessment obtained with field trials and the knowledge transfer from research to industry. The combined use of bioinsecticides (e.g., Bacillus thuringiensis and entomopathogenic nematodes) seems a promising frontier allowing to increase the efficacy of biocontrol methods by reducing the time needed to eradicate pest populations [60,61].
Another factor that is limiting the success of both organic farming and biocontrol, is the continuous and intensive use of agrochemicals in the surrounding of the fields cultivated biologically. On the one hand, residues of pesticides can contaminate biological products. Moreover, some commonly used synthetic pesticides can accumulate in the soil and interfere with the life cycles of entomopathogens used for biocontrol, such as nematodes and fungi [62,63]. This may determine an increase of the cost of biocontrol techniques or even nullify the effect of their application.
4. The “Green Hydropower” Oxymoron
4.1. Current Status of Hydropower
Hydropower is a renewable and clean energy source, since it relies on the natural water cycle and poorly contributes to greenhouse emissions into the atmosphere, but its development frequently induces significant environmental alterations. Thus, it cannot be considered a fully green energy source. Along with land-use changes, urbanization, channelization, and sediment mining, hydropower development, mainly consisting in the spread of several dams and diversions, has contributed to the relevant morphological alterations of rivers, causing substantial changes to the flow and sediment regimes [64,65].
Hydropower is still the largest source of renewable electricity globally, and is projected to grow further in the next decades. It supplied around 16% of global power in 2019 – roughly three times the generation of wind power and six times that of solar power. Global electricity production from hydropower has increased by around two-thirds since 2000. At least 3700 major dams, each with a capacity of more than 1 MW, are either planned or under construction, primarily in countries with emerging economies. These dams are predicted to increase the present global hydroelectricity capacity by 73% to about 1700 GW [66]. However, though dams provide support to economic and social development worldwide, they have affected most rivers with associated environmental impacts. The presence of dams disconnects once integrated free-flowing systems [67], and river impounding jeopardizes the biodiversity downstream [68]. Thus, the safe operation of dams has significant social, economic, and environmental relevance, and appropriate management procedures are necessary [69,70].
In the EU countries, hydropower accounts for over 14% of electricity generation, and 70% of EU hydropower is from five main countries, including Italy. Here, hydropower still accounts for more than a third of the total renewable electricity production. The total number of hydropower facilities in 2019 was 4401, almost doubled than in 2009 (2249). However, the corresponding increase in terms of total power generated was low (about 0.7%), due to the small capacity of the recently implemented projects (mini-hydropower plants). Specifically, the installation of mini-hydro began in the early 2000s, and progressed to the extent that the average size of hydropower plants in Italy fell by around a half, from 8.4 MW per plant at the beginning of the century to 4.4 MW in 2018 [71].
Among the Italian regions, Lombardy ranks first in terms of generated hydroelectricity, about 27% of the national production, and second in terms of number of hydropower stations (Table 2). As highlighted at the country scale, also in Lombardy an increase in the number of hydropower plants in recent years (from 544 in 2015 to 661 in 2019) is evident, but with negligible impact over the overall generation (Table 2). However, in developed regions such as Lombardy, hydropower is undergoing a general increase of efficiency and environmental sustainability.

Table 2.
Hydropower in Lombardy from 2015 to 2019 (data from [25,26,27,28,29]). The percentage compared to Italy is reported within brackets.
4.2. Towards Environmental Flow and Sediment Management
The increasing environmental awareness has prompted the widespread research of new management strategies aimed at mitigating the impacts of hydropower on riverine ecosystems. However, the implementation of environmental conservation measures is generally underdeveloped [72,73,74,75,76], neglecting the full range of alterations of the hydrological and sediment transport regimes.
The release of minimum flows (MFs) is yet one main measure that has been globally adopted to mitigate the hydrological alterations of regulated rivers subjected to water withdrawal [73,74,75,77,78]. However, this is an early environmental flow (e-flow) approach sufficient to maintain aquatic species during crucial low-flow periods [79]. It is based on hydrological data series, without properly considering the requirements of the aquatic communities. E-flow recommendations based on simple hydrologic rules [80,81] have been widely recognized to be inadequate for sustaining the biological structures and the functionality of aquatic ecosystems [82]. Current state-of the-art approaches, e.g., [83,84,85,86], specifically advocate that e-flow recommendations should be based on the mechanistic relationships between flows and ecological outcomes. For instance, a recent study accounting for the status of the benthic macroinvertebrate communities of both unregulated and regulated Alpine streams in Lombardy highlighted that the shift from unimpacted conditions was more significant at reaches below reservoirs than at reaches below intakes, since seasonal streamflow variation was partly preserved in the latter [87].
Besides the issues related to the e-flow, the already mature knowledge of geomorphological alteration due to river damming [88,89] is triggering the demand for managed sediment regimes [90,91,92,93]. Due to the aging of water-storage infrastructures, reservoirs siltation and related issues are receiving growing attention worldwide, and particularly in North America and Europe, where most of the dam building took place during the 1940s–1970s [94,95,96,97,98,99]. Among the sediment management techniques, sediment flushing can support tackling of the storage lost due to siltation, preserving at the same time the downstream sediment flux through dams, at least the small-sized fractions [100,101]. However, sediment flushing operations can be highly detrimental for downstream river reaches, both in terms of siltation of instream-structures and from an ecological point of view [102]. Consequently, in the last years, the research concerning sediment flushing has been focused on up-grading flushing schedule and implementation in order to reduce the downstream ecological impacts [103,104]. When sediment flushing includes objectives of downstream environmental safeguard, it can be defined as “controlled” (i.e., controlled sediment flushing operations–CSFOs). Limiting the sediment concentration of the sluiced waters is the main mitigation measure currently adopted for decreasing the flushing impact, even if thresholds for sediment concentration balancing technical, economic, and environmental aspects may be controversial [105].
Among the Italian regions, Lombardy was the first implementing MFs (since 2009), and CSFOs (since 2006). MF is currently calculated as a fixed percentage of the mean annual natural flow estimated at the intake section (Delibera Autorità di Bacino del Fiume Po n. 7/2002). The percentage set by law is usually 10%. As a result of detailed investigations, this percentage has been reduced in some cases, and a seasonal modulation was implemented, in an attempt to balancing human and environmental requirements [24,87,106]. CSFO is instead the technique most used in recent years to recover reservoir capacity in the area [41]; Lombardy accounts for approximately 470 dams, among which the largest ones (height > 15 m or reservoir capacity > 1 Mm3) are 77 (vs. 533 in Italy). The average age of these dams is 75 years, compared to 62 years of the total Italian dams.
The Roasco Stream (i.e., a tributary of the mentioned Adda River) was the first Italian watercourse where CSFOs are documented [23,105]. Impounding Roasco Stream began in 1925 by a small dam about half a kilometer below the present Valgrosina Dam, which was closed in 1960. Since 2009, mandatory MFs of 0.24 m3/s (6% of the mean annual natural flow) from November to April and 0.41 m3/s (10% of the mean annual natural flow) from May to October have been released in the Roasco Stream from an intake diverting the water of a tributary. Since 2006, CSFOs of the Valgrosina Reservoir (1.3 Mm3 capacity) have been carried out almost annually, following a consolidated protocol [23,105]. Specifically, the time of the year for executing CSFOs, the duration of the CSFO and the suspended sediment concentration averaged over the whole operation were constrained to limit downstream fish mortality. A further constraint concerned the stream quality as assessed through benthic macroinvertebrates, which would have regained pre-flushing standard by approximately six months. Thus, CSFOs of the Valgrosina Reservoir take place between August and September over approximately two weeks. The limit on the suspended sediment concentration averaged over the whole operation is 4 g/L. Each CSFO allows removing approximately 20,000 tons of sediments from the reservoir.
The comparison between the community of benthic macroinvertebrates collected before (November 2005 and March 2006) and after approximately five and ten years from the implementation of MFs and CSFOs, respectively (November 2014 and March 2015), showed that the assemblage composition remained almost unvaried (Figure 3).
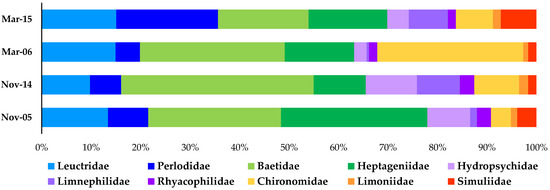
Figure 3.
Relative abundance of the main 10 families of benthic macroinvertebrates collected in the Roasco Stream before and after the implementation of minimum flows and controlled sediment flushing operations (data from [23,24]). Plecoptera families are blue-colored, Ephemeroptera are green-colored, Trichoptera are purple-colored and Diptera are orange-colored.
Specifically, total richness (17 in November and 19–20 in March), the richness of taxa belonging to the insect orders of Ephemerptera, Plecoptera and Tricopthera (11–12), including the most sensitive species, and the diversity expressed by the Shannon–Wiener index (2.0–2.2) did not change. Total density was more variable (approximately 20% less in the after samples than in the before samples) but not the relative abundance of the taxa belonging to the orders of Ephemerptera, Plecoptera and Tricopthera (83–90%, except for March 2006 that is 66% due to the high abundance of Chironomidae, Figure 3).
Accordingly, the data from pre/post monitoring of single CSFO from the Valgrosina Reservoir showed that the benthic communities were resilient enough to recover within three–six months from the end of the operation [23,107]. However, this is only an example of the effect of the current management of an Alpine regulated upland stream. In fact, site-specificities can play a key role in justifying the mentioned findings. For instance, the presence of a community that was adapted long since to the impact of hydropower exploitation, thus able to recover quickly from related alterations, and the likely recolonization from upstream unimpacted river reaches. Moreover, the availability of a wide range of microhabitats, even under minimum flow conditions, the absence of hydropeaking and thermopeaking, and the absence of a relevant sediment deposition after the CSFOs. Indeed, different impact/recovery patterns were detected when sediment deposition after the CSFOs was considerably higher, or if further anthropic impacts superimposed to those of hydropower [41]. The anthropogenic exploitation of environmental resources, described by land use classification, is indeed confirmed as a fundamental factor in predicting the status of biological communities from 42 sites, in 11 pre-Alpine rivers and streams of Lombardy. In particular, urbanization in the peri-fluvial area and agriculture, involving many related issues, such as the input of different polluting loads, are the most important limiting factor [108].
As observed by Renöfält et al. [76], in contexts highly exploited for hydropower, such as the Alps, a main challenge for river management is to identify stream reaches to prioritize for intervention (i.e., where measures involving relatively small production losses can have major ecological advantages). At the same time, advanced biomonitoring and modelling tools are required to improve quantitative prediction of the effects of hydropower [107,109], supporting a comprehensive management strategy of the water resources, aimed to sustain the production of renewable and clean energy while improving at the same time the environmental quality of exploited rivers. Moreover, water management strategies should account for the effects of current climate changes, such as the increased duration of low-flow periods [110], the increased river temperature [111] and the occurrence of catastrophic events, such as landslides increasing sediment input in regulated streams [112,113].
5. Conclusions
This paper summarizes some major issues affecting freshwater ecosystems, focusing on the most inhabited Italian region. Particularly, DDT pollution is not a matter of the past. Despite the ban of its use, the continuous monitoring of DDx should be carried on. In fact, DDx levels detected in the lakes of Lombardy in recent years are still relatively high, and both ecological and sanitary risk cannot be excluded for the next decades. In contrast, the development of organic farming and biocontrol to counteract pollution by agrochemicals is proceeding slowly. Moreover, the measures to mitigate the environmental impact of hydropower (a clean and renewable but not a fully green energy) should be improved by up-grading the current minimum flows and including sediment management into the environmental flow concept. Even if in this work pesticides pollution and hydropower were discussed in two separate sections, they can be interconnected (e.g., the mentioned potentially polluted sediments released from reservoirs in the downstream ecosystems through flushing operations) and thus their management, as well as that of other issues (e.g., morphological alteration, invasive alien species), should be carried out in a comprehensive manner. A more eco-sustainable natural resource management is necessary for pursuing the goals of the 2030 global agenda [114].
Author Contributions
Conceptualization, S.Q.; data curation, S.Q.; writing—original draft preparation, S.Q.; writing—review and editing, S.Q., P.E., S.Z., G.C., R.B., M.M.and M.F.B.; visualization, S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnell, N.W.; Gosling, S.N. The impacts of climate change on river flow regimes at the global scale. J. Hydrol. 2013, 486, 351–364. [Google Scholar] [CrossRef]
- Gudmundsson, L.; Seneviratne, S.I.; Zhang, X. Anthropogenic climate change detected in European renewable freshwater resources. Nat. Clim. Chang. 2017, 7, 813–816. [Google Scholar] [CrossRef]
- Hancock, P.J. Human impacts on the stream–groundwater exchange zone. Environ. Manag. 2002, 29, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Gleick, P.H. Global freshwater resources: Soft-path solutions for the 21st century. Science 2003, 302, 1524–1528. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Racchetti, E.; Salmaso, F.; Pinardi, M.; Quadroni, S.; Soana, E.; Sacchi, E.; Severini, E.; Celico, F.; Viaroli, P.; Bartoli, M. Is flood irrigation a potential driver of river-groundwater interactions and diffuse nitrate pollution in agricultural watersheds? Water 2019, 11, 2304. [Google Scholar] [CrossRef]
- Pericherla, S.; Karnena, M.K.; Vara, S. A review on impacts of agricultural runoff on freshwater resources. Int. J. Emerg. Tech. 2020, 11, 829–833. [Google Scholar]
- Zaccara, S.; Quadroni, S.; Vanetti, I.; Carosi, A.; La Porta, G.; Crosa, G.; Britton, R.J.; Lorenzoni, M. Morphologic and genetic variability in the Barbus fishes (Teleostei, Cyprinidae) of Central Italy. Zool. Scr. 2019, 48, 289–301. [Google Scholar] [CrossRef]
- Zaccara, S.; Quadroni, S.; De Santis, V.; Vanetti, I.; Carosi, A.; Crosa, G.; Britton, R.J.; Lorenzoni, M. Genetic and phenotypic displacement of an endemic Barbus complex by invasive European barbel Barbus barbus in central Italy. Biol. Invasions 2021, 23, 521–535. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- De Santis, V.; Quadroni, S.; Britton, R.J.; Carosi, A.; Roberts, C.G.; Lorenzoni, M.; Crosa, G.; Zaccara, S. Biological and trophic consequences of genetic introgression between endemic and invasive Barbus fishes. Biol. Invasions 2021, 23, 3351–3368. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Villéger, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- van Rees, C.B.; Waylen, K.A.; Schmidt-Kloiber, A.; Thackeray, S.J.; Kalinkat, G.; Martens, K.; Domisch, S.; Lillebø, A.I.; Hermoso, V.; Grossart, H.P.; et al. Safeguarding freshwater life beyond 2020: Recommendations for the new global biodiversity framework from the European experience. Conserv. Lett. 2021, 14, e12771. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of human impacts in freshwater ecosystems: The good, the bad and the ugly. Adv. Ecol. Res. 2011, 44, 1–68. [Google Scholar] [CrossRef]
- Salmaso, F.; Quadroni, S.; Compare, S.; Gentili, G.; Crosa, G. Benthic diatoms as bioindicators of environmental alterations in different watercourses of northern Italy. Environ. Monit. Assess. 2019, 191, 158. [Google Scholar] [CrossRef]
- Buzzi, F.; Di Piazza, R.; Genoni, P. Stato Delle Acque Superficiali in Lombardia. Laghi. Aggiornamento 2014–2019 (Revisione Luglio 2021); ARPA Lombardia, Settore Monitoraggi Ambientali: Lombardy, Italy, 2021; 45p. [Google Scholar]
- Monti, C.; Paleari, M.; Tremolada, L.; Genoni, P. Stato Delle Acque Superficiali in Regione Lombardia. Corsi D’acqua. Rapporto sessennale 2014–2019 (Marzo 2021); ARPA Lombardia, Settore Monitoraggi Ambientali: Lombardy, Italy, 2021; 105p. [Google Scholar]
- Gatto, M.; Fiorese, G.; De Leo, G. Regional impacts of global climate change on ecosystems: An analysis of the Lombardy (northern Italy) case. In Global Climate Change and the Ecology of the Next Decade; Santangelo, G., Fronzoni, L., Eds.; Edizioni ETS: Pisa, Italy, 2008; pp. 71–90. [Google Scholar]
- Bettinetti, R.; Quadroni, S.; Galassi, S.; Bacchetta, R.; Bonardi, L.; Vailati, G. Is meltwater from Alpine glaciers a secondary DDT source for lakes? Chemosphere 2008, 73, 1027–1031. [Google Scholar] [CrossRef]
- Quadroni, S.; Bettinetti, R. Health risk assessment for the consumption of fresh and preserved fish (Alosa agone) from Lago di Como (Northern Italy). Environ. Res. 2017, 156, 571–578. [Google Scholar] [CrossRef]
- Espa, P.; Castelli, E.; Crosa, G.; Gentili, G. Environmental effects of storage preservation practices: Controlled flushing of fine sediment from a small hydropower reservoir. Environ. Manag. 2013, 52, 261–276. [Google Scholar] [CrossRef]
- Quadroni, S.; Crosa, G.; Gentili, G.; Espa, P. Response of stream benthic macroinvertebrates to current water management in Alpine catchments massively developed for hydropower. Sci. Total Environ. 2017, 609, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Rama, D.; Pretolani, R. Il Sistema Agro-Alimentare Della Lombardia: Rapporto 2020; Franco Angeli Edizioni: Milan, Italy, 2021; 408p. [Google Scholar]
- Rama, D.; Pretolani, R. Il Sistema Agro-Alimentare Della Lombardia: Rapporto 2019; Franco Angeli Edizioni: Milan, Italy, 2020; 422p. [Google Scholar]
- Rama, D.; Pretolani, R. Il Sistema Agro-Alimentare Della Lombardia: Rapporto 2018; Franco Angeli Edizioni: Milan, Italy, 2019; 406p. [Google Scholar]
- Rama, D.; Pretolani, R. Il Sistema Agro-Alimentare Della Lombardia: Rapporto 2017; Franco Angeli Edizioni: Milan, Italy, 2018; 400p. [Google Scholar]
- Pieri, R.; Pretolani, R. Il Sistema Agro-Alimentare Della Lombardia: Rapporto 2016; Franco Angeli Edizioni: Milan, Italy, 2017; 434p. [Google Scholar]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Quadroni, S.; Bettinetti, R. An unnoticed issue: Organochlorine pesticides in tobacco products around the world. Chemosphere 2019, 219, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, R.; Quadroni, S.; Crosa, G.; Harper, D.; Dickie, J.; Kyalo, M.; Mavuti, K.; Galassi, S. A preliminary evaluation of the DDT contamination of sediments in Lakes Natron and Bogoria (Eastern Rift Valley, Africa). Ambio 2011, 40, 341–350. [Google Scholar] [CrossRef][Green Version]
- Li, Y.F.; Macdonald, R.W. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: A review. Sci. Total Environ. 2005, 342, 87–106. [Google Scholar] [CrossRef]
- Guzzella, L.M.; Novati, S.; Casatta, N.; Roscioli, C.; Valsecchi, L.; Binelli, A.; Parolini, M.; Solcà, N.; Bettinetti, R.; Manca, M.; et al. Spatial and temporal trends of target organic and inorganic micropollutants in Lake Maggiore and Lake Lugano (Italian-Swiss water bodies): Contamination in sediments and biota. Hydrobiologia 2018, 824, 271–290. [Google Scholar] [CrossRef]
- Marziali, L.; Guzzella, L.; Salerno, F.; Marchetto, A.; Valsecchi, L.; Tasselli, S.; Roscioli, C.; Schiavon, A. Twenty-year sediment contamination trends in some tributaries of Lake Maggiore (Northern Italy): Relation with anthropogenic factors. Environ. Sci. Pollut. Res. 2021, 28, 38193–38208. [Google Scholar] [CrossRef]
- Bettinetti, R.; Galassi, S.; Guilizzoni, P.; Quadroni, S. Sediment analysis to support the recent glacial origin of DDT pollution in Lake Iseo (Northern Italy). Chemosphere 2011, 85, 163–169. [Google Scholar] [CrossRef]
- Bettinetti, R.; Quadroni, S.; Boggio, E.; Galassi, S. Recent DDT and PCB contamination in the sediment and biota of the Como Bay (Lake Como, Italy). Sci. Total Environ. 2016, 542, 404–410. [Google Scholar] [CrossRef]
- Espa, P.; Crosa, G.; Gentili, G.; Quadroni, S.; Petts, G. Downstream ecological impacts of controlled sediment flushing in an Alpine valley river: A case study. River. Res. Appl. 2015, 31, 931–942. [Google Scholar] [CrossRef]
- Espa, P.; Brignoli, M.L.; Crosa, G.; Gentili, G.; Quadroni, S. Controlled sediment flushing at the Cancano Reservoir (Italian Alps): Management of the operation and downstream environmental impact. J. Environ. Manag. 2016, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Quadroni, S.; Brignoli, M.L.; Crosa, G.; Gentili, G.; Salmaso, F.; Zaccara, S.; Espa, P. Effects of sediment flushing from a small Alpine reservoir on downstream aquatic fauna. Ecohydrology 2016, 9, 1276–1288. [Google Scholar] [CrossRef]
- Espa, P.; Batalla, R.J.; Brignoli, M.L.; Crosa, G.; Gentili, G.; Quadroni, S. Tackling reservoir siltation by controlled sediment flushing: Impact on downstream fauna and related management issues. PLoS ONE 2019, 14, e0218822. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, R.; Galassi, S.; Guzzella, L.; Quadroni, S.; Volta, P. The role of zooplankton in DDT biomagnification in a pelagic food web of Lake Maggiore (Northern Italy). Environ. Sci. Pollut. Res. 2010, 17, 1508–1518. [Google Scholar] [CrossRef]
- Bettinetti, R.; Quadroni, S.; Manca, M.; Piscia, R.; Volta, P.; Guzzella, L.; Roscioli, C.; Galassi, S. Seasonal fluctuations of DDTs and PCBs in zooplankton and fish of Lake Maggiore (Northern Italy). Chemosphere 2012, 88, 344–351. [Google Scholar] [CrossRef]
- Bettinetti, R.; Garibaldi, L.; Leoni, B.; Quadroni, S.; Galassi, S. Zooplankton as an early warning system of persistent organic pollutants contamination in a deep lake (lake Iseo, Northern Italy). J. Limnol. 2012, 71, 335–338. [Google Scholar] [CrossRef]
- Mazzoni, M.; Boggio, E.; Manca, M.; Piscia, R.; Quadroni, S.; Bellasi, A.; Bettinetti, R. Trophic transfer of persistent organic pollutants through a pelagic food web: The case of Lake Como (Northern Italy). Sci. Total Environ. 2018, 640, 98–106. [Google Scholar] [CrossRef]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT metabolite p, p’–DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef]
- Bettinetti, R.; Croce, V.; Noè, F.; Ponti, B.; Quadroni, S.; Galassi, S. Ecotoxicity of pp’DDE to Daphnia magna. Ecotoxicology 2013, 22, 1255–1263. [Google Scholar] [CrossRef]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007, 17, 547–559. [Google Scholar]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Quadroni, S.; Toscano, A.; Mottadelli, N.; Brivio, M.F. Susceptibility to entomopathogens and modulation of basal immunity in two insect models at different temperatures. J. Therm. Biol. 2019, 79, 15–23. [Google Scholar] [CrossRef]
- Hajek, A.E.; Gardescu, S.; Delalibera, I. Summary of classical biological control introductions of entomopathogens and nematodes for insect control. Biocontrol 2021, 66, 167–180. [Google Scholar] [CrossRef]
- RRN—Rete Rurale Nazionale. Bioreport 2017–2018. In Agricoltura Biologica in Italia; Programma Rete Rurale Nazionale 2014–2020: Roma, Italy, 2019; 185p. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Trdan, S. Oulema melanopus (L.)(Coleoptera: Chrysomelidae) adults are susceptible to entomopathogenic nematodes (Rhabditida) attack: Results from a laboratory study. J. Plant Dis. Prot. 2010, 117, 30–32. [Google Scholar] [CrossRef]
- Mastore, M.; Binda Rossetti, S.; Giovannardi, S.; Scari, G.; Brivio, M.F. Inducible factors with antimicrobial activity after immune challenge in the haemolymph of Red Palm Weevil (Insecta). Innate Immun. 2015, 21, 392–405. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M. When appearance misleads: The role of the entomopathogen surface in the relationship with its host. Insects 2020, 11, 387. [Google Scholar] [CrossRef]
- De Lerma Barbaro, A.; Gariboldi, M.B.; Mastore, M.; Brivio, M.F.; Giovannardi, S. In Vivo Effects of a pro-PO system Inhibitor on the phagocytosis of Xenorhabdus nematophila in Galleria mellonella Larvae. Insects 2019, 10, 263. [Google Scholar] [CrossRef]
- Mastore, M.; Quadroni, S.; Brivio, M.F. Susceptibility of Drosophila suzukii larvae to the combined administration of the entomopathogens Bacillus thuringiensis and Steinernema carpocapsae. Sci. Rep. 2021, 11, 8149. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Caramella, S.; Quadroni, S.; Brivio, M.F. Drosophila suzukii Susceptibility to the Oral Administration of Bacillus thuringiensis, Xenorhabdus nematophila and Its Secondary Metabolites. Insects 2021, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Poprawski, J.T.; Majchrowicz, I. Effects of herbicides on in vitro vegetative growth and sporulation of entomopathogenic fungi. Crop Prot. 1995, 14, 81–87. [Google Scholar] [CrossRef]
- Özdemir, E.; İnak, E.; Evlice, E.; Laznik, Z. Compatibility of entomopathogenic nematodes with pesticides registered in vegetable crops under laboratory conditions. J. Plant Dis. Prot. 2020, 127, 529–535. [Google Scholar] [CrossRef]
- Surian, N.; Rinaldi, M. Morphological response to river engineering and management in alluvial channels in Italy. Geomorphology 2003, 50, 307–326. [Google Scholar] [CrossRef]
- Comiti, F. How natural are Alpine mountain rivers? Evidence from the Italian Alps. Earth Surf. Processes Landf. 2012, 37, 693–707. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Graf, W.L. Dam nation: A geographic census of American dams and their largescale hydrologic impacts. Water Resour. Res. 1999, 35, 1305–1311. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Pisaniello, J.D.; Tingey-Holyoak, J.; Burritt, R.L. Appropriate small dam management for minimizing catchment-wide safety threats: International benchmarked guidelines and demonstrative cases studies. Water Resour. Res. 2012, 48, W01546. [Google Scholar] [CrossRef]
- Bocchiola, D.; Rosso, R. Safety of Italian dams in the face of flood hazard. Adv. Water Resour. 2014, 71, 23–31. [Google Scholar] [CrossRef]
- Available online: https://www.enelgreenpower.com/learning-hub/renewable-energies/hydroelectric-energy/italy (accessed on 6 October 2021).
- Grantham, T.E.; Viers, J.H.; Moyle, P.B. Systematic screening of dams for environmental flow assessment and implementation. Bioscience 2014, 64, 1006–1018. [Google Scholar] [CrossRef]
- Mezger, G.; De Stefano, L.; del Tánago, M.G. Assessing the establishment and implementation of environmental flows in Spain. Environ. Manag. 2019, 64, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Opperman, J.J.; Kendy, E.; Barrios, E. Securing environmental flows through system reoperation and management: Lessons from case studies of implementation. Front. Environ. Sci. 2019, 7, 104. [Google Scholar] [CrossRef]
- Ramos, V.; Formigo, N.; Maia, R. Environmental flows under the WFD implementation. Water Resour. Manag. 2018, 32, 5115–5149. [Google Scholar] [CrossRef]
- Renöfält, B.M.; Jansson, R.; Nilsson, C. Effects of hydropower generation and opportunities for environmental flow management in Swedish riverine ecosystems. Freshw. Biol. 2010, 55, 49–67. [Google Scholar] [CrossRef]
- Ma, L.; Wang, H.; Qi, C.; Zhang, X.; Zhang, H. Characteristics and adaptability assessment of commonly used ecological flow methods in water storage and hydropower projects, the case of Chinese river basins. Water 2019, 11, 2035. [Google Scholar] [CrossRef]
- Wu, M.; Chen, A.; Zhang, X.; McClain, M.E. A comment on Chinese policies to avoid negative impacts on river ecosystems by hydropower projects. Water 2020, 12, 869. [Google Scholar] [CrossRef]
- Tennant, D.L. Instream flow regimens for fish, wildlife, recreation and related environmental resources. Fisheries 1976, 1, 6–10. [Google Scholar] [CrossRef]
- McKay, S.K. Quantifying tradeoffs associated with hydrologic environmental flow methods. J. Am. Water Resour. Assoc. 2015, 51, 1508–1518. [Google Scholar] [CrossRef]
- Acreman, M. Environmental flows—Basics for novices. WIREs Water 2016, 3, 622–628. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Poff, N.L.; Richter, B.D.; Arthington, A.H.; Bunn, S.E.; Naiman, R.J.; Kendy, E.; Acreman, M.; Apse, C.; Bledsoe, B.P.; Freeman, M.C.; et al. The ecological limits of hydrologic alteration (ELOHA): A new framework for developing regional environmental flow standards. Freshw. Biol. 2010, 55, 147–170. [Google Scholar] [CrossRef]
- Richter, B.D.; Davis, M.M.; Apse, C.; Konrad, C. A presumptive standard for environmental flow protection. River Res. Appl. 2012, 28, 1312–1321. [Google Scholar] [CrossRef]
- Yin, X.A.; Yang, Z.F.; Petts, G.E. Optimizing environmental flows below dams. River Res. Appl. 2012, 28, 703–716. [Google Scholar] [CrossRef]
- Yarnell, S.M.; Petts, G.E.; Schmidt, J.C.; Whipple, A.A.; Beller, E.E.; Dahm, C.N.; Goodwin, P.; Viers, J.H. Functional flows in modified riverscapes: Hydrographs, habitats and opportunities. Bioscience 2015, 65, 963–972. [Google Scholar] [CrossRef]
- Quadroni, S.; Salmaso, F.; Gentili, G.; Crosa, G.; Espa, P. Response of benthic macroinvertebrates to different hydropower off-stream diversion schemes. Ecohydrology 2021, 14, e2267. [Google Scholar] [CrossRef]
- Graf, W.L. Downstream hydrologic and geomorphic effects of large dams on American rivers. Geomorphology 2006, 79, 336–360. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Wilcock, P.R. Metrics for assessing the downstream effects of dams. Water Resour. Res. 2008, 44, W04404. [Google Scholar] [CrossRef]
- Wohl, E.; Bledsoe, B.P.; Jacobson, R.B.; Poff, N.L.; Rathburn, S.L.; Walters, D.M.; Wilcox, A.C. The natural sediment regime in rivers: Broadening the foundation for ecosystem management. BioScience 2015, 65, 358–371. [Google Scholar] [CrossRef]
- Gabbud, C.; Lane, S.N. Ecosystem impacts of Alpine water intakes for hydropower: The challenge of sediment management. WIREs Water 2016, 3, 41–61. [Google Scholar] [CrossRef]
- Chamoun, S.; De Cesare, G.; Schleiss, A.J. Management of turbidity current venting in reservoirs under different bed slopes. J. Environ. Manag. 2017, 204, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Gabbud, C.; Robinson, C.T.; Lane, S.N. Summer is in winter: Disturbance-driven shifts in macroinvertebrate communities following hydroelectric power exploitation. Sci. Total Environ. 2019, 650, 2164–2180. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.L.; Wohl, E.; Sinha, T.; Sabo, J.L. Sedimentation and sustainability of western American reservoirs. Water Resour. Res. 2010, 46, W12535. [Google Scholar] [CrossRef]
- Juracek, K.E. The aging of America’s reservoirs: In-reservoir and downstream physical changes and habitat implications. J. Am. Water Resour. Assoc. 2015, 51, 168–184. [Google Scholar] [CrossRef]
- George, M.W.; Hotchkiss, R.H.; Huffaker, R. Reservoir sustainability and sediment management. J. Water Res. Plan Man. 2016, 143, 04016077. [Google Scholar] [CrossRef]
- Schleiss, A.J.; Franca, M.J.; Juez, C.; De Cesare, G. Reservoir sedimentation. J. Hydraul. Res. 2016, 54, 595–614. [Google Scholar] [CrossRef]
- Ho, M.; Lall, U.; Allaire, M.; Devineni, N.; Kwon, H.H.; Pal, I.; Raff, D.; Wegner, D. The future role of dams in the United States of America. Water Resour Res. 2017, 53, 982–998. [Google Scholar] [CrossRef]
- Hauer, C.; Wagner, B.; Aigner, J.; Holzapfel, P.; Flödl, P.; Liedermann, M.; Tritthart, M.; Sindelar, C.; Pulg, U.; Klösch, M.; et al. State of the art, shortcomings and future challenges for a sustainable sediment management in hydropower: A review. Renew. Sust. Energ. Rev. 2018, 98, 40–55. [Google Scholar] [CrossRef]
- Kondolf, G.M.; Gao, Y.; Annandale, G.W.; Morris, G.L.; Jiang, E.; Zhang, J.; Cao, Y.; Carling, P.; Fu, K.; Guo, Q.; et al. Sustainable sediment management in reservoirs and regulated rivers: Experiences from five continents. Earth’s Future 2014, 2, 256–280. [Google Scholar] [CrossRef]
- Wang, H.W.; Kondolf, M.; Tullos, D.; Kuo, W.C. Sediment Management in Taiwan’s Reservoirs and Barriers to Implementation. Water 2018, 10, 1034. [Google Scholar] [CrossRef]
- Morris, G.L.; Fan, J. Reservoir Sedimentation Handbook: Design and Management of Dams, Reservoir, and Watersheds for Sustainable Use; McGraw-Hill Book Co.: New York, NY, USA, 1997; 848p. [Google Scholar]
- Annandale, G.W.; Morris, G.L.; Karki, P. Extending the Life of Reservoirs; World Bank Publications: Washington, DC, USA, 2016; 193p. [Google Scholar] [CrossRef]
- Reckendorfer, W.; Badura, H.; Schütz, C. Drawdown flushing in a chain of reservoirs—Effects on grayling populations and implications for sediment management. Ecol. Evol. 2019, 9, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Crosa, G.; Castelli, E.; Gentili, G.; Espa, P. Effects of suspended sediments from reservoir flushing on fish and macroinvertebrates in an Alpine stream. Aquat. Sci. 2010, 72, 85–95. [Google Scholar] [CrossRef]
- Salmaso, F.; Crosa, G.; Espa, P.; Gentili, G.; Quadroni, S.; Zaccara, S. Benthic macroinvertebrates response to water management in a lowland river: Effects of hydro-power vs irrigation off-stream diversions. Environ. Monit. Assess. 2018, 190, 33. [Google Scholar] [CrossRef]
- Doretto, A.; Piano, E.; Fenoglio, S.; Bona, F.; Crosa, G.; Espa, P.; Quadroni, S. Beta-diversity and stressor specific index reveal patterns of macroinvertebrate community response to sediment flushing. Ecol. Indic. 2021, 122, 107256. [Google Scholar] [CrossRef]
- Calabrese, S.; Mezzanotte, V.; Marazzi, F.; Canobbio, S.; Fornaroli, R. The influence of multiple stressors on macroinvertebrate communities and ecosystem attributes in Northern Italy pre-Alpine rivers and streams. Ecol. Indic. 2020, 115, 106408. [Google Scholar] [CrossRef]
- Larsen, S.; Bruno, M.C.; Zolezzi, G. WFD ecological status indicator shows poor correlation with flow parameters in a large Alpine catchment. Ecol. Indic. 2019, 98, 704–711. [Google Scholar] [CrossRef]
- Salmaso, F.; Crosa, G.; Espa, P.; Quadroni, S. Climate Change and Water Exploitation as Co-Impact Sources on River Benthic Macroinvertebrates. Water 2021, 13, 2778. [Google Scholar] [CrossRef]
- Salmaso, F.; Quadroni, S.; Gentili, G.; Crosa, G. Thermal regime of a highly regulated Italian river (Ticino River) and implications for aquatic communities. J. Limnol. 2017, 76, 23–33. [Google Scholar] [CrossRef]
- Salmaso, F.; Crosa, G.; Espa, P.; Gentili, G.; Quadroni, S. The year after an extraordinary sedimentation event in a regulated Alpine river: The impact on benthic macroinvertebrate communities. River Res. Appl. 2020, 36, 1656–1667. [Google Scholar] [CrossRef]
- Salmaso, F.; Espa, P.; Crosa, G.; Quadroni, S. Impacts of fine sediment input on river macroinvertebrates: The role of the abiotic characteristics at mesohabitat scale. Hydrobiologia 2021, 848, 4189–4209. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations General Assembly: New York, NY, USA, 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).