Abstract
Biological invasions can affect ecosystems in different ways. Invasive forest species, such as Acacia dealbata Link., affect forests’ productivity, because they compete directly with native species for access to light and nutrients, contributing to the loss of biodiversity. In this study, an area occupied by A. dealbata, located in Casal do Rei (Seia, Portugal) was studied to evaluate the influence of fire in the dispersion of this species, analyzing the historical occurrence of rural fires in the region, as well as through the determination of its annual biomass production and comparing its growth with other species using satellite images. The research shows a competitive advantage for A. dealbata, even when compared to species, such as Eucalyptus globulus and Pinus pinaster, which practically disappeared from the location under study after a significant fire occurred in 2005, while A. dealbata continued to thrive.
1. Introduction
The Mediterranean region has co-existed with fires since it was occupied by people. The region’s climate determined the forests of the region and agricultural practices [1]. The concurrence of fires has shaped the ways of life of the people of the region. They have adapted to the seasonal climate, and frequent fires, and used those to their benefit [2]. Over time, this coexistence has become more complex. The frequency of fires has changed from an occasional to frequent occurrence [3]. In Portugal, the season at which fires occurs is defined and civil protection forces annually plan their mitigation efforts accordingly [4]. Fires are designated in different ways, depending on the type of area in which they may occur. The designation “forest fire” is often applied in a wrong way and is used when areas with other types of non-forest land use burn, such as are the shrubs and pastures areas. In this study, the designation “rural fire” is used referring to fires that occur in a rural environment, not distinguishing forest fires from those occurring in agriculture or shrubs and pastures areas.
The analysis of the data that are presented in the provisional report on rural fires of the Instituto para a Conservação da Natureza e da Floresta (ICNF) (a summary is shown in Figure 1) indicates that, in Portugal, rural fires are mainly caused by human activity. Natural causes, such as lightning strikes, only accounted for 2% of the occurrences in 2020, while the average over the decade 2010 to 2019 was just 1%. The causes attributed to human activity, whether negligent or arson, represent most of the events analyzed, with 76% during the year 2020. This value is in line with the average over the referred decade, for which the value reached 72%. However, occurrences with arson origin have significant weight when analyzed individually, representing 37% of the events during 2020, a significant increase from the average for 2010 to 2019, i.e., 27% of the occurrences.
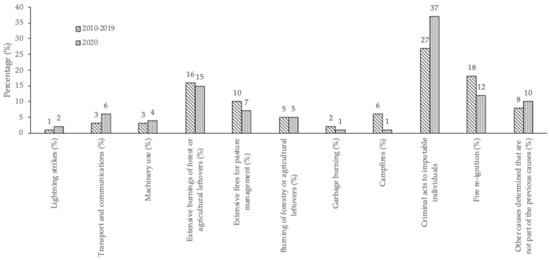
Figure 1.
Frequent causes of rural fires in Portugal (data collected from http://www.icnf.pt/portal/florestas/dfci/relat/rel-if/2020, accessed on 12 January 2021).
The misuse of fire continues to be problematic. Such mainly negligent use causes a high percentage of rural fires and it is associated with practices, such as extensive burning for pasture management, the burning of waste materials resulting from forestry and agriculture, the burning of garbage, or holding bonfires for leisure activities, totalizing 29% of the occurrences in 2020. Despite this high percentage, there was a decrease when compared to the decade 2010–2019, which was 39%. The reduction may be associated with intensive public awareness activities, and restriction and control measures implemented after the fires occurred in 2017. During 2020, these measures were accentuated by the impact of the COVID-19 pandemic by reducing the economic and leisure activities of the population in a generalized way.
Contrasting with previous periods, during which fires were seen as a way of renewing agro–silvo–pastoral systems, serving to eliminate plagues, revitalizing meadows and pastures, and fertilizing soil through the deposition of ashes, rural fires are seen today as a difficult problem to manage. The occurrence cycles are shorter and are not long enough to allow the regeneration of the systems [5]. For example, when successive occurrences are recorded in a certain area with annual repetition cycles, the forest species that can settle and start to prosper will be only those adapted to post-fire conditions. These are usually invasive species coming from arid environments where fire contributes to their spreading. In Portugal, there are some species that proliferated in post-fire scenarios, where those of the genus Acacia stand out.
In Portugal, the spreading of species of the genus Acacia has been the subject of several studies, mainly contributing to the mitigation and control of their dispersion since there has been an increment of the occupied areas. Acacia dealbata Link. aroused significant interest due to its dispersion capacity in burnt environments since it is a pyrophyte species [6]. As shown in Figure 2, the increase in the area occupied by species of the genus Acacia occurred precisely when the rise in the number of rural fires started (in the mid-1990s), because of several circumstances, such as the abandonment of rural environment by the population searching better living conditions; the agricultural labor force transferred to the civil construction sector with the advent of public works made possible by European Union funds and by the forest reorganization to produce raw materials in a monoculture regime [6].
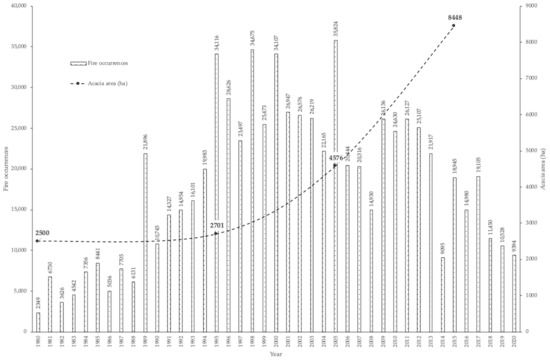
Figure 2.
Evolution of the A. dealbata area (data collected from www.icnf.pt, accessed on 12 January 2021, and [7,8,9]).
The growth of the area has been a problem, especially after large fires, since A. dealbata, being pyrophyte, benefits from the destruction of the vegetation cover, allowing for its seeds, deposited in the soil for long periods, to benefit from direct exposure to sunlight. Because A. dealbata is heliophilous, it can develop much faster than other plants and, due to its capacity for allelopathy, can prevent or hinder the development of other species [10,11,12]. These plants can occupy the area and thrive with incredible speed to produce seeds, which are released in great abundance onto the soil [13]. As new rural fires have decreased, acacia stands are growing in the direct proportion to their capacity to reach maturity and produce seeds in between new events [14,15]. Thus, their capacity for territorial expansion depends directly on the number of seeds that each specimen can produce and its ability to disperse them around its location [16]. Afterwards, is only a matter of time to the occurrence of a new fire, which will unblock the access to sunlight activating the germination of the seeds [17,18].
Analyzing a case study in Casal do Rei (Seia, Portugal) is the purpose of this article, where the occurrence of rural fires during the last two decades led to a profound transformation of the forest, with the establishment of several A. dealbata stands that are prospering, contrary to what is happening with other species. In this article, the sequence of occurrences of rural fires in the region is characterized, and a comparative analysis of the growth cycles of the specimens from one of these stands is made to demonstrate how this species benefits in these new circumstances of climate change and most frequent post-fire scenarios.
2. Materials and Methods
2.1. Location of the Study Area
Casal do Rei is in the União de Freguesias de Vide e Cabeça, which is the result of the merge of two parishes in the municipality of Seia (Portugal). The parishes of Cabeça and Vide were unified in the administrative reform of the territory, which was approved by Law No. 56/2012 of 8 November and Law No. 11-A/2013 of 28 January. Figure 3 shows the location of the area under study and its regional and national framework. The site is located on the opposite slope to the villages of Cabeça and Casal do Rei (Figure 3).
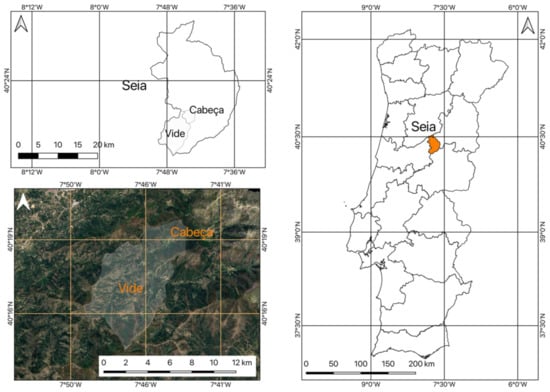
Figure 3.
Location of the area under study.
The stands of A. dealbata analyzed in this study are visible from the road that connects Casal do Rei to Cabeça, located on the opposite slope of the Ribeira de Loriga valley, as shown in Figure 4.
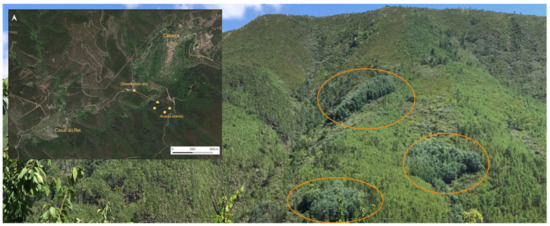
Figure 4.
A. dealbata stands (highlighted) on the opposite slope to the road that connects Casal do Rei to Cabeça.
2.2. Framework of the Area under Study
2.2.1. Geology and Geomorphology
The region mainly consists of extensive granite outcrops, aged between 340 and 280 million years, interspersed with metamorphic rocks, such as shale and graywacke, aged between 650 and 500 million years. These dominant geological formations are intercepted by numerous veins of quartz, granitic pegmatites, and dolerites. In the granite areas, the landscape is dominated by extensive plateaus that are bounded by steep slopes. In these areas, watercourses take advantage of the existing network of tectonic faults and fractures, presenting straight lines. In places where the action of glaciers was felt, erosive forms can be observed, as well as deposition structures. In areas that are not covered by the ice masses, the characteristic features of granite landscapes are evident from the action of different geological agents, such as water, wind, chemical phenomena, and temperature differences. In the areas where shales and greywackes dominate, which are materials whose impermeable nature facilitates surface runoff, a succession of wavy forms originated through a dense and winding drainage network can be observed.
2.2.2. Climatology
Regarding the climatological characterization, data were searched in the meteorological stations closest geographically to the study area with data that are available on the website of Instituto Português do Mar e da Atmosfera (IPMA). These stations were located in Viseu, Guarda and Castelo Branco. The total absence of thermopluviometric stations in the target area made it impossible to characterize the climate locally. However, there may be significant differences in the conditions observed, mainly due to the difference in altitude at which these thermopluviometric stations are in relation to the target zone. The climatic information for Casal do Rei was simulated through the METEOBLUE platform, available at https://www.meteoblue.com, accessed in 14 January 2021. Figure 5 presents the data collected for the three locations and the data modelled for the target area. The Viseu station is at an altitude of 443 m, with the coordinates 40°39′ N and 7°54′ W. The Guarda station is at an altitude of 1019 m, with the coordinates 40°31′ N and 7°15′ W. The Castelo Branco station is at an altitude of 386 m, with the coordinates 39°50′ N and 7°28′ W. The geographical coordinates of Casal do Rei are 40°31′ N and 7°55′ W and the altitude is 470 m.
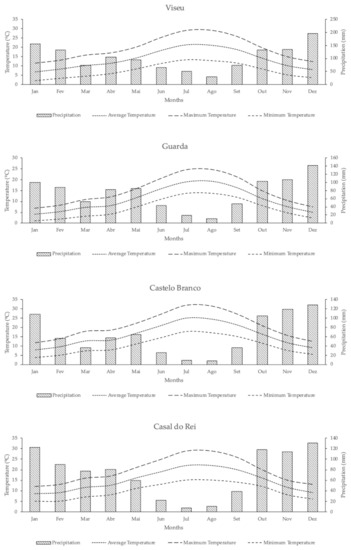
Figure 5.
Data that were collected for the three selected stations, Viseu, Guarda and Castelo Branco, and the data modelled for Casal do Rei.
Despite the geographic proximity between with Penhas da Saúde weather station, it was decided not to use the data from this station. The influence of altitude, especially during winter, can differ significantly from the conditions that were verified at Casal do Rei. The region has a Mediterranean climate, with two mild seasons (spring and autumn) being separated by two extreme seasons (a hot and dry season, summer, and a cold and wet season, winter). In the case of the Ribeira de Loriga valley, the patterns of precipitation and temperature are further conditioned by the effect of the orientation of the slope and the surrounding relief. Thus, the embedded valley and exposure to the north determine that, in the area selected for the present study, relatively mild temperatures and high humidity are felt.
2.3. Study Species
The genus Acacia comprises about 1380 species, 1000 of which are native to Australia, with the rest being native to the remaining continents of the Southern Hemisphere, except for a few species that are originally from North America [19]. There are divergences in the classification of the family to which the acacias belong. On the one hand, some of the authors place them in the large family Leguminosae, although some authors claim that it belongs to an independent family, the Mimosaceae [20,21]. Like other leguminous plants, acacia seeds grow in small pods, which open, releasing the seed [19]. Among these many species, there is one that, mainly due to the spectacular nature of its flowers, quickly attracted attention as an ornamental plant. A. dealbata presents bright yellow globular inflorescences in bloom [22]. As an essentially Australian species, acacias are very well adapted to hot and dry climates, with the regular occurrence of rural fires, many of which may be designated as pyrophyte species, which is, species for which fire acts as a stimulus for growth and dispersion [22]. Thus, it is seen that, in areas where the climate is hot and dry, as Southern Europe, acacias are very well prepared to compete with native species for resources, such as space, water and nutrients, in addition to having the collaboration of seasonal fires to facilitate dispersion [14].
Humans have been an excellent dispersing agent of species worldwide, and species that were previously restricted to an area can be easily transported to another side of the world. These non-native plants, which are described as exotic, may not even cause significant problems. In fact, in many cases, the new habitat does not have the ideal conditions that the species had in its original habitat. However, sometimes the exotic species have biological characteristics, such as rapid growth, the formation of a more significant number of seeds, alteration of the features of the environment, among others, which make it a formidable competitor in a place where it is not native, beginning to proliferate. In these cases, it becomes an invasive species, and the phenomenon is called biological invasion [23]. In Portugal and most Southern Europe, the most disturbing species are A. melanoxylon, A. longifolia, and A. dealbata, which is the most problematic of all; it forms the most significant clusters and is the species more dispersion [24].
For a long time, A. dealbata and other acacias continued to be seen as species of exceptional economic or botanical value. Although observations on their invasive nature were already beginning to be made, it was not until 1937 that the first legislation to control the plantation of A. dealbata. However, it only controlled minimum distances for sensitive lands, such as pastures, agricultural crops, or embankments [25,26]. It was only in the 1970s and 1980s that this problem started to be seen as something serious, but, at this time, the main focuses of expansion were related not to plantations, but to forest fires that allow this and other acacias to quickly colonize previously occupied land by native species [27]. Once colonized by acacias, these areas are unlikely to have conditions for native species to develop again, because, in addition to proliferating, creating an extensive seed bank in the soil that allows rapid recolonization in the event of disturbances, such as fires or removal of vegetation, its leaves contain toxic compounds that inhibit the growth of other plants when they accumulate in the soil (a phenomenon called allelopathy [28]), and they mainly change the chemical composition of the soil a lot [29,30].
Some studies that were carried out in Spain and Portugal, where soils with A. dealbata and soils occupied with Quercus robur were characterized, demonstrated the differences that these soils suffered [31,32,33]. In these studies, it was found that soils invaded by A. dealbata have less biodiversity, are more prone to other exotic species, present fewer ferns and mosses, are richer in nitrogen, and have a more acidic pH [34]. These changes in soils are reflected in the biodiversity, in the water cycle and in all other processes and functions of an ecosystem [35]. The cutting of the acacias combined with herbicides can be a way to control them or be dehulled to cause dehydration. However, given the seed production capacity that these species have, even if all of the plants were uprooted at a given time, there would still be enough seeds to continue to burst for several years [36].
2.4. Data Acquisition
2.4.1. Occurrences of A. dealbata Stands in the União de Freguesia de Cabeça e Vide
The data on A. dealbata stands in the territory corresponding to the União de Freguesia de Cabeça and Vide were collected using satellite images available on the platform https://www.sentinel-hub.com, accessed on 14 January 2021, which were subsequently processed in the open-source Geographic Information System (GIS) QGIS 3.16 Hannover. The images were collected by the Sentinel-2 satellite during August 2019 and true color L2A images were selected. In these images, A. dealbata stands were delimited.
2.4.2. Rural Fires Occurrences
The data relating to rural fires in the União de Freguesias de Vide e Cabeça were obtained through the database of the Instituto para a Conservação da Natureza e da Floresta (ICNF) at http://www.icnf.pt, accessed on 16 January 2021. The data were downloaded in the form of shapefiles and used in GIS QGIS 3.16 Hannover. They were overlaid on the territory boundaries that corresponded to the União de Freguesias de Vide e Cabeça, so that it was possible to visualize the area that is affected by the successive fires in this region.
2.4.3. A. dealbata Stand Selection
The area for the study was selected following an accessibility criterion. Afterwards, two circular-shaped parcels were delimited with a radius of 15 m, corresponding to an area slightly larger than 700 m2 for each one of the parcels. Given the relatively small size of the area, which is approximately 1960 m2, dispersed over several terraces, and with some difficult to access, there was a slight overlap between the two parcels. Where this overlap occurred, the trees were counted as part of Parcel 1. Subsequently, all the existing trees in each of the parcels were counted, and their relative positions were determined by measuring the distance to the center of Parcels 1 and 2, as shown in Figure 6.
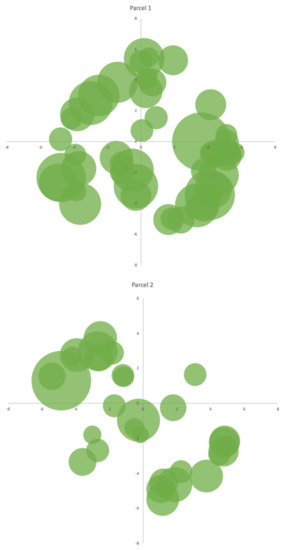
Figure 6.
Distribution of A. dealbata trees in Parcels 1 and 2.
The diameter at breast height (DBH) was also measured for all trees. This diameter was determined as the average between the largest and smallest diameters that were measured at 1.30 m from the ground. Using the DBH measurement, it was possible to classify the trees into categories. Thus, eight classes were defined according to the following intervals:
- Class 5—DBH less than 5 cm;
- Class 10—DBH between 5 and 10 cm;
- Class 15—DBH between 10 and 15 cm;
- Class 20—DBH between 15 and 20 cm;
- Class 25—DBH between 20 and 25 cm;
- Class 30—DBH between 25 and 30 cm;
- Class 35—DBH between 30 and 35 cm;
- Class 40—DBH between 35 and 40 cm.
In Parcels 1 and 2, 83 trees were accounted, with 79 belonging to the species A. dealbata, two belonging to the species Pinus pinaster, and two others belonging to the species Arbutus unedo. The classification within the representative classes of the DBH was only carried out for the specimens of the species A. dealbata, since this was the species of focus. After counting, locating, and framing the existing specimens in the parcels, samples were selected to represent each of the classes. In cases where it became difficult or impossible to collect the chosen specimens, they were replaced by other specimens that were considered equivalent; first, because they had the same diameter class, and, second, for presenting a similar morphology in aspects, such as height, canopy width, and density of branches and foliage. The trees were cut as close as possible to the ground, using chainsaws that were operated by Forest Sappers. The measurements, namely, the height of the trunk, the height of the crown, the diameter of the crown, the weight of the trunk, and the weight of the branches and foliage were then made. Slices of the trunks were also collected, with a thickness of approximately 2 to 3 cm to determine the age of each specimen.
3. Results and Discussion
3.1. Occurrences of A. dealbata in the União de Freguesias de Vide e Cabeça
The A. dealbata stands that, in the União de Freguesias de Vide e Cabeça, are of a dispersed nature and occur exclusively in areas that have already been affected by rural fires. These stands, which can still be considered negligible, represent about 11.85 ha in a territory of about 5647.41 ha. However, the potential for expansion is high, given the number of dispersed stands that have already been identified, with 40 throughout the territory, but with a preferential concentration, in number and size, in the territory of the former parish of Cabeça. In this area, stands are located on the opposite hillside to the villages of Cabeça and Casal do Rei, with several stands exceeding 2000 m2 (Figure 7).
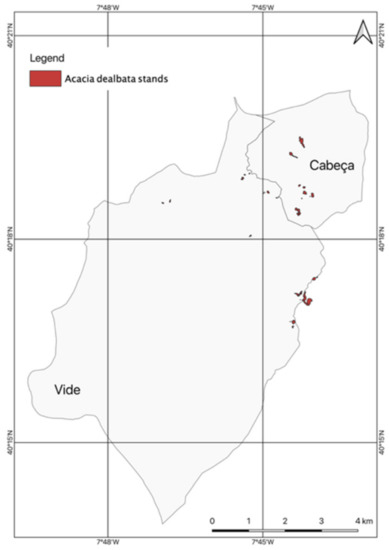
Figure 7.
The areas occupied by A. dealbata stands in the União de Freguesias de Vide e Cabeça.
3.2. Occurrences of Rural Fires in the União de Freguesias de Vide e Cabeça
There is already a long history of rural fires in the União de Freguesias de Vide e Cabeça, with a few years since 1975 for which there were no registered fires. When there were no fires in the region, they occurred nearby, as shown by the overlapping of the burnt areas shown in Figure 8. Of all the occurrences verified, the periods in which the largest fires occurred was in 2005, followed by 2017. The difference between the two occurrences is that, in 2017, the fire only stayed in the territory of Vide and did not reach Cabeça. In 2005, the fire hit the two territories violently, but fires in the decade from 2000 to 2009 did not stop with the occurrence in 2005, since, in 2001, 2002, 2003, and 2004, fires also plowed through the slopes of Cabeça and Vide in such a way that practically the only parts of the territory that did not burn were the urbanized parts and those where agriculture did not allow the accumulation of fuel. After a period in which there was an extensive occurrence, which affected a significant part of the territory, the probability of new occurrences in subsequent years decreased, most likely because an extended period was necessary for the fuel load to recover, as can be seen in the sequence of overlaps.
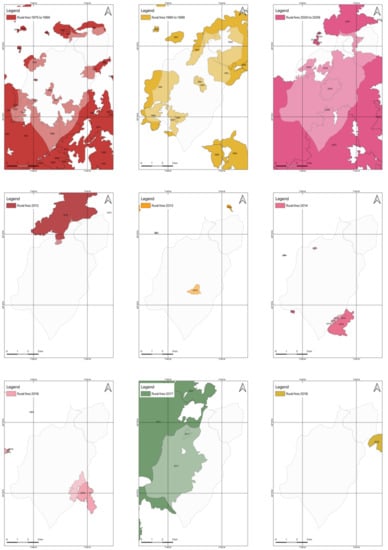
Figure 8.
Burnt areas between 1975 and 2019 in União de Freguesias de Vide e Cabeça.
However, this reality can change, since invasive species, such as A. dealbata (and others already identified in the area under study, such as Hakea sericea), can react rapidly in the post-fire period, producing large amounts of biomass. This ability to recover may contribute to the increased risk of rural fires occurring, mainly by reducing the recovery period between occurrences that were directly related to the recovery capacity of native species. It should be remembered that the study area is on the slope of Serra da Estrela and is, therefore, a terrain in altitude, where low temperatures may occur and associated with poor soils, do not constitute an environment that is conducive to the exuberance of vegetation. The major milestone regarding the occurrence of rural fires in the region was the year 2005, which was marked in the local population memory. 2005 was a dark year for the municipality of Seia, with a total of 276 fires and a total burnt area of 17,260.95 ha. That is, 39.62% of the territory of the municipality was directly affected by rural fires. Concerning União de Freguesias de Vide e Cabeça, in the period between 1975 and 2018, it was found that practically the entire region was at some point burnt. In Figure 9, the overlap of all areas that are affected by rural fires can be seen. As shown, only urbanized areas occupied by the villages of Cabeça and Casal do Rei were not affected.
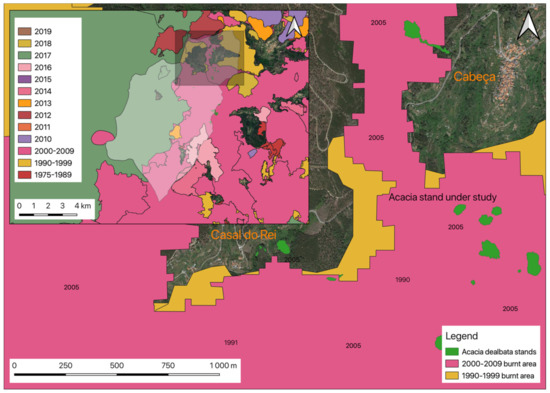
Figure 9.
Overlapping of the burnt areas in 1975–2019 in União de Freguesias de Vide e Cabeça.
3.3. The Productivity of A. dealbata in the Casal do Rei Region
The samples of A. dealbata selected in Parcels 1 and 2 were processed, as described in Section 2.4.3. The slices collected (Figure 10) were used to determine the age of the trees. The height of the trunks and tree crowns were measured and the weight of all the material, obtaining the results that are shown in Table 1.

Figure 10.
Slices used in the age determination of the specimens by counting the growth rings.

Table 1.
Measurements and annual biomass increment calculated for the samples collected in Casal do Rei.
With the data acquired, the annual biomass increment was calculated expressed in kg.year−1. Some of the specimens with ages prior to the tremendous rural fire of 2005 were found, most likely because they are in a more secure location on the slope, being closer to the watercourse that runs nearby (Ribeira de Loriga). These also correspond to the larger specimens; namely, sample 8 from parcel 1, with a total height of 22.9 m, a total weight of 594.8 kg and an age of 20 years; sample 26 from parcel 1, with a total height of 25.1 m, a total weight of 249.1 kg and an age of 14 years; sample 33, of parcel 1, with a total height of 23.4 m, a total weight of 223.9 kg and an age of 14 years old; sample 9 from parcel 2, with a total height of 19.9 m, a total weight of 138.9 kg and an age of 15 years; and, sample 15 from parcel 2, with a total height of 32.7 m, a total weight of 333.4 kg and an age of 17 years. Despite having started its growth prior to 2005, the only specimen that did not show the same rates of development was sample 6 of parcel 1. Although its development in height was in line with the other specimens at 13.2 m, it did not maintain the same development concerning the annual biomass increase, since its total weight of 33.5 kg only represents an annual biomass increase of 2.4 kg.year−1.
The average value of the annual increase in biomass that was observed for the different specimens was 8.73 ± 4.09 kg.year−1. Other studies, such as the one that was conducted by Albaugh et al. (2017) in Chile on the use of A. dealbata and Eucalyptus globulus as energy crops, reached values of 2.4 kg.year−1 for plantations of 15,000 trees.ha−1, with a total of 108 t.ha−1, with a rotation of three years, while, for a plantation of 5000 trees.ha−1, obtained an annual biomass increment of 5.05 kg.year−1 [37]. Another study, which was conducted by Gouws and Shackleton (2019) in South Africa, determined a value of 0.57 kg.year−1 for the annual increment in biomass, with planting densities of 7000 trees.ha−1 [38]. This difference in values is particularly noticeable for the case study that was carried out in South Africa, since, in the case of the study carried out in Chile, for a plantation density of 5000 trees.ha−1, the presented values are within the confidence interval calculated. These differences may be related to the levels of rainfall recorded in different locations, since the growth of A. dealbata is directly related to the access to water, as shown by Hunt and Beadle (1998), Forrester et al. (2010), or Vertessy et al. (2001) [39,40,41].
The dispersion of A. dealbata is being enhanced by the increase in occurrences of forest fires in the study area. From Sentinel-2 satellite images analysis, as shown in Figure 11, in the year immediately after the 2005 fire (more specifically in October 2006), the vegetation was emerging. However, according to the testimony of local inhabitants, there are visible patches of trees as they existed, who reported a continuous patch of dense pine forest up to a certain level. From that level upwards, the vegetation decreases in size, with a predominance of shrub species, such as Cytisus striatus, until this also disappears and gives rise to smaller species, such as Erica australis. In the following images, which were obtained in May 2013, August 2017, and May 2019, the vegetation is slowly recovering. However, recovery by A. dealbata is much faster than any other species, only contrasted by the occurrence of some eucalyptus that competes for the best area with A. dealbata.
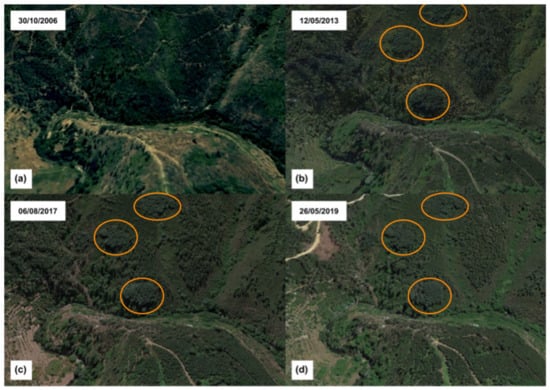
Figure 11.
Sentinel-2 satellite images of the A. dealbata stands located on the opposite slope to the villages of Cabeça and Casal do Rei. (a) image obtained on 30 October 2006; (b) image obtained on 12 May 2013; (c) image on 6 August 2017; and, (d) image obtained on 26 May 2019. The observation positioning was chosen to reflect the same view used in the previous Figure 4.
As seen in the satellite images, A. dealbata is the only species showing an increasing development, since it is the only species that started its growth immediately after the fire. One year after the fire occurred in the summer of 2005, A. dealbata already has a set of stands that remain until today, as can be seen in the satellite image of October 2006. However, the high density that is caused by the initial fast growth also led to discrepancies in each tree, varying in accordance with its exposure to sunlight, which is reason why trees that started growing in the same year do not show the same development since those that acquire the status of dominance do not allow others to grow at the same rate.
This reaction to fire is in accordance with the studies that were carried out by Bowd et al. (2019), which point to fire as one of the auxiliaries that benefit the dispersion of A. dealbata in a study on long-term changes in soils that were caused by fire, as well as in previous studies, such as that carried out by Je (2006), where the fire management impacts on invasive plant species in the western United States is analyzed [42,43]. Another study, which was carried out by Florentine et al. (2008), points to fire as the activating process that awakens seeds in pyrophyte species [44]. Gordon et al. (2017) addressed the response of species of the genus Acacia to high severity fires, indicating the regeneration potential of these species in the post-fire period [45,46]. The results that were observed in Casal do Rei show a capacity for recovery from fire superior to other species, mainly concerning the natives, since they may find competition from other exotic species, such as Hakea sericea, which has already been observed in the vicinity of these stands. This capacity for growth and area occupation is visible in Figure 12, which was captured on March 15, 2021, where the A. dealbata stands photographed from the same observation point used in Figure 4 and Figure 11, show a progression mainly following the water lines. This occupation of the water lines is, most likely, related to the fact that the seeds are dragged by rainwater and because those are areas where the humidity is higher, thus enhancing their development.

Figure 12.
Stands of A. dealbata (photo obtained on 15 March 2021). The yellow color of the flowers makes the identification of the stands evident. It is possible to observe the development of the stand in the waterline.
4. Conclusions
Biological invasions are a problem that intensively affects ecosystems, mainly when associated with other factors, which may have a supporting effect on the dispersion of invasive species. One of these factors is fire, which, in in the case of A. dealbata, operates as a disruptor for its dispersion and as an activator of its germination. Being a heliophilous species, A. dealbata benefits in the first moment with the elimination of the vegetation cover, with, in a second moment, the seeds to be activated by the fire itself, promoting germination and a quick conquer of the space. In Portugal, the productivity of A. dealbata is high when compared with other stands in different latitudes, such as South Africa or Chile, and very high when compared with the Portuguese native species, with which competes for area and nutrients with a clear advantage, as was verified in this study. Thus, it can be concluded that fire is an enhancing agent for the expansion of A. dealbata with the area that is occupied by this species growing significantly in recent years, following the trend of an increasing number of rural fires. This results from several factors, e.g., climate change and the abandonment of agriculture and forest land.
Author Contributions
Conceptualization, L.J.R.N. and C.I.R.M.; methodology, L.J.R.N., N.M.C.A.R. and C.J.P.G.; validation, L.J.R.N., M.A.M.R. and C.J.P.G.; formal analysis, L.J.R.N., C.I.R.M. and M.A.M.R.; investigation, L.J.R.N., M.A.M.R., C.I.R.M., C.J.P.G. and N.M.C.A.R.; resources, L.J.R.N.; data curation, L.J.R.N., M.A.M.R. and C.I.R.M.; writing—original draft preparation, L.J.R.N., M.A.M.R. and C.I.R.M.; writing—review and editing, L.J.R.N., M.A.M.R., C.I.R.M., C.J.P.G. and N.M.C.A.R.; supervision, N.M.C.A.R. and C.J.P.G.; project administration, N.M.C.A.R. and C.J.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
L.J.R.N. was supported by proMetheus—Research Unit on Energy, Materials and Environment for Sustainability—UIDP/05975/2020, funded by national funds through FCT—Fundação para a Ciência e Tecnologia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available per request to corresponding author.
Acknowledgments
The authors would like to thank the group of forest sappers from Manteigas for their support in opening access to the site under study, and the União de Freguesias de Vide e Cabeça for their support during the entire investigation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhillon, R.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- MacDonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Lazpita, J.G.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manag. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O. Impacts of global change on Mediterranean forests and their services. Forests 2017, 8, 463. [Google Scholar] [CrossRef]
- Nunes, A.; Lourenço, L.; Meira, A.C. Exploring spatial patterns and drivers of forest fires in Portugal (1980–2014). Sci. Total Environ. 2016, 573, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Estevão, C.; Costa, C.; Nunes, S.; Peraboa, F. Tourism sector competitiveness in central Portugal following the 2017 forest and rural fires: Evaluating the situation and its future implications. Rev. Tur. Desenvolv. 2020, 77–99. [Google Scholar] [CrossRef]
- Gomes, J. Forest fires in Portugal: How they happen and why they happen. Int. J. Environ. Stud. 2006, 63, 109–119. [Google Scholar] [CrossRef]
- Fernandes, M.; Devy-Vareta, N.; Rangan, H. Plantas exóticas invasoras e instrumentos de gestão territorial. O caso paradigmático do género Acacia em Portugal. Rev. Geogr. Ordenam. Territ. 2013, 1, 83–107. [Google Scholar] [CrossRef][Green Version]
- Nunes, L.J.; Meireles, C.I.; Pinto Gomes, C.J.; Almeida Ribeiro, N. Historical development of the portuguese forest: The introduction of invasive species. Forests 2019, 10, 974. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renew. Sustain. Energy Rev. 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Pinto Gomes, C.J.; Ribeiro, N.; Almeida, M. Control of Invasive Forest Species through the Creation of a Value Chain: Acacia dealbata Biomass Recovery. Environments 2020, 7, 39. [Google Scholar] [CrossRef]
- Minuto, L.; Casazza, G.; Dagnino, D.; Guerrina, M.; Macrì, C.; Zappa, E.; Mariotti, M.G. REPRODUCTIVE TRAITS OF THE INVASIVE SPECIES ACACIA DEALBATA LINK. IN THE NORTHERN MEDITERRANEAN BASIN. Ann. Bot. 2020, 10, 13–20. [Google Scholar]
- Martins, F.; Alegria, C.; Gil, A. Mapping invasive alien Acacia dealbata Link using ASTER multispectral imagery: A case study in central-eastern of Portugal. For. Syst. 2016, 25, 13. [Google Scholar] [CrossRef]
- Correia, M.; Montesinos, D.; French, K.; Rodríguez-Echeverría, S. Evidence for enemy release and increased seed production and size for two invasive Australian acacias. J. Ecol. 2016, 104, 1391–1399. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Johnson, D.W. The use of fire as a tool for controlling invasive plants. Cal-IPC Publ. 2006, 1, 56. [Google Scholar]
- Lorenzo, P.; Pazos-Malvido, E.; Reigosa, M.J.; González, L. Differential responses to allelopathic compounds released by the invasive Acacia dealbata Link (Mimosaceae) indicate stimulation of its own seed. Aust. J. Bot. 2010, 58, 546–553. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and identification of chemical compounds released from the invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Gibson, M.R.; Richardson, D.M.; Marchante, E.; Marchante, H.; Rodger, J.G.; Stone, G.N.; Byrne, M.; Fuentes-Ramírez, A.; George, N.; Harris, C. Reproductive biology of Australian acacias: Important mediator of invasiveness? Divers. Distrib. 2011, 17, 911–933. [Google Scholar] [CrossRef]
- Orchard, A.E.; Maslin, B.R. (1584) Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved type. Taxon 2003, 52, 362–363. [Google Scholar] [CrossRef]
- Bouchenak-Khelladi, Y.; Maurin, O.; Hurter, J.; Van der Bank, M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol. Phylogenet. Evol. 2010, 57, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P. Das mimosas e outras acácias. In Histórias da Vida e da Terra—Um blog de História Natural; Wordpress.com: San Francisco, CA, USA, 2012; Volume 2019. [Google Scholar]
- Aguiar, F.; Ferreira, M. Plant invasions in the rivers of the Iberian Peninsula, south-western Europe: A review. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 147, 1107–1119. [Google Scholar] [CrossRef]
- Fernandes, P.M. Combining forest structure data and fuel modelling to classify fire hazard in Portugal. Ann. For. Sci. 2009, 66, 1–9. [Google Scholar] [CrossRef]
- Fernandes, M.M. Acácias e geografia histórica: Rotas de um percurso global (parte1). Cad. Curso Doutor. Geogr. 2012, 4, 23–40. [Google Scholar]
- Fernandes, M. Recuperação Ecológica de Áreas Invadidas por Acacia Dealbata Link no Vale do rio Gerês: Um Trabalho de Sísifo. Master’s Thesis, Universidade de Trás-os-Montes e Alto-Douro, Vila Real, Portugal, 2008. [Google Scholar]
- Simões, C.A.d.C.P. A Degradação da Paisagem e a Sua Perceção Após Invasão pela Espécie Acacia Dealbata Link.: O Caso da Região do Alto Ceira. Master’s Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- González, L.; Souto, X.C.; Reigosa, M. Allelopathic effects of Acacia melanoxylon R. Br. phyllodes during their decomposition. For. Ecol. Manag. 1995, 77, 53–63. [Google Scholar] [CrossRef]
- Ramoliya, P.; Patel, H.; Pandey, A. Effect of salinisation of soil on growth and macro-and micro-nutrient accumulation in seedlings of Acacia catechu (Mimosaceae). Ann. Appl. Biol. 2004, 144, 321–332. [Google Scholar] [CrossRef]
- Martins-Corder, M.P.; Borges, R.Z.; Junior, N.B. Fotoperiodismo e quebra de dormência em sementes de acácia-negra (Acacia mearnsii De Wild.). Ciência Florest. 1999, 9, 71–77. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Antunes, P.M.; Martins-Loução, M.A.; Klironomos, J.N. Disturbance influences the outcome of plant–soil biota interactions in the invasive Acacia longifolia and in native species. Oikos 2010, 119, 1172–1180. [Google Scholar] [CrossRef]
- Coelho, S.I.D.B.F. Factores Facilitadores da Invasibilidade de Acacia Dealbata em Função do Uso do Solo. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2014. [Google Scholar]
- González-Muñoz, N.; Costa-Tenorio, M.; Espigares, T. Invasion of alien Acacia dealbata on Spanish Quercus robur forests: Impact on soils and vegetation. For. Ecol. Manag. 2012, 269, 214–221. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Wilson, J.R.; Gairifo, C.; Gibson, M.R.; Arianoutsou, M.; Bakar, B.B.; Baret, S.; Celesti-Grapow, L.; DiTomaso, J.M.; Dufour-Dror, J.M.; Kueffer, C. Risk assessment, eradication, and biological control: Global efforts to limit Australian acacia invasions. Divers. Distrib. 2011, 17, 1030–1046. [Google Scholar] [CrossRef]
- Albaugh, T.J.; Rubilar, R.A.; Maier, C.A.; Acuna, E.A.; Cook, R.L. Biomass and nutrient mass of Acacia dealbata and Eucalyptus globulus bioenergy plantations. Biomass Bioenergy 2017, 97, 162–171. [Google Scholar] [CrossRef]
- Gouws, A.J.; Shackleton, C.M. Abundance and correlates of the Acacia dealbata invasion in the northern Eastern Cape, South Africa. For. Ecol. Manag. 2019, 432, 455–466. [Google Scholar] [CrossRef]
- Hunt, M.A.; Beadle, C.L. Whole-tree transpiration and water-use partitioning between Eucalyptus nitens and Acacia dealbata weeds in a short-rotation plantation in northeastern Tasmania. Tree Physiol. 1998, 18, 557–563. [Google Scholar] [CrossRef]
- Forrester, D.I.; Theiveyanathan, S.; Collopy, J.J.; Marcar, N.E. Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For. Ecol. Manag. 2010, 259, 1761–1770. [Google Scholar] [CrossRef]
- Vertessy, R.A.; Watson, F.G.; Sharon, K. Factors determining relations between stand age and catchment water balance in mountain ash forests. For. Ecol. Manag. 2001, 143, 13–26. [Google Scholar] [CrossRef]
- Bowd, E.J.; Banks, S.C.; Strong, C.L.; Lindenmayer, D.B. Long-term impacts of wildfire and logging on forest soils. Nat. Geosci. 2019, 12, 113–118. [Google Scholar] [CrossRef]
- Je, K. Fire management impacts on invasive plant species in the western United States. Conserv. Biol. 2006, 20, 375–384. [Google Scholar]
- Florentine, S.; Milberg, P.; Gibson, M.; Westbrooke, M. Post-wildfire seedling colonisation patterns in a Eucalyptus delegatensis (Myrtaceae) windthrow site at Snowy River National Park, Victoria. Aust. For. 2008, 71, 48–53. [Google Scholar] [CrossRef]
- Gordon, C.E.; Price, O.F.; Tasker, E.M.; Denham, A.J. Acacia shrubs respond positively to high severity wildfire: Implications for conservation and fuel hazard management. Sci. Total Environ. 2017, 575, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.L.; Torres, R.C.; Renison, D. Do wildfires promote woody species invasion in a fire-adapted ecosystem? Post-fire resprouting of native and non-native woody plants in central Argentina. Environ. Manag. 2016, 57, 308–317. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).