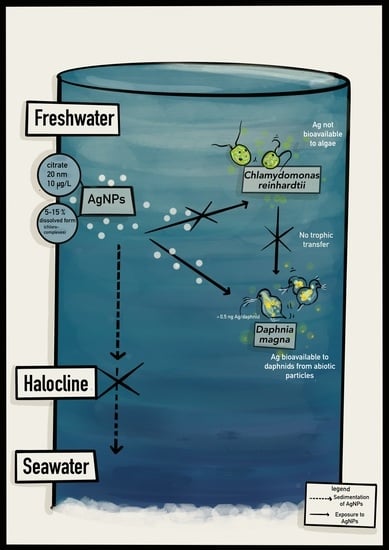
Dissolution of Silver Nanoparticles in Stratified Estuarine Mesocosms and Silver Accumulation in a Simple Planktonic Freshwater Trophic Chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesocosm Design
2.2. Determination of Dissolved Ag in the Surface Freshwater Layer
2.3. Algal Culture and Cell Density in Mesocosms
2.4. Silver Bioaccumulation by the Green Algae
2.5. Silver Bioaccumulation in Daphnia Magna
3. Results and Discussion
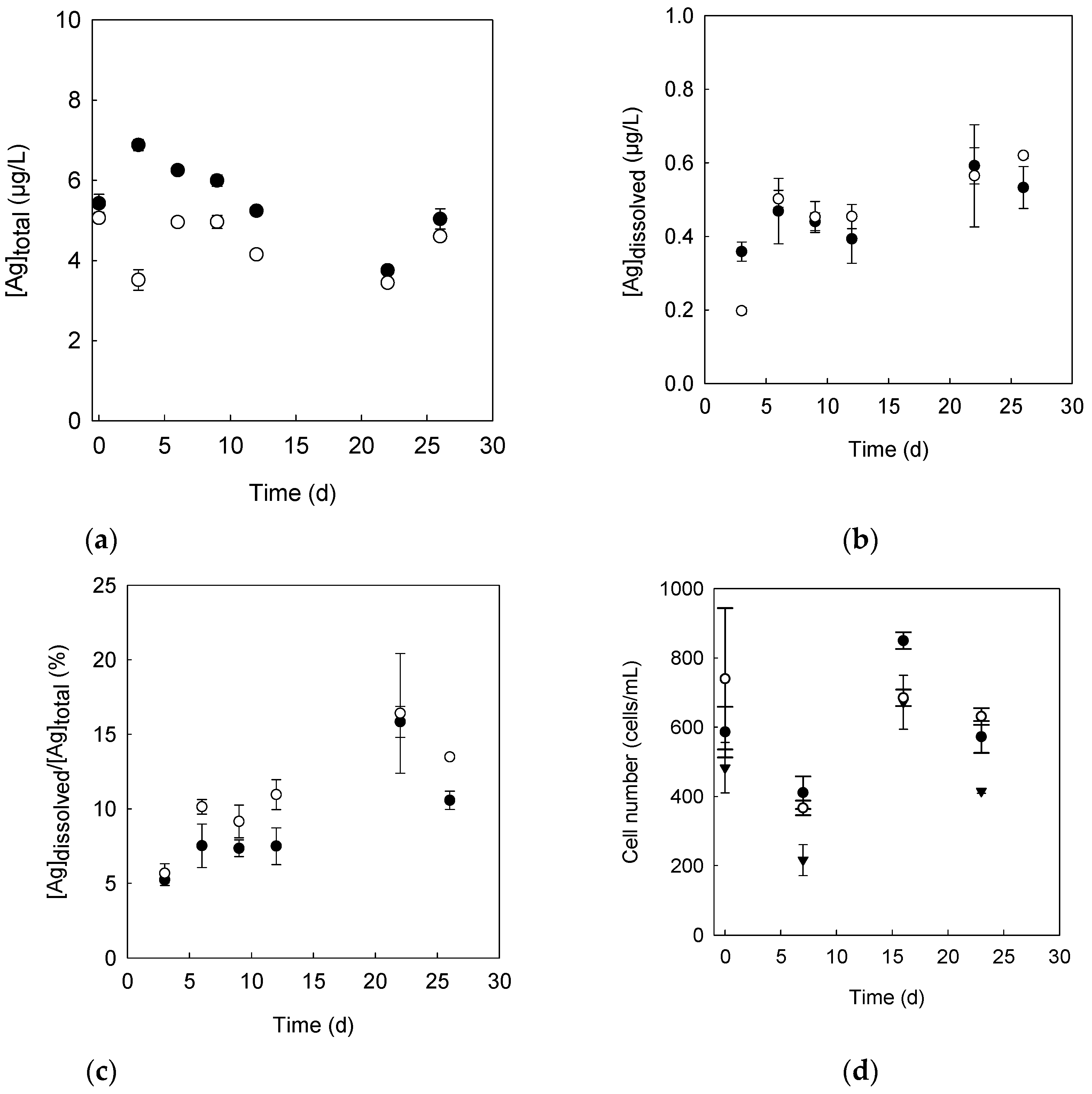
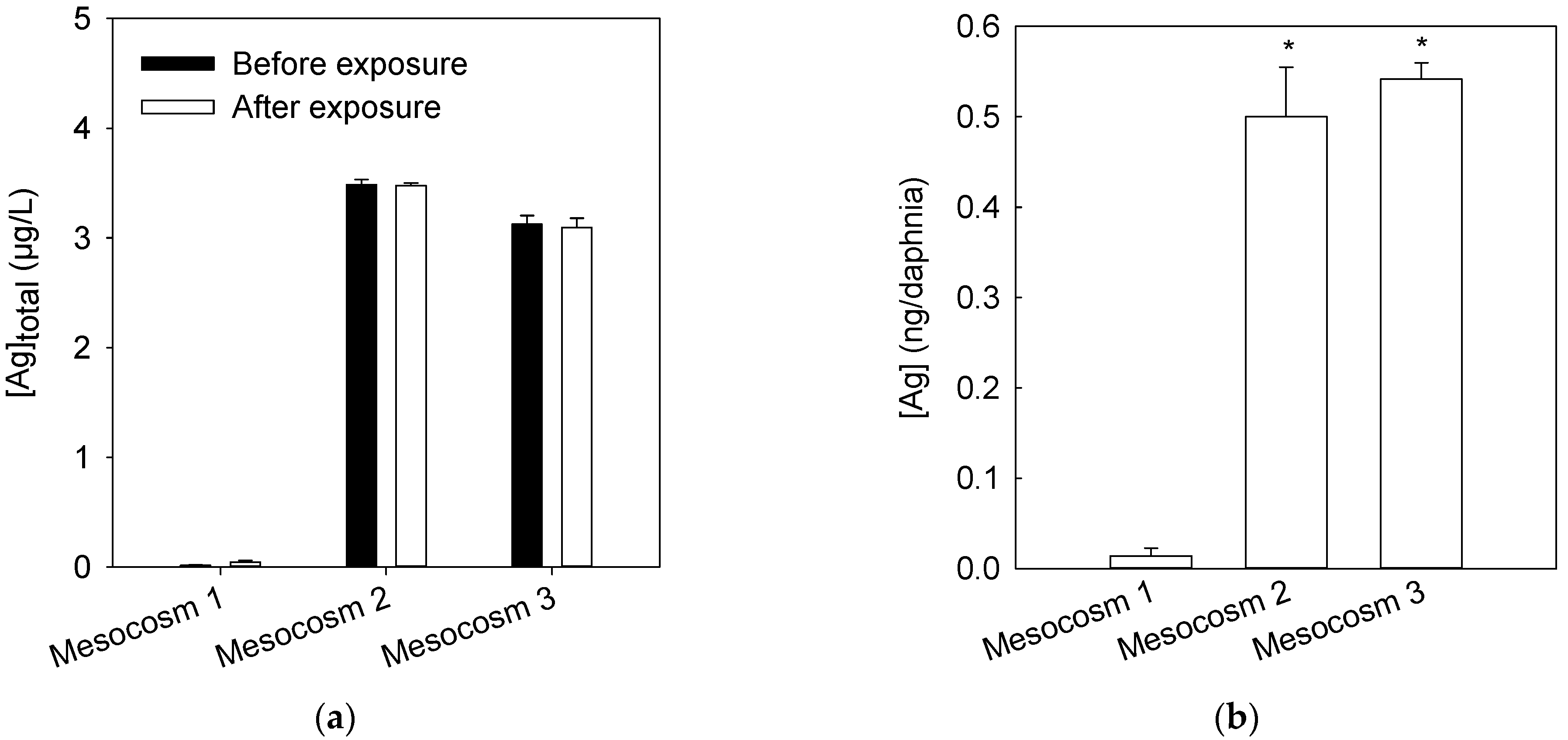
3.1. Dissolution of c-AgNPs in the Surface Freshwater Layer
3.2. Determination of Ag Concentrations in Green Algae
3.3. Determination of Ag Concentration in Daphnia Magna
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Azimzada, A.; Jreije, I.; Hadioui, M.; Shaw, P.; Farner, J.M.; Wilkinson, K.J. Quantification and characterization of Ti-, Ce-, and Ag-nanoparticles in global surface waters and precipitation. Environ. Sci. Technol. 2021, 55, 9836–9844. [Google Scholar] [CrossRef] [PubMed]
- Wigger, H.; Kägi, R.; Wiesner, M.; Nowack, B. Exposure and possible risks of engineered nanomaterials in the environment—Current knowledge and directions for the future. Rev. Geophys. 2020, 58, e2020RG000710. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Millour, M.; Gagné, J.-P.; Doiron, K.; Lemarchand, K.; Pelletier, É. Silver nanoparticles aggregative behavior at low concentrations in aqueous solutions. Colloid Surf. A 2020, 603, 125191. [Google Scholar] [CrossRef]
- Bathi, J.R.; Moazeni, F.; Upadhyayula, V.K.K.; Chowdhury, I.; Palchoudhury, S.; Potts, G.E.; Gadhamshetty, V. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021, 793, 148560. [Google Scholar] [CrossRef]
- Li, P.; Su, M.; Wang, X.; Zou, X.; Sun, X.; Shi, J.; Zhang, H. Environmental fate and behavior of silver nanoparticles in natural estuarine systems. J. Environ. Sci. 2020, 88, 248–259. [Google Scholar] [CrossRef]
- Behra, R.; Sigg, L.; Clift, M.J.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R Soc. Interface 2013, 10, 20130396. [Google Scholar] [CrossRef]
- Guilleux, C.; Campbell, P.G.C.; Fortin, C. Interactions between silver nanoparticles/silver ions and liposomes: Evaluation of the potential passive diffusion of silver and effects of speciation. Arch. Environ. Contam. Toxicol. 2018, 75, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Osterheld, K.; Millour, M.; Pelletier, É.; Magesky, A.; Doiron, K.; Lemarchand, A.; Gagné, J.-P. Nanotoxicity of silver nanoparticles: From environmental spill to effects on organisms. In Environmental Toxicity of Nanomaterials; Kumar, V., Dasgupta, N., Ranjan, S., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–240. [Google Scholar]
- Liu, W.; Worms, I.A.; Slaveykova, V.I. Interactions of metal-containing nanomaterials with microorganisms. In Interfaces between Nanomaterials and Microbes; Gupta, M.N., Kumar Khare, S., Sinha, R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 38–58. [Google Scholar]
- Stegemeier, J.P.; Avellan, A.; Lowry, G.V. Effect of initial speciation of copper- and silver-based nanoparticles on their long-term fate and phytoavailability in freshwater wetland mesocosms. Environ. Sci. Technol. 2017, 51, 12114–12122. [Google Scholar] [CrossRef]
- Auffan, M.; Santaella, C.; Brousset, L.; Tella, M.; Morel, E.; Ortet, P.; Barakat, M.; Chaneac, C.; Issartel, J.; Angeletti, B.; et al. The shape and speciation of Ag nanoparticles drive their impacts on organisms in a lotic ecosystem. Environ. Sci. Nano 2020, 7, 3167–3177. [Google Scholar] [CrossRef]
- Cleveland, D.; Long, S.E.; Pennington, P.L.; Cooper, E.; Fulton, M.H.; Scott, G.I.; Brewer, T.; Davis, J.; Petersen, E.J.; Wood, L. Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Sci. Total Environ. 2012, 421–422, 267–272. [Google Scholar] [CrossRef]
- Buffet, P.E.; Zalouk-Vergnoux, A.; Chatel, A.; Berthet, B.; Metais, I.; Perrein-Ettajani, H.; Poirier, L.; Luna-Acosta, A.; Thomas-Guyon, H.; Risso-de Faverney, C.; et al. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: The ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci. Total Environ. 2014, 470–471, 1151–1159. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook Second Edition—Introduction to Chlamydomonas and Its Laboratory Use; Academic Press–Elsevier: San Diego, CA, USA, 2009; Volume 1. [Google Scholar]
- Fortin, C.; Campbell, P.G.C. Silver uptake by the green alga Chlamydomonas reinhardtii in relation to chemical speciation: Influence of chloride. Environ. Toxicol. Chem. 2000, 19, 2769–2778. [Google Scholar] [CrossRef]
- Piccapietra, F.; Allué, C.G.; Sigg, L.; Behra, R. Intracellular silver accumulation in Chlamydomonas reinhardtii upon exposure to carbonate coated silver nanoparticles and silver nitrate. Environ. Sci. Technol. 2012, 46, 7390–7397. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.P.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Porcher, C.; Campbell, P.G.C.; Fortin, C. Influence of humic acid on algal uptake and toxicity of ionic silver. Environ. Sci. Technol. 2013, 47, 8835–8842. [Google Scholar] [CrossRef]
- Fortin, C.; Campbell, P.G.C. Thiosulfate enhances silver uptake by a green alga: Role of anion transporters in metal uptake. Environ. Sci. Technol. 2001, 35, 2214–2218. [Google Scholar] [CrossRef]
- Environment Canada. Biological Test Method—Acute Lethality Test Using Daphnia spp. 1990. Available online: https://publications.gc.ca/site/eng/453426/publication.html (accessed on 24 January 2022).
- Millour, M.; Doiron, K.; Lemarchand, K.; Gagne, J.P. Does the bacterial media culture chemistry affect the stability of nanoparticles in nanotoxicity assays? J. Xenobiot. 2015, 5, 5772. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Campbell, P.G.C.; Fortin, C. Silver binding by humic acid as determined by equilibrium ion-exchange and dialysis. J. Phys. Chem. A 2012, 116, 6532–6539. [Google Scholar] [CrossRef] [PubMed]
- Hadioui, M.; Leclerc, S.; Wilkinson, K.J. Multimethod quantification of Ag+ release from nanosilver. Talanta 2013, 105, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Millour, M. Comportement et Devenir des Nanoparticules D’Argent Durant une Transition Estuarienne. Ph.D. Thesis, Université du Québec à Rimouski, Rimouski, QC, Canada, 2018. [Google Scholar]
- Klučáková, M. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 2018, 6, 235. [Google Scholar] [CrossRef]
- Repligen. Pore Size Chart. Available online: https://www.repligen.com/application/files/3115/4023/7659/PoreSize_chart.jpg (accessed on 23 December 2021).
- Lowry, G.V.; Espinasse, B.P.; Badireddy, A.R.; Richardson, C.J.; Reinsch, B.C.; Bryant, L.D.; Bone, A.J.; Deonarine, A.; Chae, S.; Therezien, M.; et al. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ. Sci. Technol. 2012, 46, 7027–7036. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.E.; Tipping, E.; Hamilton-Taylor, J. Comparison of measured and modelled copper binding by natural organic matter in freshwaters. Comp. Biochem. Physiol. C 2002, 133, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Millour, M.; Gagné, J.-P.; Doiron, K.; Marcotte, I.; Arnold, A.A.; Pelletier, É. Effects of concentration and chemical composition of natural organic matter on the aggregative behavior of silver nanoparticles. Colloid Surf. A 2021, 623, 126767. [Google Scholar] [CrossRef]
- Zhou, D.; Abdel-Fattah, A.I.; Keller, A.A. Clay particles destabilize engineered nanoparticles in aqueous environments. Environ. Sci. Technol. 2012, 46, 7520–7526. [Google Scholar] [CrossRef]
- Ribeiro, F.; Van Gestel, C.A.M.; Pavlaki, M.D.; Azevedo, S.; Soares, A.; Loureiro, S. Bioaccumulation of silver in Daphnia magna: Waterborne and dietary exposure to nanoparticles and dissolved silver. Sci. Total Environ. 2017, 574, 1633–1639. [Google Scholar] [CrossRef]
- McTeer, J.; Dean, A.P.; White, K.N.; Pittman, J.K. Bioaccumulation of silver nanoparticles into Daphnia magna from a freshwater algal diet and the impact of phosphate availability. Nanotoxicology 2014, 8, 305–316. [Google Scholar] [CrossRef]
- Yan, N.; Wang, W.-X. Novel imaging of silver nanoparticle uptake by a unicellular alga and trophic transfer to Daphnia magna. Environ. Sci. Technol. 2021, 55, 5143–5151. [Google Scholar] [CrossRef]
- Simčič, T.; Brancelj, A. Electron transport system (ETS) activity and respiration rate in five Daphnia species at different temperatures. Hydrobiologia 1997, 360, 117–125. [Google Scholar] [CrossRef]


| Exposure | Total (µg) | Filtrate (µg) | Wash Solution (µg) | Filter 1 (µg) | Filter 2 (µg) | Recovery (%) | |
|---|---|---|---|---|---|---|---|
| Mesocosm 2 | Without algae | 1.39 ± 0.10 | 1.04 ± 0.06 | 0.082 ± 0.019 | 0.107 ± 0.053 | 0.018 ± 0.004 | 89.8 ± 2.2 |
| With algae | 1.29 ± 0.05 | 0.96 ± 0.04 | 0.062 ± 0.009 | 0.086 ± 0.014 | 0.021 ± 0.004 | 88.0 ± 1.6 | |
| Mesocosm 3 | Without algae | 1.19 ± 0.10 | 0.84 ± 0.06 | 0.075 ± 0.022 | 0.084 ± 0.026 | 0.011 ± 0.003 | 84.5 ± 1.9 |
| With algae | 1.08 ± 0.01 | 0.74 ± 0.05 | 0.064 ± 0.013 | 0.101 ± 0.021 | 0.014 ± 0.004 | 85.3 ± 3.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilleux, C.; Chen, Z.; Campbell, P.G.C.; Fortin, C. Dissolution of Silver Nanoparticles in Stratified Estuarine Mesocosms and Silver Accumulation in a Simple Planktonic Freshwater Trophic Chain. Environments 2022, 9, 20. https://doi.org/10.3390/environments9020020
Guilleux C, Chen Z, Campbell PGC, Fortin C. Dissolution of Silver Nanoparticles in Stratified Estuarine Mesocosms and Silver Accumulation in a Simple Planktonic Freshwater Trophic Chain. Environments. 2022; 9(2):20. https://doi.org/10.3390/environments9020020
Chicago/Turabian StyleGuilleux, Camille, Zhongzhi Chen, Peter G. C. Campbell, and Claude Fortin. 2022. "Dissolution of Silver Nanoparticles in Stratified Estuarine Mesocosms and Silver Accumulation in a Simple Planktonic Freshwater Trophic Chain" Environments 9, no. 2: 20. https://doi.org/10.3390/environments9020020
APA StyleGuilleux, C., Chen, Z., Campbell, P. G. C., & Fortin, C. (2022). Dissolution of Silver Nanoparticles in Stratified Estuarine Mesocosms and Silver Accumulation in a Simple Planktonic Freshwater Trophic Chain. Environments, 9(2), 20. https://doi.org/10.3390/environments9020020