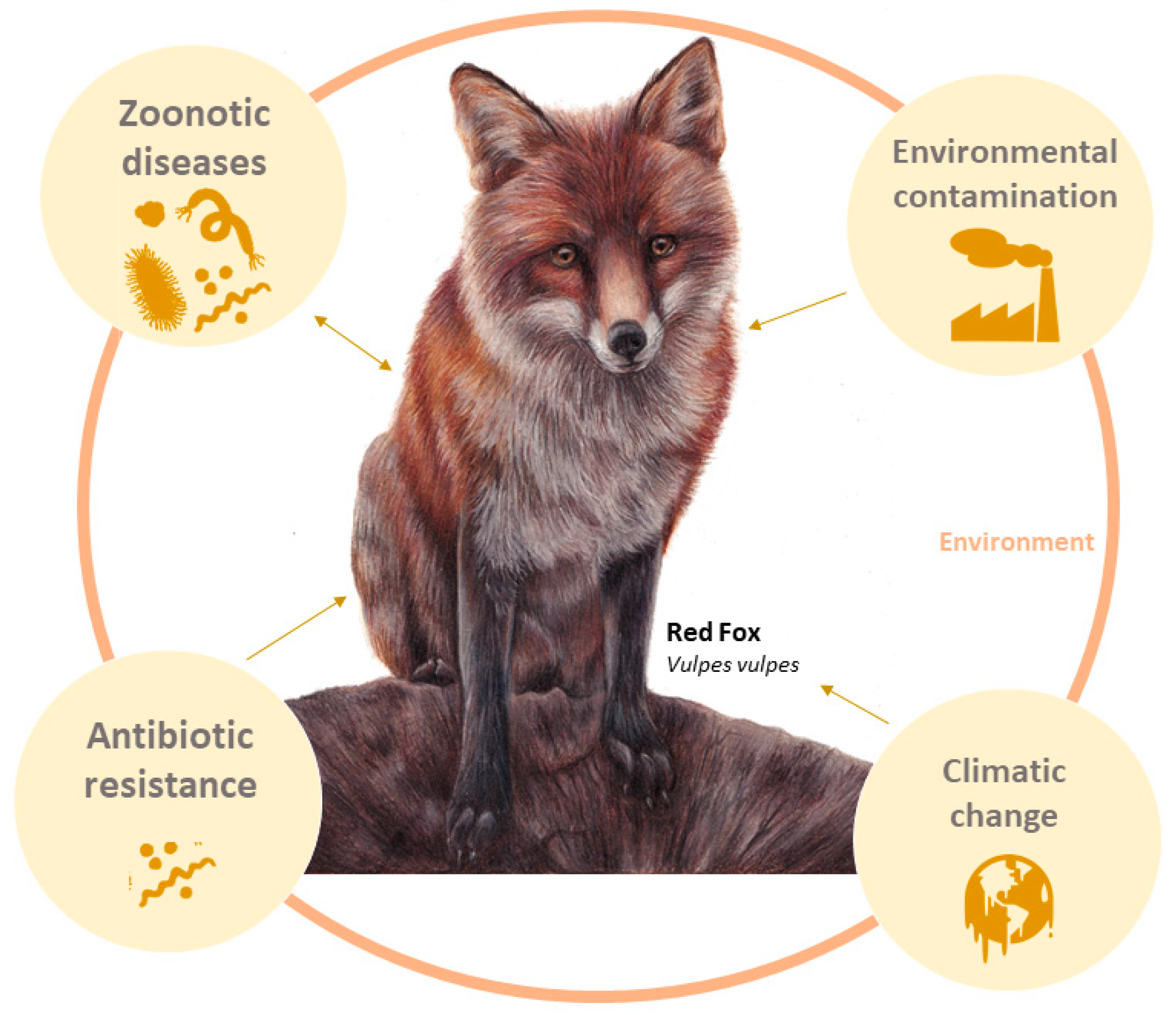
Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health
Abstract
1. Introduction
2. Material and Methods
3. Results Obtained from the Consulted Papers
3.1. Red Fox as a Sentinel of Environmental Contamination
| Substance | Number of Animals | Origin Animal | Sample | Country | Year | Reference |
|---|---|---|---|---|---|---|
| Cr, Cu, Ni, Pb, Zn | 20 | Wild and Fur farm | Hair and skin | Poland | 2011 | [17] |
| Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn | 48 | Wild | Small intestines | Czech Republic | 2010–2011 | [34] |
| Cd, Pb, Cu, Zn | 87 | Wild | Kidney and liver | Switzerland | 1997–1998 | [35] |
| Pb, Cd, Hg | 30 | Fur Farm | Kidney | Poland | 2008 | [36] |
| Cu, Ni, Zn, Co, Cd, Pb | 10 | Wild | Kidney | Hungary | 2008 | [37] |
| Hg | 37 | Fur farm | Hair and skin | Poland | 2014 | [17] |
| Hg, Pb, Cd, Cr, As | 18 | Wild | Liver, kidneys, and muscles | Slovak Republic | 1998–1999 | [12] |
| Cd, Pb, Zn | 250 | Wild | Kidney | Spain | 2003–2011 | [38] |
| Cd, Pb, Zn. | 36 | Wild | Kidney, liver and muscle | Poland | 2002–2003 | [39] |
| Hg | 6 | Wild | Liver and kidney | Russia | 2007–2011 | [14] |
| Al, Ca, Cr, Cu, Fe, Mg, Mn, Ni, Pb | 56 | Wild | Liver | Romania | May–September 2014 | [40] |
| Hg | 200 | Wild | Liver, muscle, kidney, hair, bone | Alaska | 2010–2011 | [28] |
| Zn, Cu, Pb, Cd, Hg | 30 | Wild | Cartilage, compact bone, and spongy bone | Poland | 2008–2009 | [41] |
| Pb, Cu | 42 | Wild | Muscle and skin | Italy | 2010 | [13] |
| Cd, Pb, Cr, Cu, Zn, Mn, Ni | 27 | Wild | Intestine | Czech Republic | 2009 to 2010 | [42] |
| Hg | 27 | Wild | Liver, muscle, and kidney | Poland | 2004–2006 | [29] |
| Mn, Fe, Sr | 38 | Wild | Bone | Poland | 2008–2009 | [43] |
| Hg, Cd, Pb | 46 | Wild | Liver | Italy | 1992 | [30] |
| As, Cd, Cu, Pb, Hg | 28 | Wild | Liver, kidney and muscle | Croatia | 2008–2009 | [44] |
| Pb, Cd, Cr, Hg | Unknown | Wild | Heart, liver, diaphragm, kidney, muscle, and adipose tissue | Italy | 1994–1995 | [10] |
| PCB, DDE | Unknown | Wild | Heart, liver, diaphragm, kidney, muscle, and adipose tissue | Italy | 1994–1995 | [10] |
| PCB, Dieldrin, DDT, Endosulfan, HCB, Heptachlor | 192 | Wild | Perirenal adipose tissue, Kidney | Switzerland | 1999–2000 | [25] |
| PCB | 80 | Wild | Muscle | Germany | 1983–1991. | [24] |
| PCBs, DDT | 23 | Wild | Adipose tissue | Italy | 1991–1992 | [3] |
| HCB, DDT, PBC | 57 | Wild | Muscle and adipose tissue | Italy | 1992–1993 | [3] |
| PBDEs | 33 | Wild | Adipose tissue, liver, and muscle | Belgium | 2003–2004 | [45] |
| HCB, DDT, PCB | 36 | Wild | Adipose tissues and muscle | Italy | 1992 | [30] |
| PCB | 20 | Wild | Liver, lungs | Poland | 2008–2009 | [26] |
| Aldrin, cis-chlordane, trans-chlordane, DDE, DDD, DDT, dieldrin, endosulfan, endrin, HCB, heptachlor, heptachlor-exo-epoxide, iso-drin, methoxychlor, mirex, PBC | 18 | Wild | Plasma, liver, and adipose tissue | Spain | 2004–2006 | [46] |
| Fluoride | 32 | Wild | Bone | Poland | 2014 | [32] |
| Fluoride | 34 | Wild | Teeth | Poland | Unknown | [31] |
| Fluoride | 182 | Wild | Mandible | Great Britain | Unknown | [31] |
| Fluoride | 35 | Wild | Teeth | Poland | 2004/2005 and 2005/2006 | [7] |
| 90Sr, 238,239+240Pu, 241Am and 137Cs | 183 | Wild | Jaw bones | Poland | 2008 | [33] |
3.2. Red Foxes as a Sentinel of Antimicrobial Resistance (AMR)
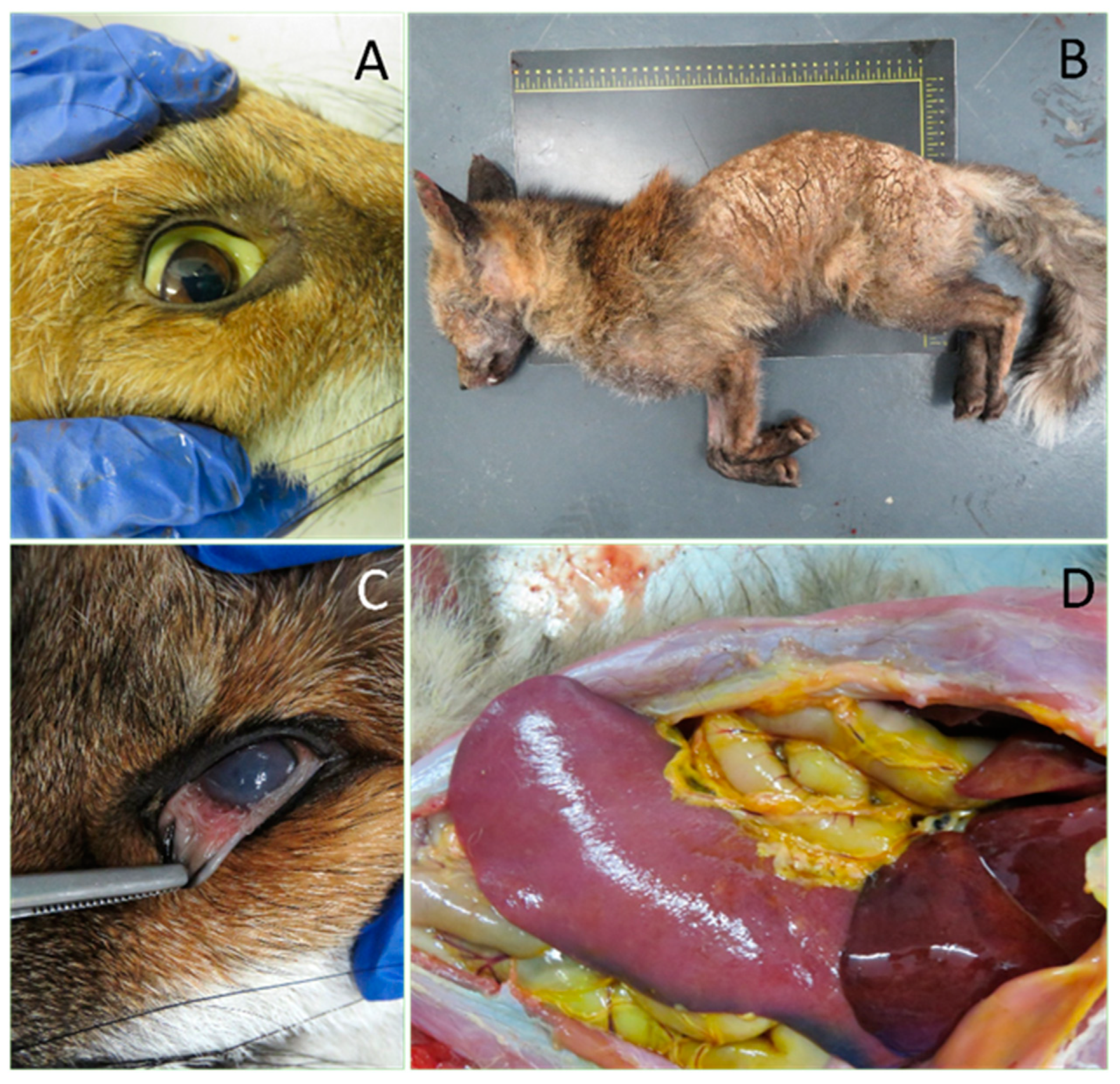
3.3. Red Foxes as a Sentinel of Zoonotic Diseases
3.4. Red Fox as Sentinel of Climatic Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hoffmann, M.; Sillero-Zubiri, C. Vulpes vulpes (amended version of 2016 assessment). The IUCN Red List of Threatened Species 2021: e.T23062A193903628. 2021. Available online: https://www.iucnredlist.org/species/23062/193903628 (accessed on 10 August 2021).
- Brash, M. Foxes. In BSAVA Manual of Wildlife Casualties; British Small Animal Veterinary Association: Gloucester, UK, 2003; pp. 154–166. [Google Scholar]
- Corsolini, S.; Burrini, L.; Focardi, S.; Lovari, S. How Can We Use the Red Fox as a Bioindicator of Organochlorines? Arch. Environ. Contam. Toxicol. 2000, 39, 547–556. [Google Scholar] [CrossRef]
- Harris, S.; Yalden, D. Mammals of the British Isles: Handbook, 4th ed.; Mammal Society: Southampton, UK, 2008; ISBN 0906282659. [Google Scholar]
- Kelly, T.R.; Sleeman, J.M.; Box, P.O. Morbidity and Mortality of Red Foxes (Vulpes vulpes) and Gray Foxes (Urocyon Ciner-eoargenteus) Admitted to the Wildlife Center of Virginia, 1993–2001. J. Wildl. Dis. 2003, 39, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Turnover, A. Annual Turnover of Fox Populations in Europe *. Zbl. Vet. Med. 1976, 589, 580–589. [Google Scholar]
- Kalisińska, E.; Palczewska-Komsa, M. Teeth of the red fox Vulpes vulpes (L., 1758) as a bioindicator in studies on fluoride pollution. Acta Thériol. 2011, 56, 343–351. [Google Scholar] [CrossRef][Green Version]
- A LeBlanc, G.; Bain, L.J. Chronic toxicity of environmental contaminants: Sentinels and biomarkers. Environ. Heal. Perspect. 1997, 105, 65–80. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Animals as Monitors of Environmental Hazards. Animals as Sentinels of Environmental Health Hazards; National Academies Press (US): Washington, DC, USA, 1991; ISBN 0-309-59489-8. [Google Scholar]
- Alleva, E.; Francia, N.; Pandolfi, M.; De Marinis, A.M.; Chiarotti, F.; Santucci, D. Organochlorine and Heavy-Metal Contaminants in Wild Mammals and Birds of Urbino-Pesaro Province, Italy: An Analytic Overview for Potential Bioindicators. Arch. Environ. Contam. Toxicol. 2006, 51, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Wang-Andersen, G.; Skaare, J.U.; Prestrud, P.; Steinnes, E. Levels and congener pattern of PCBs in arctic fox, Alopex lagopus, in Svalbard. Environ. Pollut. 1993, 82, 269–275. [Google Scholar] [CrossRef]
- Piskoroyá, L.; Vasilková, Z.; Krupicer, I. Heavy Metal Residues in Tissues of Wild Boar (Sus Scrofa) and Red Fox (Vulpes vulpes) in the Central Zemplin Region of the Slovak Republic. Czech J. Anim. Sci. 2003, 48, 134–138. [Google Scholar]
- Naccari, C.; Giangrosso, G.; Macaluso, A.; Billone, E.; Cicero, A.; D’Ascenzi, C.; Ferrantelli, V. Red foxes (Vulpes vulpes) bioindicator of lead and copper pollution in Sicily (Italy). Ecotoxicol. Environ. Saf. 2013, 90, 41–45. [Google Scholar] [CrossRef]
- Komov, V.T.; Ivanova, E.S.; Gremyachikh, V.A.; Poddubnaya, N.Y. Mercury Content in Organs and Tissues of Indigenous (Vulpes vulpes L.) and Invasive (Nyctereutes procyonoides Gray.) Species of Canids from Areas Near Cherepovets (North-Western Industrial Region, Russia). Bull. Environ. Contam. Toxicol. 2016, 97, 480–485. [Google Scholar] [CrossRef]
- Köhler, H.-R.; Triebskorn, R.; Meierbachtol, T.; Harper, J.; Humphrey, N. Wildlife Ecotoxicology of Pesticides: Can We Track Effects to the Population Level and Beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Filistowicz, A.; Dobrzański, Z.; Przysiecki, P.; Nowicki, S.; Filistowicz, A. Concentration of heavy metals in hair and skin of silver and red foxes (Vulpes vulpes). Environ. Monit. Assess. 2011, 182, 477–484. [Google Scholar] [CrossRef]
- Rogstad, T.W.; Sonne, C.; Villanger, G.D.; Øystein, A.; Fuglei, E.; Muir, D.C.; Jørgensen, E.; Jenssen, B.M. Concentrations of vitamin A, E, thyroid and testosterone hormones in blood plasma and tissues from emaciated adult male Arctic foxes (Vulpes lagopus ) dietary exposed to persistent organic pollutants (POPs). Environ. Res. 2017, 154, 284–290. [Google Scholar] [CrossRef]
- Bocharova, N.; Treu, G.; Czirják, G.Á.; Krone, O.; Stefanski, V.; Wibbelt, G.; Unnsteinsdóttir, E.R.; Hersteinsson, P.; Schares, G.; Doronina, L.; et al. Correlates between Feeding Ecology and Mercury Levels in Historical and Modern Arctic Foxes (Vulpes lagopus). PLoS ONE 2013, 8, e60879. [Google Scholar] [CrossRef]
- Pedersen, K.E.; Styrishave, B.; Sonne, C.; Dietz, R.; Jenssen, B.M. Accumulation and potential health effects of organohalogenated compounds in the arctic fox (Vulpes lagopus)—A review. Sci. Total. Environ. 2015, 502, 510–516. [Google Scholar] [CrossRef]
- Andersen, M.S.; Fuglei, E.; König, M.; Lipasti, I.; Pedersen, Å.Ø.; Polder, A.; Yoccoz, N.; Routti, H. Levels and temporal trends of persistent organic pollutants (POPs) in arctic foxes (Vulpes lagopus) from Svalbard in relation to dietary habits and food availability. Sci. Total. Environ. 2015, 511, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; White, P.A.; Burrows, D.G.; Lundin, J.I.; Ylitalo, G.M. Food resources influence levels of persistent organic pollutants and stable isotopes of carbon and nitrogen in tissues of Arctic foxes (Vulpes lagopus) from the Pribilof Islands, Alaska. Polar Res. 2017, 36, 12. [Google Scholar] [CrossRef]
- Mullineaux, E.; Best, D.; Cooper, J.E. BSAVA Manual of Wildlife Casualties; British Small Animal Veterinary Association: Gloucester, UK, 2003; ISBN 0905214633. [Google Scholar]
- Georgii, S.; Bachour, G.; Failing, K.; Eskens, U.; Elmadfa, I.; Brunn, H. Polychlorinated biphenyl congeners in Foxes in Germany from 1983 to 1991. Arch. Environ. Contam. Toxicol. 1994, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dip, R.; Hegglin, D.; Deplazes, P.; Dafflon, O.; Koch, H.; Naegeli, H. Age- and sex-dependent distribution of persistent organochlorine pollutants in urban foxes. Environ. Heal. Perspect. 2003, 111, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Tomza-Marciniak, A.; Pilarczyk, B.; Bakowska, M.; Tylkowska, A.; Marciniak, A.; Ligocki, M.; Udala, J. Polychlorinated Biphenyl (PCBS) Residues in Suburban Red Foxes (Vulpes vulpes)-Preliminary Study. Pol. J. Environ. Stud. 2012, 21, 193–199. [Google Scholar]
- Corsolini, S.; Focardi, S.; Kannan, K.; Tanabe, S.; Tatsukawa, R. Isomer-specific analysis of polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin equivalents (TEQs) in red fox and human adipose tissue from central Italy. Arch. Environ. Contam. Toxicol. 1995, 29, 61–68. [Google Scholar] [CrossRef]
- Dainowski, B.; Duffy, L.; McIntyre, J.; Jones, P. Hair and bone as predictors of tissular mercury concentration in the western Alaska red fox, Vulpes vulpes. Sci. Total. Environ. 2015, 518-519, 526–533. [Google Scholar] [CrossRef]
- Kalisinska, E.; Lisowski, P.; Kosik-Bogacka, D.I. Red Fox Vulpes vulpes (L., 1758) as a Bioindicator of Mercury Contamination in Terrestrial Ecosystems of North-Western Poland. Biol. Trace Element Res. 2012, 145, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Corsolini, S.; Focardi, S.; Leonzio, C.; Lovari, S.; Monaci, F.; Romeo, G. Heavy Metals and Chlorinated Hydrocarbon Concentrations in the Red Fox in Relation to Some Biological Paramaters. Environ. Monit. Assess. 1999, 54, 87–100. [Google Scholar] [CrossRef]
- Palczewska-Komsa, M.; Wilk, A.; Stogiera, A.; Chlubek, D.; Buczkowska-Radlińska, J.; Wiszniewska, B. Animals in Bio-monitoring Studies of Environmental Fluoride Pollution. Fluoride 2016, 49, 279–292. [Google Scholar]
- Palczewska-Komsa, M.; Kalisinska, E.; Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N.; Budis, H.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Fluoride in the Bones of Foxes (Vulpes vulpes Linneaus, 1758) and Raccoon Dogs (Nyctereutes procyonoides Gray, 1834) from North-Western Poland. Biol. Trace Element Res. 2014, 160, 24–31. [Google Scholar] [CrossRef]
- Mietelski, J.W.; Kitowski, I.; Tomankiewicz, E.; Gaca, P.; Blażej, S. Plutonium, americium, 90Sr and 137Cs in bones of red fox (Vulpes vulpes) from Eastern Poland. J. Radioanal. Nucl. Chem. 2007, 275, 571–577. [Google Scholar] [CrossRef]
- Sedláková, J.; Řezáč, P.; Fišer, V.; Hedbávný, J. Red Fox, Vulpes vulpes L., as a Bioindicator of Environmental Pollution in the Countryside of Czech Republic. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 447–452. [Google Scholar] [CrossRef]
- Dip, C.S.R.; Stieger, C.; Deplazes, P.; Hegglin, D.; Müller, U.; Dafflon, O.; Koch, H.; Naegeli, H.; Dip, R. Comparison of Heavy Metal Concentrations in Tissues of Red Foxes from Adjacent Urban, Suburban, and Rural Areas. Arch. Environ. Contam. Toxicol. 2001, 40, 551–556. [Google Scholar] [CrossRef]
- Cybulski, W.; Andrzej, J. Content of lead, cadmium, and mercury in the liver and kidneys of silver foxes (Vulpes vulpes ) in relation to age and reproduction disorders. Bull Vet Inst Pulawy 2009, 53, 63–69. [Google Scholar]
- Heltai, M.; Markov, G. Red Fox (Vulpes vulpes Linnaeus, 1758) as Biological Indicator for Environmental Pollution in Hungary. Bull. Environ. Contam. Toxicol. 2012, 89, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, M.; Rodríguez, F.S.; Hernández-Moreno, D.; Rigueira, L.; Fidalgo, L.E.; Beceiro, A.L. Bioaccumulation of cadmium, lead and zinc in liver and kidney of red fox (Vulpes vulpes) from NW Spain: Influence of gender and age. Toxicol. Environ. Chem. 2016, 98, 109–117. [Google Scholar] [CrossRef]
- Ziętara, J.; Wierzbowska, I.A.; Gdula-Argasińska, J.; Gajda, A.; Laskowski, R. Concentrations of cadmium and lead, but not zinc, are higher in red fox tissues than in rodents—pollution gradient study in the Małopolska province (Poland). Environ. Sci. Pollut. Res. 2019, 26, 4961–4974. [Google Scholar] [CrossRef]
- Farkas, A.; Bidló, A.; Bolodár-Varga, B.; Jánoska, F. Accumulation of Metals in Liver Tissues of Sympatric Golden Jackal (Canis aureus) and Red Fox (Vulpes vulpes) in the Southern Part of Romania. Bull. Environ. Contam. Toxicol. 2017, 98, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Lanocha-Arendarczyk, N.; Kalisinska, E.; Kosik-Bogacka, D.I.; Budis, H.; Noga-Deren, K. Trace metals and micronutrients in bone tissues of the red fox Vulpes vulpes (L., 1758). Acta Thériol. 2012, 57, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Borkovcova, M.; Fiser, V.; Bednarova, M.; Havlicek, Z.; Adámková, A.; Mlcek, J.; Jurikova, T.; Balla, S.; Adámek, M. Effect of Accumulation of Heavy Metals in the Red Fox Intestine on the Prevalence of Its Intestinal Parasites. Animals 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Budis, H.; Kalisinska, E.; Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I. The Concentration of Manganese, Iron and Strontium in Bone of Red Fox Vulpes vulpes (L. 1758). Biol. Trace Element Res. 2013, 155, 361–369. [Google Scholar] [CrossRef]
- Bilandžić, N.; Dežđek, D.; Sedak, M.; Đokić, M.; Solomun, B.; Varenina, I.; Knežević, Z.; Slavica, A. Concentrations of Trace Elements in Tissues of Red Fox (Vulpes vulpes) and Stone Marten (Martes foina) from Suburban and Rural Areas in Croatia. Bull. Environ. Contam. Toxicol. 2010, 85, 486–491. [Google Scholar] [CrossRef]
- Voorspoels, S.; Covaci, A.; Lepom, P.; Escutenaire, S.; Schepens, P. Remarkable Findings Concerning PBDEs in the Terres-trial Top-Predator Red Fox (Vulpes vulpes). Environ. Sci. Technol. 2006, 40, 2937–2943. [Google Scholar] [CrossRef]
- Mateo, R.; Millán, J.; Rodríguez-Estival, J.; Camarero, P.R.; Palomares, F.; Ortiz-Santaliestra, M.E. Levels of organochlorine pesticides and polychlorinated biphenyls in the critically endangered Iberian lynx and other sympatric carnivores in Spain. Chemosphere 2012, 86, 691–700. [Google Scholar] [CrossRef]
- Plaza-Rodríguez, C.; Alt, K.; Grobbel, M.; Hammerl, J.A.; Irrgang, A.; Szabo, I.; Stingl, K.; Schuh, E.; Wiehle, L.; Pfefferkorn, B.; et al. Wildlife as Sentinels of Antimicrobial Resistance in Germany? Front. Vet. Sci. 2021, 27, 627821. [Google Scholar] [CrossRef]
- Caprioli, A.; Busani, L.; Martel, J.L.; Helmuth, R. Monitoring of antibiotic resistance in bacteria of animal origin: Epidemiological and microbiological methodologies. Int. J. Antimicrob. Agents 2000, 14, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Igrejas, G.; Gonçalves, A.; Estepa, V.; Sargo, R.; Torres, C.; Poeta, P. Molecular characterization of extended-spectrum-beta-lactamase-producing Escherichia coli isolates from red foxes in Portugal. Arch. Microbiol. 2013, 195, 141–144. [Google Scholar] [CrossRef]
- Lineages, C.; Resistance, A. Clonal Lineages, Antibiotic Resistance and Virulence Factors in Vancomycin-Resistant Enterococci Isolated from Fecal Samples of Red Foxes ( Vulpes vulpes ). J. Wildl. Dis. 2011, 47, 769–773. [Google Scholar]
- Mo, S.S.; Urdahl, A.M.; Madslien, K.; Sunde, M.; Nesse, L.L.; Slettemeås, J.S.; Norström, M. What does the fox say? Monitoring antimicrobial resistance in the environment using wild red foxes as an indicator. PLoS ONE 2018, 13, e0198019. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Igrejas, G.; Gonçalves, A.; Pacheco, R.; Monteiro, R.; Sargo, R.; Brito, F.; Torres, C.; Poeta, P. Antimicrobial resistance and virulence genes in Escherichia coli and enterococci from red foxes (Vulpes vulpes). Anaerobe 2013, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.; Meredith, A.; Shaw, D.J.; Giotis, E.S.; Lloyd, D.H.; Loeffler, A. Foxes As a Potential Wildlife Reservoir formecA-Positive Staphylococci. Vector-Borne Zoonotic Dis. 2012, 12, 583–587. [Google Scholar] [CrossRef]
- Chiari, M.; Ferrari, N.; Giardiello, D.; Lanfranchi, P.; Zanoni, M.; Lavazza, A.; Alborali, L.G. Isolation and identification of Salmonella spp. from red foxes (Vulpes vulpes) and badgers (Meles meles) in northern Italy. Acta Veter-Scand. 2014, 56, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.P.; Vila-Viçosa, M.J.; Coutinho, T.; Cardoso, L.; Gottstein, B.; Müller, N.; Cortes, H.C. Trichinella britovi in a red fox (Vulpes vulpes) from Portugal. Vet. Parasitol. 2015, 12, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, B.; Ravel, A.; Leighton, P.; Stephen, C.; Iqbal, A.; Ndao, M.; Konecsni, K.; Fernando, C.; Jenkins, E. Foxes (Vulpes vulpes) as sentinels for parasitic zoonoses, Toxoplasma gondii and Trichinella nativa, in the northeastern Canadian Arctic. Int. J. Parasitol. Parasites Wildl. 2018, 7, 391–397. [Google Scholar] [CrossRef]
- Barrera, J.P.; Carmena, D.; Rodríguez, E.; Checa, R.; López, A.M.; Fidalgo, L.E.; Gálvez, R.; Marino, V.; Fuentes, I.; Miró, G.; et al. The red fox ( Vulpes vulpes ) as a potential natural reservoir of human cryptosporidiosis by Cryptosporidium hominis in Northwest Spain. Transbound. Emerg. Dis. 2020, 67, 2172–2182. [Google Scholar] [CrossRef]
- Papini, R.A.; Verin, R. Giardia and Cryptosporidium in Red Foxes (Vulpes Vulpes): Screening for Coproantigens in a Population of Central Italy and Mini-Review of the Literature. Maced. Veter-Rev. 2019, 42, 101–106. [Google Scholar] [CrossRef]
- Ebani, V.V.; Rocchigiani, G.; Nardoni, S.; Bertelloni, F.; Vasta, V.; Papini, R.A.; Verin, R.; Poli, A.; Mancianti, F. Molecular detection of tick-borne pathogens in wild red foxes (Vulpes vulpes) from Central Italy. Acta Trop. 2017, 172, 197–200. [Google Scholar] [CrossRef]
- Gicik, Y.; Kara, M.; Sari, B.; Kiliç, K.; Arslan, M.Ö. Intestinal Parasites of Red Foxes (Vulpes vulpes) and Their Zoonotic Im-portance for Humans in Kars Province. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 135–140. [Google Scholar]
- Dipineto, L.; Manna, L.; Baiano, A.; Gala, M.; Fioretti, A.; Gravino, A.E.; Menna, L.F. Presence of Leishmania infantum in Red Foxes (Vulpes vulpes) in Southern Italy. J. Wildl. Dis. 2007, 43, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, S.; Ntais, P.; Messaritakis, I.; Tsirigotakis, N.; Dokianakis, E.; Antoniou, M. Detection of Leishmania Infantumin red foxes (Vulpes vulpes) in Central Greece. Parasitology 2015, 142, 1574–1578. [Google Scholar] [CrossRef]
- Smith, G.C. Prevalence of zoonotic important parasites in the red fox (Vulpes vulpes) in Great Britain. Veter-Parasitol. 2003, 118, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sargo, R.; Loureiro, F.; Catarino, A.L.; Valente, J.; Silva, F.; Cardoso, L.; Otranto, D.; Maia, C. First report of thelazia callipaedain red foxes (Vulpes vulpes) from Portugal. J. Zoo Wildl. Med. 2014, 45, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carrasco, C.; Ruiz De Ybáñez, M.R.; Sagarminaga, J.L.; Garijo, M.M.; Moreno, F.; Acosta, I.; Hernández, S.; Alonso, F.D. Parasites of the Red Fox (Vulpes vulpes Linnaeus, 1758) in Murcia, Southeast Spain. Med. Vet. 2007, 158, 331–335. [Google Scholar]
- Dybing, N.A.; Fleming, P.A.; Adams, P.J. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int. J. Parasitol. Parasites Wildl. 2013, 2, 165–172. [Google Scholar] [CrossRef]
- Shamsi, S.; Mcspadden, K.; Baker, S.; Jenkins, D.J. Occurrence of tongue worm, Linguatula cf. serrata (Pentastomida: Linguatulidae) in wild canids and livestock in south-eastern Australia. Int. J. Parasitol. Parasites Wildl. 2017, 6, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.M.; Baker, R.; Tomlinson, A.; Berg, M.J.; Charman, N.; Tolhurst, B.A. Spatial distribution of sarcoptic mange (Sarcoptes scabiei) in urban foxes (Vulpes vulpes) in Great Britain as determined by citizen science. Urban Ecosyst. 2020, 23, 1127–1140. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; Iossa, G.; Baker, P.J.; Cole, N.C.; Funk, S.M.; Harris, S. The impact of sarcoptic mange Sarcoptes scabiei on the British fox Vulpes vulpes population. Mammal Rev. 2007, 37, 278–296. [Google Scholar] [CrossRef][Green Version]
- Dietary control of exertional rhabdomyolysis in horses. J. Equine Veter-Sci. 1998, 18, 450. [CrossRef]
- Bezerra-Santos, M.A.; Nguyen, V.-L.; Iatta, R.; Manoj, R.R.S.; Latrofa, M.S.; Hodžić, A.; Dantas-Torres, F.; Mendoza-Roldan, J.A.; Otranto, D. Genetic variability of Ehrlichia canis TRP36 in ticks, dogs, and red foxes from Eurasia. Veter-Microbiol. 2021, 255, 109037. [Google Scholar] [CrossRef]
- Niedringhaus, K.D.; Brown, J.D.; Sweeley, K.M.; Yabsley, M.J. A review of sarcoptic mange in North American wildlife. Int. J. Parasitol. Parasites Wildl. 2019, 9, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Serieys, L.E.K.; Stephenson, N.; Riley, S.; Foley, C.; Jennings, M.; Wengert, G.; Vickers, W.; Boydston, E.; Lyren, L.; et al. A synthetic review of notoedres species mites and mange. Parasitology 2016, 143, 1847–1861. [Google Scholar] [CrossRef]
- Vos, A.; Müller, T.; Neubert, L.; Zurbriggen, A.; Botteron, C.; Pöhle, D.; Schoon, H.; Haas, L.; Jackson, A.C. Rabies in Red Foxes (Vulpes vulpes) Experimentally Infected with European Bat Lyssavirus Type 1. J. Veter-Med. Ser. B 2004, 51, 327–332. [Google Scholar] [CrossRef]
- Chautan, M.; Pontier, D.; Artois, M. Role of rabies in recent demographic changes in Red Fox (Vulpes vulpes) populations in Europe. Mammalia 2000, 64, 391–410. [Google Scholar] [CrossRef]
- Escutenaire, S.; Pastoret, P.-P.; Sjölander, K.B.; Lundkvist, Å.; Heyman, P.; Brochier, B. Evidence of Puumala Hantavirus infection in red foxes (Vulpes vulpes ) in Belgium. Veter-Rec. 2000, 147, 365–366. [Google Scholar] [CrossRef]
- Campbell, S.J.; Ashley, W.; Gil-Fernandez, M.; Newsome, T.M.; Di Giallonardo, F.; Ortiz-Baez, A.S.; E Mahar, J.; Towerton, A.L.; Gillings, M.; Holmes, E.C.; et al. Red fox viromes in urban and rural landscapes. Virus Evol. 2020, 6, veaa065. [Google Scholar] [CrossRef]
- Bourg, M.; Nobach, D.; Herzog, S.; Lange-Herbst, H.; Nesseler, A.; Hamann, H.-P.; Becker, S.; Höper, D.; Hoffmann, B.; Eickmann, M.; et al. Screening red foxes (Vulpes vulpes) for possible viral causes of encephalitis. Virol. J. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Haemig, P.D.; Lithner, S.; De Luna, S.S.; Lundkvist, Å.; Waldenström, J.; Hansson, L.; Arneborn, M.; Olsen, B. Red fox and tick-borne encephalitis (TBE) in humans: Can predators influence public health? Scand. J. Infect. Dis. 2008, 40, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; Habela, M.Á.; De Arellano, E.R.; Díez-Fuertes, F.; Al., A.N.E.; López, P.; Sarriá, A.; Labiod, N.; Calero-Bernal, R.; Arenas, M.; et al. Survey of Crimean-Congo Hemorrhagic Fever Enzootic Focus, Spain, 2011–2015. Emerg. Infect. Dis. 2019, 25, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Tatum, L.M.; Pacy, J.M.; Frazier, K.S.; Weege, J.F.; Baldwin, C.A.; Hullinger, G.A.; Bossart, G.D.; Altman, N.H. Canine LaCrosse Viral Meningoencephalomyelitis with Possible Public Health Implications. J. Veter-Diagn. Investig. 1999, 11, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; Van Amerongen, G.; Van De Bildt, M.W.; Rimmelzwaan, G.F.; Dobson, A.P.; Osterhaus, A.D.; Kuiken, T. Highly Pathogenic Avian Influenza Virus (H5N1) Infection in Red Foxes Fed Infected Bird Carcasses. Emerg. Infect. Dis. 2008, 14, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Lledó, L.; Serrano, J.L.; Giménez-Pardo, C.; Gegúndez, I. Wild Red Foxes (Vulpes vulpes) as Sentinels of Rodent-Borne Hantavirus and Lymphocytic Choriomeningitis Virus in the Province of Soria, Northern Spain. J. Wildl. Dis. 2020, 56, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Slavica, A.; Deždek, D.; Konjević, D.; Cvetnić, Ž.; Sindicic, M.; Stanin, D.; Habus, J.; Turk, N. Prevalence of leptospiral antibodies in the red fox (Vulpes vulpes) population of Croatia. Veterinární Med. 2011, 56, 209–213. [Google Scholar] [CrossRef]
- Kingscote, B.F. Leptospirosis in red foxes in Ontario. J. Wildl. Dis. 1986, 22, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Żmudzki, J.; Arent, Z.; Jabłoński, A.; Nowak, A.; Zębek, S.; Stolarek, A.; Bocian, Łukasz; Brzana, A.; Pejsak, Z. Seroprevalence of 12 serovars of pathogenic Leptospira in red foxes (Vulpes vulpes) in Poland. Acta Veter-Scand. 2018, 60, 34. [Google Scholar] [CrossRef]
- Barrat, J.; Blancou, J.; Demantke, C.; Gerard, Y. β Hemolytic streptococcal infection in red foxes (Vulpes vulpes L.) in France: The natural disease and experimental studies. J. Wildl. Dis. 1985, 21, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; De Cruz, K.; Hénault, S.; Tambosco, J.; Richomme, C.; Réveillaud, Édouard; Gares, H.; Moyen, J.-L.; Boschiroli, M.L. Mycobacterium bovis Infection of Red Fox, France. Emerg. Infect. Dis. 2018, 24, 1150–1153. [Google Scholar] [CrossRef]
- Steinparzer, R.; Stanclova, G.; Bagó, Z.; Revilla-Fernández, S.; Leth, C.; Hofer, E.; Pohl, B.; Schmoll, F. Generalized Tuberculosis due to Mycobacterium caprae in a Red Fox (Vulpes vulpes) in Austria. J. Wildl. Dis. 2020, 56, 956–958. [Google Scholar] [CrossRef]
- Matos, A.C.; Figueira, L.; Martins, M.H.; Loureiro, F.; Pinto, M.L.; Matos, M.; Coelho, A.C. Survey of mycobacterium avium subspecies paratuberculosis in road-killed wild carnivores in Portugal. J. Zoo Wildl. Med. 2014, 45, 775–781. [Google Scholar] [CrossRef]
- Matos, A.; Figueira, L.; Martins, M.H.; Matos, M.; Morais, M.; Dias, A.P.; Pinto, M.; Coelho, A. Disseminated Mycobacterium bovis Infection in Red Foxes (Vulpes vulpes) with Cerebral Involvement Found in Portugal. Vector-Borne Zoonotic Dis. 2014, 14, 531–533. [Google Scholar] [CrossRef]
- Millán, J.; Jiménez, M.Á.; Viota, M.; Candela, M.G.; Peña, L.; León-Vizcaíno, L. Disseminated Bovine Tuberculosis in a Wild Red Fox (Vulpes vulpes) in Southern Spain. J. Wildl. Dis. 2008, 44, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Víchová, B.; Majláthová, V.; Hapunik, J.; Pet’ko, B. Anaplasma Phagocytophilum Infection of Red Foxes (Vulpes vulpes). Ann. Agric. Environ. Med. 2009, 16, 299–300. [Google Scholar] [PubMed]
- Liu, G.; Zhao, S.; Tan, W.; Hornok, S.; Yuan, W.; Mi, L.; Wang, S.; Liu, Z.; Zhang, Y.; Hazihan, W.; et al. Rickettsiae in red fox (Vulpes vulpes), marbled polecat (Vormela peregusna) and their ticks in northwestern China. Parasites Vectors 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Kawabata, H.; Masuzawa, T.; Yanagihara, Y.; Kimura, K.; Sakai, T.; Azuma, Y.; Fujii, N.; Ohno, S. Lyme Disease Spirochetes in a Wild Fox (Vulpes vulpes schrencki) and in Ticks. J. Wildl. Dis. 1994, 30, 439–444. [Google Scholar] [CrossRef]
- Ortuño, A.; Sanfeliu, I.; Nogueras, M.-M.; Pons, I.; López-Claessens, S.; Castellà, J.; Antón, E.; Segura, F. Detection of Rickettsia massiliae/Bar29 and Rickettsia conorii in red foxes (Vulpes vulpes) and their Rhipicephalus sanguineus complex ticks. Ticks Tick-borne Dis. 2018, 9, 629–631. [Google Scholar] [CrossRef]
- Szyfres, B.; González Tomé, J.; Tomé, J.G. Natural Brucella Infection in Argentine Wild Foxes*. Bull. Org. mond. Sante 1966, 1, 919–923. [Google Scholar]
- Morgan, W.J.B. The Examination of Brucella Cultures for Lysis by Phage. J. Gen. Microbiol. 1963, 30, 437–443. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, Y.; Ren, Y.; Liu, Z.; Li, Z. A Retrospective Survey of the Abortion Outbreak Event Caused by Brucellosis at a Blue Fox Breeding Farm in Heilongjiang Province, China. Front. Veter-Sci. 2021, 8, 409. [Google Scholar] [CrossRef]
- Scholz, H.C.; Revilla-Fernández, S.; Al Dahouk, S.; Hammerl, J.A.; Zygmunt, M.; Cloeckaert, A.; Koylass, M.; Whatmore, A.; Blom, J.; Vergnaud, G.; et al. Brucella vulpis sp. nov., isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Int. J. Syst. Evol. Microbiol. 2016, 66, 2090–2098. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Collinge, S.K.; Bai, Y.; Ray, C. Are carnivores universally good sentinels of plague? Vector Borne Zoonotic Dis. 2009, 9, 491–497. [Google Scholar] [CrossRef]
- Scholz, H.C.; Hofer, E.; Vergnaud, G.; Le Flèche, P.; Whatmore, A.M.; Al Dahouk, S.; Pfeffer, M.; Krüger, M.; Cloeckaert, A.; Tomaso, H. Isolation of Brucella microti from Mandibular Lymph Nodes of Red Foxes, Vulpes vulpes, in Lower Austria. Vector-Borne Zoonotic Dis. 2009, 9, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Hofer, E.; Revilla-Fernández, S.; Al Dahouk, S.; Riehm, J.M.; Nöckler, K.; Zygmunt, M.S.; Cloeckaert, A.; Tomaso, H.; Scholz, H.C. A potential novel Brucella species isolated from mandibular lymph nodes of red foxes in Austria. Veter-Microbiol. 2012, 155, 93–99. [Google Scholar] [CrossRef]
- Nikolova, S.; Tzvetkov, Y.; Najdenski, H.; Vesselinova, A. Isolation of Pathogenic Yersiniae from Wild Animals in Bulgaria. J. Veter-Med. Ser. B 2001, 48, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lempp, C.; Jungwirth, N.; Grilo, M.L.; Reckendorf, A.; Ulrich, A.; van Neer, A.; Bodewes, R.; Pfankuche, V.M.; Bauer, C.; Osterhaus, A.D.M.E.; et al. Pathological findings in the red fox (Vulpes vulpes), stone marten (Martes foina) and raccoon dog (Nyctereutes procyonoides), with special emphasis on infectious and zoonotic agents in Northern Germany. PLoS ONE 2017, 12, e0175469. [Google Scholar] [CrossRef]
- Malmasi, A.; Khosravi, A.R.; Selk Ghaffari, M.; Shojaee Tabrizi, A. Microsporum Canis Infection in a Red Fox (Vulpes vulpes). Iran. J. Vet. Res. 2009, 10, 189–191. [Google Scholar] [CrossRef]
- Knudtson, W.U.; Gates, C.E.; Ruth, G.K.; Haley, L.D. Trichophyton mentagrophytes dermatophytosis in wild fox. J. Wildl. Dis. 1980, 16, 465–468. [Google Scholar] [CrossRef]
- Riebold, D.; Lubig, J.; Wolf, P.; Wolf, C.; Russow, K.; Loebermann, M.; Slevogt, H.; Mohr, E.; Feldhusen, F.; Reisinger, E.C. First molecular detection of Pneumocystis spp. in red foxes (Vulpes vulpes linnaeus, 1758) and raccoon dogs (Nyctereutes procyonoides gray, 1834). Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101531. [Google Scholar] [CrossRef]
- Letková, V.; Lazar, P.; Čurlík, J.; Goldová, M.; Košuthová, L.; Mojžišová, J. The Red Fox (Vulpes vulpes L.) as a Source of Zoonoses. Veterinarski Arhiv. 2006, 76, 73–81. [Google Scholar]
- Pisano, S.R.R.; Zimmermann, F.; Rossi, L.; Capt, S.; Akdesir, E.; Bürki, R.; Kunz, F.; Origgi, F.C.; Ryser-Degiorgis, M.-P. Spatiotemporal spread of sarcoptic mange in the red fox (Vulpes vulpes) in Switzerland over more than 60 years: Lessons learnt from comparative analysis of multiple surveillance tools. Parasites Vectors 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Sukara, R.; Juwaid, S.; Ćirović, D.; Penezić, A.; Mihaljica, D.; Veinović, G.; Radojičić, S.; Hodžić, A.; Duscher, G.G.; Tomanović, S. Candidatus Neoehrlichia sp. (FU98) and Borrelia burgdorferi Sensu Lato in Red Foxes (Vulpes vulpes) from Serbia. Acta Vet. 2019, 69, 312–324. [Google Scholar] [CrossRef]
- Ebani, V.V.; Verin, R.; Fratini, F.; Poli, A.; Cerri, D. Molecular Survey of Anaplasma phagocytophilum and Ehrlichia canis in Red Foxes (Vulpes vulpes) from Central Italy. J. Wildl. Dis. 2011, 47, 699–703. [Google Scholar] [CrossRef]
- Rabies-OIE-World Organisation for Animal Health. Available online: https://www.oie.int/en/disease/rabies/ (accessed on 15 August 2021).
- Rabies and the Red Fox | Wildlife Online. Available online: https://www.wildlifeonline.me.uk/articles/view/rabies-and-the-red-fox (accessed on 15 August 2021).
- Meredith, A.L.; Cleaveland, S.C.; Denwood, M.J.; Brown, J.K.; Shaw, D.J. Coxiella burnetii(Q-Fever) Seroprevalence in Prey and Predators in the United Kingdom: Evaluation of Infection in Wild Rodents, Foxes and Domestic Cats Using a Modified ELISA. Transbound. Emerg. Dis. 2014, 62, 639–649. [Google Scholar] [CrossRef]
- Millán, J.; Proboste, T.; de Mera, I.G.F.; Chirife, A.D.; de la Fuente, J.; Altet, L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human–wildlife interface. Ticks Tick-borne Dis. 2016, 7, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Anaplasma phagocytophilum Infection of Red Foxes (Vulpes vulpes). Available online: http://www.aaem.pl/Anaplasma-phagocytophilum-infection-of-red-foxes-Vulpes-vulpes-,71577,0,2.html (accessed on 21 September 2021).
- Dumitrache, M.O.; Matei, I.A.; Ionică, A.M.; Kalmar, Z.; D’Amico, G.; Sikó-Barabási, S.; Ionescu, D.T.; Gherman, C.M.; Mihalca, A.D. Molecular detection of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato genospecies in red foxes (Vulpes vulpes) from Romania. Parasites Vectors 2015, 8, 1–5. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Campbell, G.D.; Oesterle, P.T.; Shirose, L.; McEwen, B.; Jardine, C.M. Red Fox as Sentinel forBlastomyces dermatitidis, Ontario, Canada. Emerg. Infect. Dis. 2016, 22, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Aguiló, E.; Alonso, S.; Cobelas, M.Á.; Anadón, R.; Ballester, F.; Benito, G.; Catalán, J.; de Castro, M.; Cendrero, A.; et al. A Preliminary General Assessment of the Impacts in Spain Due to the Effects of Climate Change. Ambiente. Ministry of Environment 2005. Available online: https://www.miteco.gob.es/es/cambio-climatico/temas/impactos-vulnerabilidad-y-adaptacion/Full%20report_tcm30-178514.pdf (accessed on 15 August 2021).
- Fuglei, E.; Ims, R.A. Global Warming and effects on the Arctic Fox. Sci. Prog. 2008, 91, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.I.; Blanton, J.D.; Gilbert, A.; Castrodale, L.; Hueffer, K.; Slate, D.; Rupprecht, C.E. A conceptual model for the impact of climate change on fox rabies in Alaska, 1980-2010. Zoonoses Public Heal. 2013, 61, 72–80. [Google Scholar] [CrossRef] [PubMed]
| Infectious Agent | Reference | |
|---|---|---|
| Parasites | Cryptosporidium parvum, C. hominis | [57,58] |
| Babesia spp. | [59] | |
| Hepatozoon canis | [59] | |
| Giardia spp. | [58] | |
| Capillaria hepatica, C. aerophilia | [60] | |
| Leishmania infantum | [61,62] | |
| Trichinella spiralis, Trichinella britovi | [55,63] | |
| Toxocara canis, T. leonina, T. gondii | [60,63] | |
| Uncinaria stenocephala | [63] | |
| Thelazia callipaeda | [64] | |
| Mesocestoides lineatus | [34] | |
| Echinococcus granulosus, E. multilocularis | [55,60,63] | |
| Dipylidium caninum | [65,66] | |
| Alaria alata | [60] | |
| Linguatula serrata | [60,67] | |
| Ixodes spp. | [2] | |
| Sarcoptes scabiei | [68,69,70] | |
| Demodex folliculorum | [69] | |
| Rhipicephalus sanguineus sensu lato (s.l.). | [71] | |
| Notoedres spp. | [72,73] | |
| Virus | Lyssavirus | [74,75] |
| Puumala Hantavirus | [76] | |
| Picornaviridae | [77] | |
| Picobirnaviruses | [77] | |
| Astrovirus | [77] | |
| Hepeviridae | [77] | |
| Borna disease virus 1 (BoDV-1) | [78] | |
| Tick-borne encephalitis (TBE) | [79] | |
| Crimean-Congo hemorrhagic fever virus (CCHFV) | [80] | |
| LaCrosse virus (LACV) encephalitis | [81] | |
| Avian Influenza Virus (H5N1) | [82] | |
| Lymphocytic Choriomeningitis Virus (LCMV) | [83] | |
| Bacteria | Leptospira interrogans, L. canicola, L. icterohaemorrhagica | [83] |
| Streptococcus spp. | [84,85,86] | |
| Salmonella spp. | [87] | |
| Coxiella burnetii | [54] | |
| Mycobacterium avium subsp. paratuberculosis, M. bovis, M. caprae | [88,89,90,91] | |
| Anaplasma phagocytophilum | [88,89,91,92] | |
| Borrelia valaisiana, B. burgdorferi s.l. | [93] | |
| Rickettsia conori | [94,95] | |
| Escherichia coli | [96] | |
| Brucella suis biovar 2, B. microti, B. vulpis | [97,98,99,100] | |
| Yersinia pseudotuberculosis, Y. pestis | [101,102,103] | |
| Listeria monocytogenes | [104] | |
| Ehrlichia canis | [71] | |
| Fungi | Microsporum spp. | [105] |
| Trichophyton spp. | [106] | |
| Pneumocystis carinii | [107] | |
| Blastomyces dermatitidis | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcês, A.; Pires, I. Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health. Environments 2021, 8, 103. https://doi.org/10.3390/environments8100103
Garcês A, Pires I. Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health. Environments. 2021; 8(10):103. https://doi.org/10.3390/environments8100103
Chicago/Turabian StyleGarcês, Andreia, and Isabel Pires. 2021. "Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health" Environments 8, no. 10: 103. https://doi.org/10.3390/environments8100103
APA StyleGarcês, A., & Pires, I. (2021). Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health. Environments, 8(10), 103. https://doi.org/10.3390/environments8100103







