Temporal and Spatial Study of Water Quality and Trophic Evaluation of a Large Tropical Reservoir
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Water Quality
2.3. Statistical Analysis
2.4. Trophic Status
3. Results and Discussion
3.1. Hydrological Conditions
3.2. Temporal and Spatial Variations of Water Quality in the ALM Reservoir
3.3. Spatial Classification of Parameters by Probabilistic Neural Network
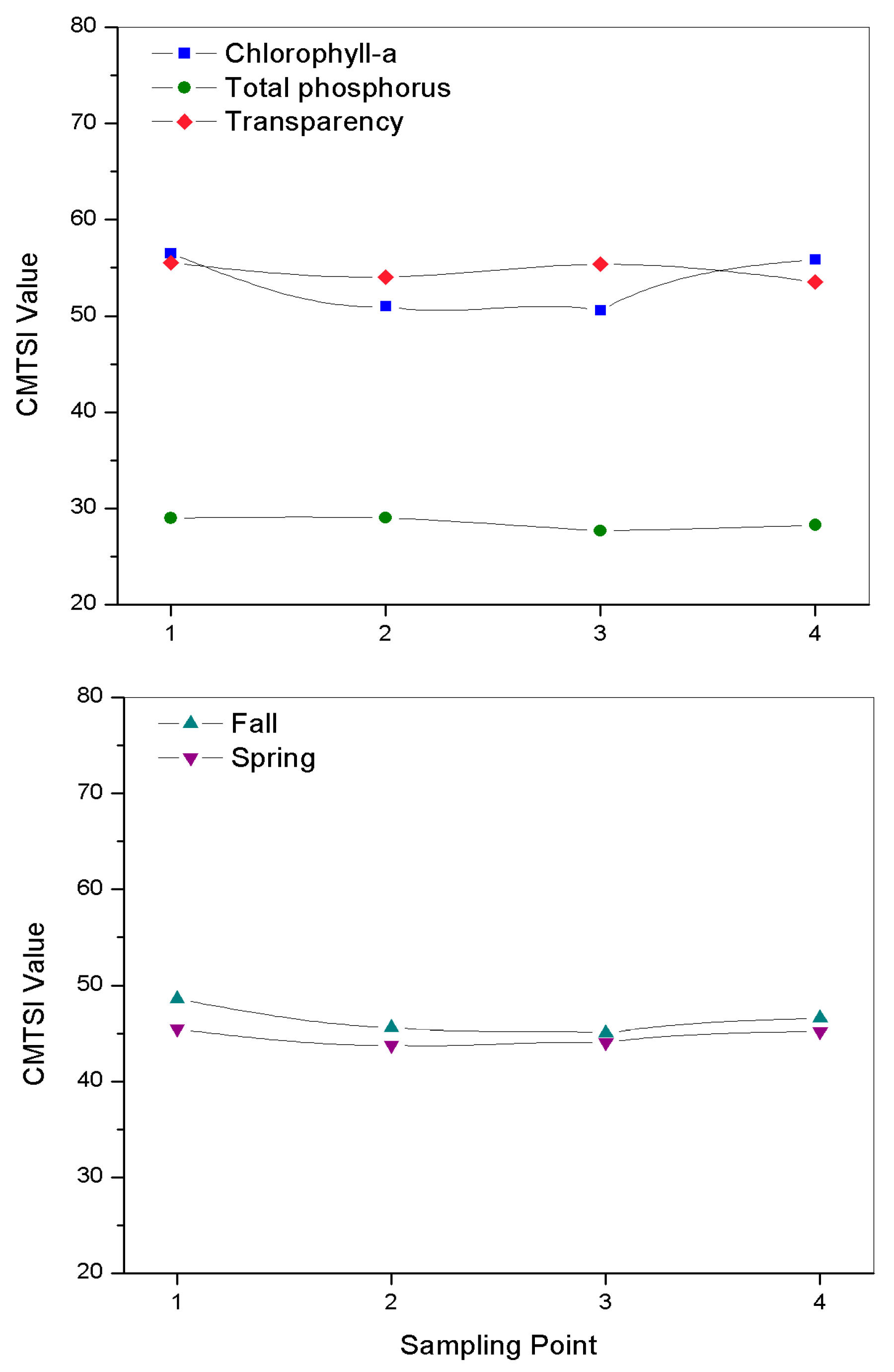
3.4. Trophic Status of the Reservoir
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mori, N.; Sugitani, K.; Yamamoto, M.; Tomioka, R.; Sato, M.; Harada, N. Major and minor elemental compositions of streambed biofilms and its implications of riverine biogeochemical cycles. Environ. Pollut. 2018, 243, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.M.; Rodić, M.N.; Vidović, M.U.; Trajković, I.S.; Jovanić, S.Z. Assessment of the Trophic Status by Monitoring of Reservoir’s Water Quality. J. Water Resour. Prot. 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Qiu, J.; Shen, Z.; Huang, M.; Zhang, X. Exploring effective best management practices in the miyun reservoir watershed, china. Ecol. Eng. 2018, 123, 30–42. [Google Scholar] [CrossRef]
- Klymiuk, V.; Barinova, S.; Fatiukha, A. Algal bio-indication in assessment of hydrological impact on ecosystem in wetlands of “slavyansky resort”. Transylv. Rev. Syst. Ecol. 2015, 17, 63–70. [Google Scholar] [CrossRef]
- Zhao, G.; Kondolf, G.M.; Mu, X.; Han, M.; He, Z.; Rubin, Z.; Sun, W. Sediment yield reduction associated with land use changes and check dams in a catchment of the loess plateau, china. Catena 2017, 148, 126–137. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, B.; Xu, Y.; Liu, D.; Ma, J.; Ji, D. Stable isotopes in water indicate sources of nutrients that drive algal blooms in the tributary bay of a subtropical reservoir. Sci. Total Environ. 2018, 634, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nayna, O.; Begum, M.; Chea, E.; Hartmann, J.; Keil, R.; Yu, R. Reviews and syntheses: Anthropogenic perturbations to carbon fluxes in asian river systems—Concepts, emerging trends, and research challenges. Biogeosciences 2018, 15, 3049–3069. [Google Scholar] [CrossRef]
- Nyairo, W.N.; Owuor, P.O.; Kengara, F.O. Effect of anthropogenic activities on the water quality of Amala and Nyangores tributaries of River Mara in Kenya. Environ. Monit. Assess. 2015, 187, 691. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, W.; Liu, W.; Qin, N.; Ouyang, H.; Wang, Q.; Kong, X.; He, Q.; Yang, C.; Yang, B.; et al. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol. Indic. 2014, 40, 58–67. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Ma, W.; Qin, C. Dynamic characteristics of nutrients and causal analysis in eutrofic reservoir: A case study of Shibianyu reservoir. Desalin. Water Treat. 2014, 52, 1624–1635. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, T.; Bi, L.; Tempero, G.; Hamilton, D.P.; Pan, G. Combating hypoxia/anoxia at sediment-water interfaces: A preliminary study of oxygen nanobubble modified clay materials. Sci. Total Environ. 2018, 637, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Semedo-Aguiar, A.; Pereira-Leal, J.; Leite, R. Microbial diversity and toxin risk in tropical freshwater reservoirs of cape verde. Toxins 2018, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Baker, K.; Zamor, R.; Nikolai, S.; Elshahed, M.; Youssef, N. Spatiotemporal analysis of microbial community dynamics during seasonal stratification events in a freshwater lake (grand lake, OK, USA). PLoS ONE 2017, 12, e0177488. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Quevedo, C.M.; Da Silva, P.W. Detergents as a source of phosphorus in sewage: The current situation in Brazil. Water Air Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]
- Gillett, N.; Pan, Y.; Asarian, J.; Kann, J. Spatial and temporal variability of river periphyton below a hypereutrophic lake and a series of dams. Sci. Total Environ. 2016, 541, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Zhou, Y.; Liu, M.; Shi, K.; Yu, Z. Seasonal-spatial distribution and long-term variation of transparency in xin’anjiang reservoir: Implications for reservoir management. Int. J. Environ. Res. Public. Health. 2015, 12, 9492–9507. [Google Scholar] [CrossRef] [PubMed]
- CONAGUA. Water Statistics in Mexico, 2015th ed.; Ministry of Environment and Natural Resources: Coyoacan, Mexico, 2015. Available online: http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/EAM2015_ing.pdf (accessed on 13 May 2018).
- Sanhouse-Garcia, A.; Bustos-Terrones, Y.; Rangel-Peraza, J.; Quevedo-Castro, A.; Pacheco, C. Multi-temporal analysis for land use and land cover changes in an agricultural region using open source tools. Remote Sens. Appl. Soc. Environ. 2017, 8, 278–290. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Rangayyan, R.M.; Wu, Y. Screening of knee-joint vibroarthrographic signals using probability density functions estimated with parzen windows. Biomed. Signal Process. Control 2010, 5, 53–58. [Google Scholar] [CrossRef]
- Lewandowski, V.; Bridi, V.; Bittencourt, F.; Signor, A.; Boscolo, W.; Feiden, A. Spatial and temporal limnological changes of an aquaculture area in a neotropical reservoir. Ann. Limnol. Int. J. Lim. 2018, 54, 27. [Google Scholar] [CrossRef]
- Maznah, W.; Makhlough, A. Water quality of tropical reservoir based on spatio-temporal variation in phytoplankton composition and physico-chemical analysis. Int. J. Environ. Sci. Technol. 2014, 12, 221–2232. [Google Scholar] [CrossRef]
- Molisani, M.; Barroso, H.; Becker, H.; Parente, M.; Grossi, C.; Maia do Monte, T.; Harrison, G. Trophic state, phytoplankton assemblages and limnological diagnosis of the Castanhao Reservoir, CE Brazil. Acta Limnol. Bras. 2010, 22, 1–12. [Google Scholar] [CrossRef]
- Vollenwieder, R.A. The scientific basis of lake and stream eutrophication with particular reference to phosphorus and nitrogen as eutrophication factors. OECD 1998, 27, 1–182. [Google Scholar]
- Kubiak, J.; Machula, S.; Stepanowska, K.; Biernaczyk, M. Evaluation of trophic level of the largest dimictic lakes of western Pomerania based on the Carlson criteria. Limnol. Rev. 2012, 12, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.; Tucker, C. Water Quality and Pond Soil Analyses for Aquaculture; Auburn University: Auburn, AL, USA, 1992. [Google Scholar]
- James, R.T.; Havens, K.; Zhu, G.; Qin, B. Comparative analysis of nutrients, chlorophyll and transparency in two large shallow lakes (Lake Taihu, P.R. china and Lake Okeechobee, USA). Hydrobiologia 2009, 627, 211–231. [Google Scholar] [CrossRef]
- Begliutti, B.; Buscarinu, P.; Marras, G.; Sechi, G.; Sulis, A. Reservoirs water-quality characterization for optimization modelling under drought conditions. Part 1—reservoirs trophic state characterization. Methods Tools Drought Anal. Manag. 2007, 62, 239–261. [Google Scholar]
- Carlson, R. A trophic state index for lakes. Limnol. Oceanogr. 1977, 2, 361–369. [Google Scholar] [CrossRef]
- Prasad, D.; Siddaraju, P. Carlson’s Trophic State Index for the assessment of trophic status of two Lakes in Mandya district. Adv. Appl. Sci. Res. 2012, 3, 2992–2996. [Google Scholar]
- Rangel-Peraza, J.; de Anda, J.; González-Farías, F.A.; Rode, M.; Sanhouse-García, A.; Bustos-Terrones, Y. Assessment of heavy metals in sediment of Aguamilpa dam, México. Environ. Monit. Assess. 2015, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pestana, I.; Bastos, W.; Almeida, M.; de Carvalho, D.; Rezende, C.; Souza, C. Spatial-temporal dynamics and sources of total hg in a hydroelectric reservoir in the western amazon, Brazil. Environ. Sci. Pollut. Res. 2016, 23, 9640–9648. [Google Scholar] [CrossRef]
- Chittoor, V.; Molson, J.; Schirmer, M. Does river restoration affect diurnal and seasonal changes to surface water quality? A study along the thur river, Switzerland. Sci. Total Environ. 2015, 532, 91–102. [Google Scholar] [CrossRef]
- Monteiro, D.; Nogueira, M.; Ayroza, M.; Marques, L.; Carvalho, E.; Ferraudo, S.; Camargo, A. Temporal and spatial variability of limnological characteristics in areas under the influence of tilapia cages in the chavantes reservoir, paranapanema river, Brazil. J. World Aquacult. Soc. 2013, 44, 814–825. [Google Scholar]
- Toor, G.; Han, L.; Stanley, C.D. Temporal variability in water quality parameters—a case study of drinking water reservoir in Florida, USA. Environ. Monit. Assess. 2013, 185, 4305–4320. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Nadeem, M. Spatial and temporal characterization of trace elements and nutrients in the rawal lake reservoir, Pakistan using multivariate analysis techniques. Environ. Geochem. Health 2011, 33, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Mwanamoki, P.; Devarajan, N.; Thevenon, F.; Atibu, E.; Tshibanda, J.; Ngelinkoto, P.; Mpiana, P.; Prabakar, K.; Mubedi, J.; Kabele, C.; et al. Assessment of pathogenic bacteria in water and sediment from a water reservoir under tropical conditions (Lake Ma Vallée), Kinshasa democratic republic of Congo. Environ. Monit. Assess. 2014, 186, 6821–6830. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xua, Z.; Wuc, W.; Zuoa, D. Effect of land use types on stream water quality under seasonal variation and topographic characteristics in the Wei River basin, China. Ecol. Indic. 2016, 60, 202–212. [Google Scholar] [CrossRef]
- Varol, M.; Gökot, B.; Bekleyen, A.; Şen, B. Spatial and temporal variations in surface water quality of the dam reservoirs in the tigris river basin, Turkey. Catena 2012, 92, 11–21. [Google Scholar] [CrossRef]
- Palma, P.; Ledo, L.; Soares, S.; Barbosa, I.; Alvarenga, P. Spatial and temporal variability of the water and sediments quality in the alqueva reservoir (guadiana basin; southern portugal). Sci. Total Environ. 2014, 470, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ciric, M.; Gavrilović, B.; Simić, G.S.; Krizmanić, J.; Vidović, M.; Zebić, G. Driving factors affecting spatial and temporal variations in the structure of phytoplankton functional groups in a temperate reservoir. Oceanol. Hydrobiol. Stud. 2015, 44, 431–444. [Google Scholar] [CrossRef]
- Li, W.; Gao, L.; Shi, Y.; Wang, Y.; Liu, J.; Cai, Y. Spatial distribution, temporal variation and risks of parabens and their chlorinated derivatives in urban surface water in Beijing, China. Sci. Total Environ. 2016, 539, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yao, R.; He, W.; Zhou, F.; Zhou, M.; Chen, C.; Lin, Y. Impacts of sanitation upgrading to the decrease of fecal coliforms entering into the environment in china. Environ. Res. 2016, 149, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.; Shelton, D. Escherichia coli and fecal coliforms in freshwater and estuarine sediments. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1067–1110. [Google Scholar] [CrossRef]
- Alweshah, M.; Abdullah, S. Hybridizing firefly algorithms with a probabilistic neural network for solving classification problems. Appl. Soft Comput. 2015, 35, 513–524. [Google Scholar] [CrossRef]
- Santhanam, H.; Raj, S. Carlson’s index, a poor cursor of trophic status assessment of pulicat lagoon, southeast coast of India. Int. J. Environ. Stud. 2010, 67, 17–25. [Google Scholar] [CrossRef]
- Phillips, G.; Pietiläinen, O.; Carvalho, L.; Solimini, A.; Solheim, A.L.; Cardoso, A. Chlorophyll—nutrient relationships of different lake types using a large European dataset. Aquat. Ecol. 2008, 42, 213–226. [Google Scholar] [CrossRef]
- Quevedo-Castro, A.; Lopez, J.; Rangel-Peraza, J.; Bandala, E.; Bustos-Terrones, Y. Study of the Water Quality of a Tropical Reservoir. Environments 2019, 6, 7. [Google Scholar] [CrossRef]
- Vollenweider, R.A. Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters, with Particular Reference to Nitrogen and Phosphorus as Factors in Eutrophication. Available online: http://garfield.library.upenn.edu/classics1987/A1987J648000001.pdf (accessed on 1 June 2018).
- Sakka, A.; Chikhaoui, M.; El Grami, B.; Hadj Mabrouk, H. Effects of N and P supply on phytoplankton in bizerte lagoon (western mediterranean). J. Exp. Mar. Biol. Ecol. 2006, 333, 79–96. [Google Scholar] [CrossRef]
- Perkins, R.G.; Underwood, G. Gradients of chlorophyll a and water chemistry along a eutrophic reservoir with determination of the limiting nutrient by in situ nutrient addition. Water Res. 2000, 34, 713–724. [Google Scholar] [CrossRef]


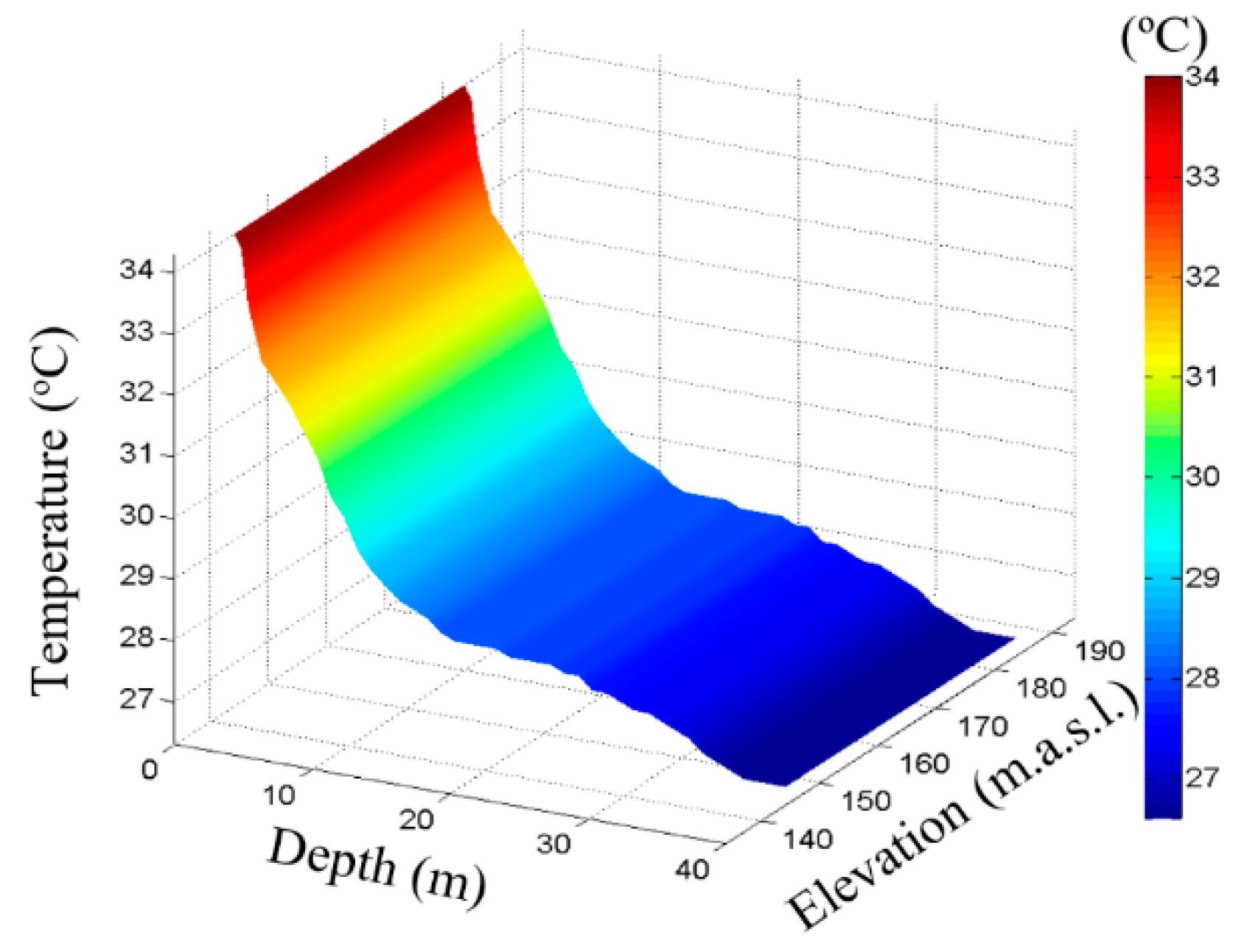
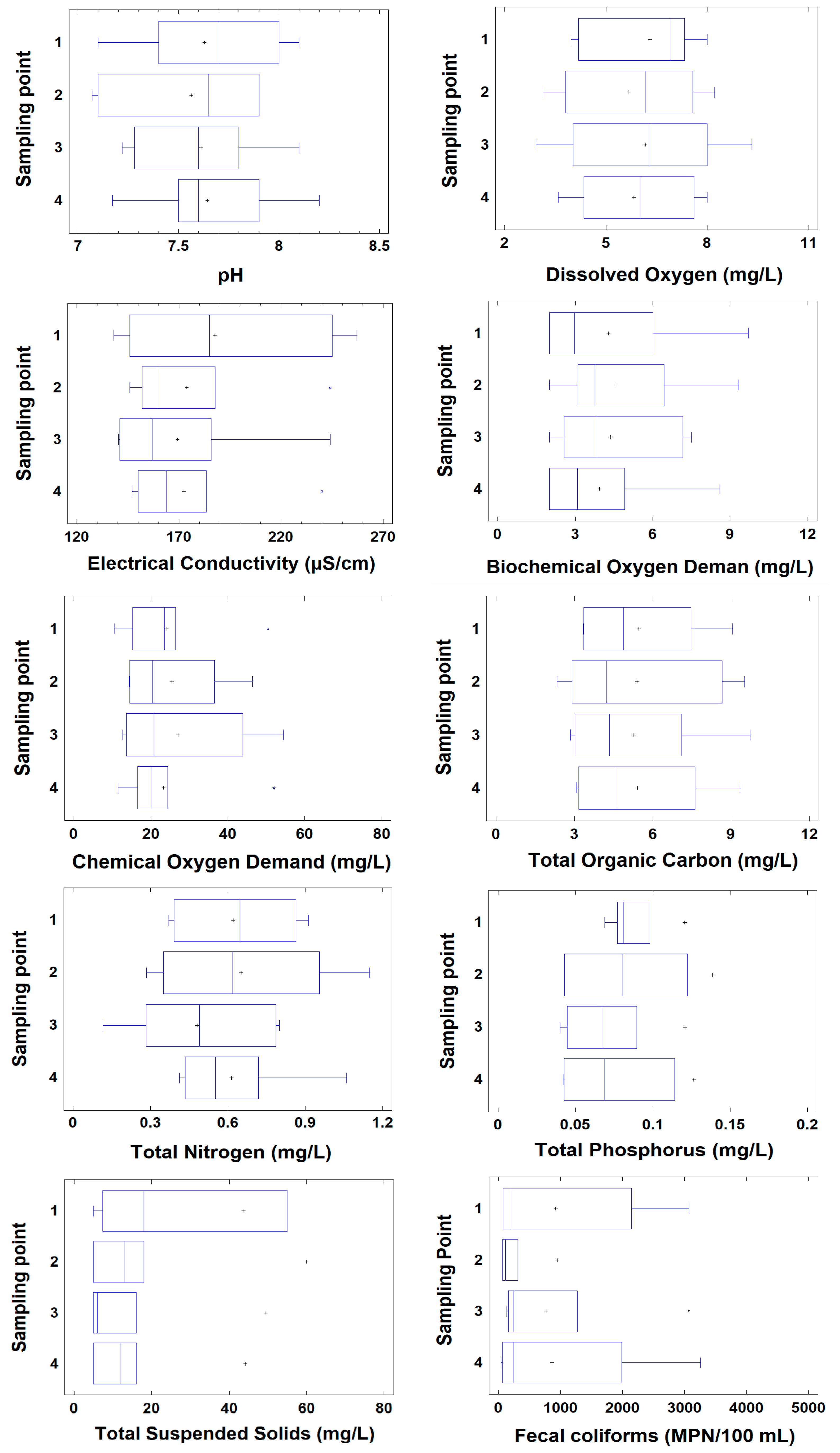
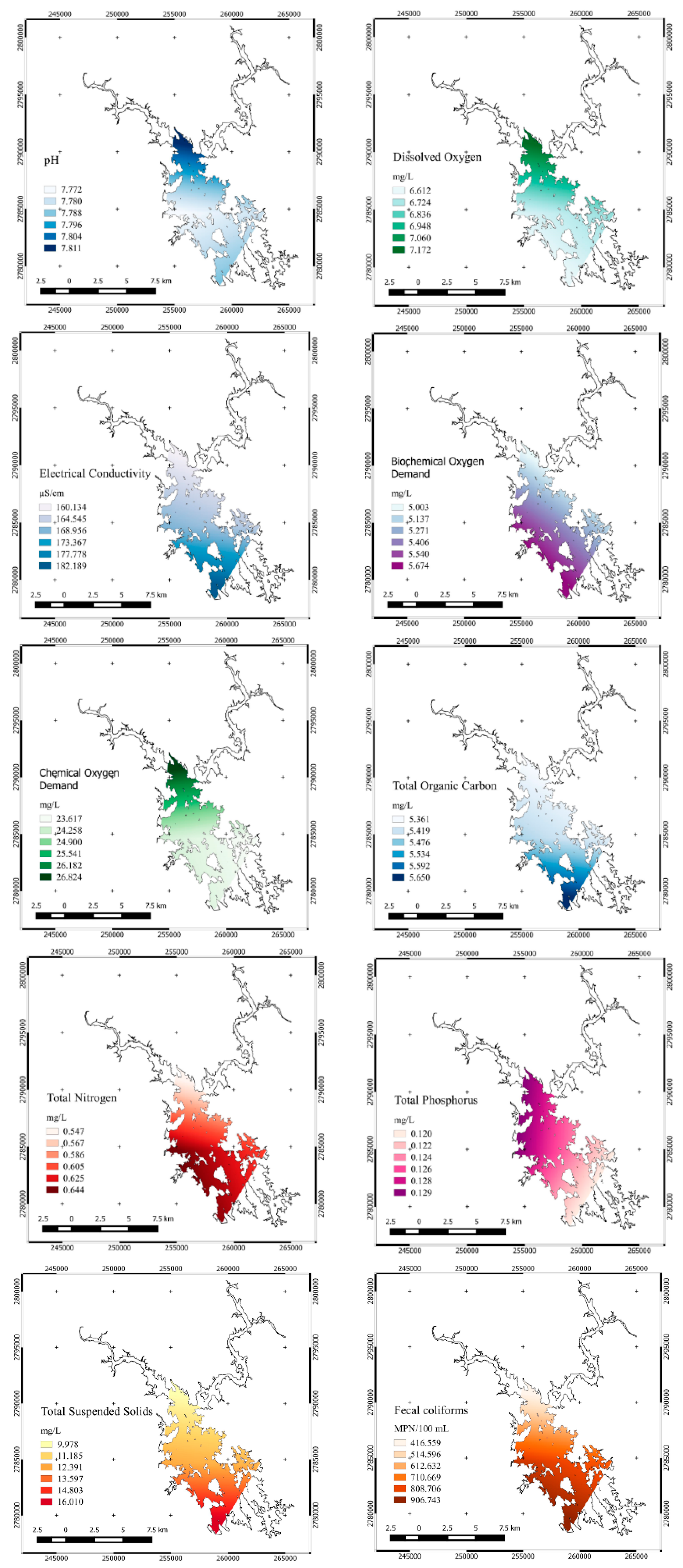
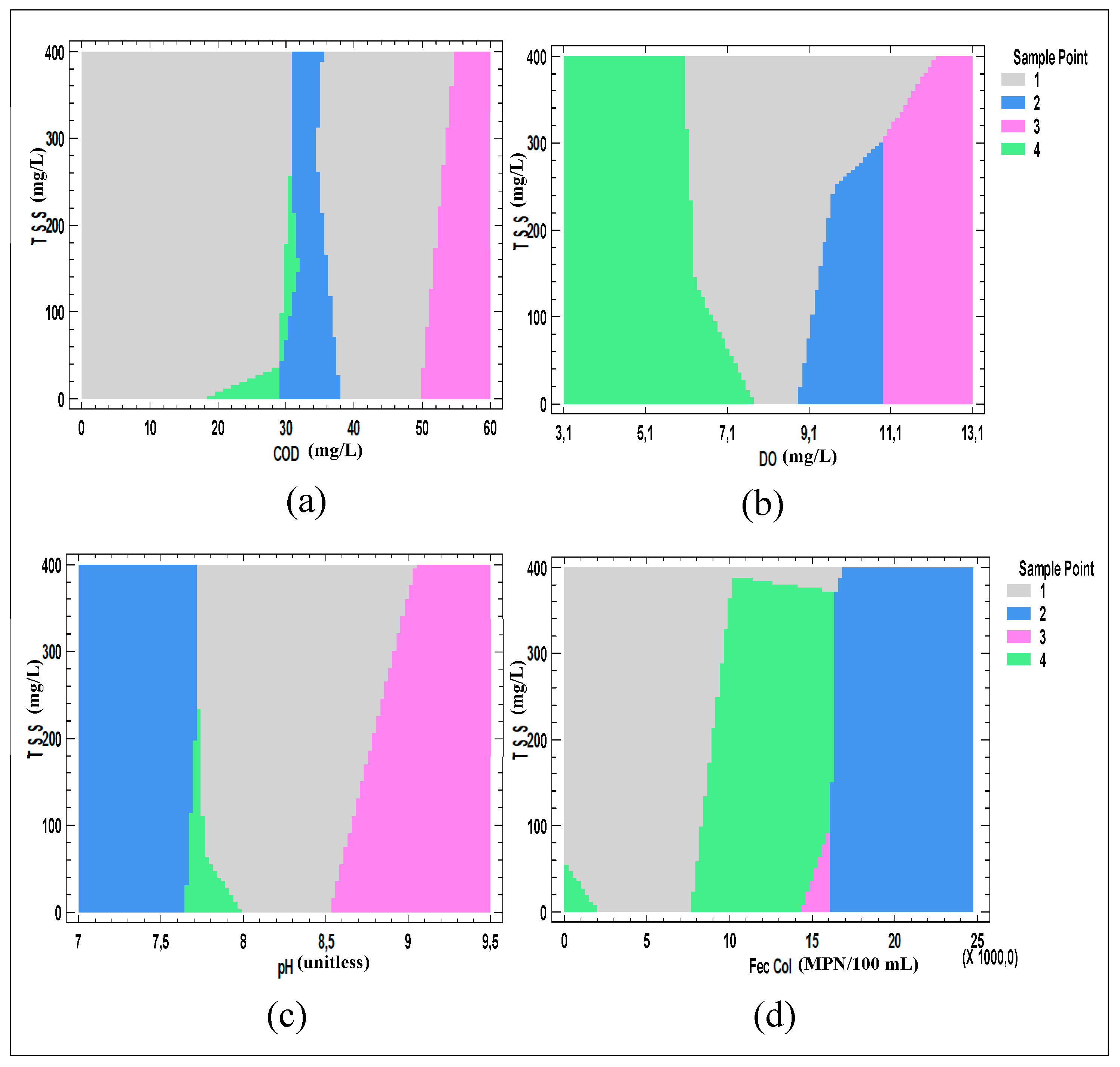

| Parameter | Units | n | Average | Standard Deviation | Variation Coefficient | Minimum | Maximum | ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Reason-F | Value-P | ||||||||
| Temperature | °C | 9360 | 19.858 | 4.897 | 24.6% | 10.6 | 28.5 | 0.78 | 0.76 |
| Precipitation | mm | 9360 | 2.477 | 3.615 | 145.9% | 0 | 18 | 0.28 | 0.99 |
| Evaporation | mm | 9360 | 5.588 | 2.084 | 37.3% | 2.1 | 10.6 | 0.54 | 0.96 |
| Parameter | Units | n | Average | SD | Variation Coefficient | Minimum | Maximum | ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Reason-F | Value-P | ||||||||
| pH | unitless | 20 | 7.704 | 0.443 | 5.757% | 7.07 | 8.4 | 0.08 | 0.9704 |
| DO | mg/L | 20 | 7.33 | 2.210 | 30.139% | 3.81 | 10.4 | 0.07 | 0.9759 |
| Turbidity | NTU | 20 | 3.4675 | 5.1537 | 148.63% | 0.88 | 22.0 | 0.80 | 0.5150 |
| EC | µS/cm | 20 | 160.46 | 29.707 | 18.502% | 127.0 | 257.0 | 1.70 | 0.2200 |
| TSS | mg/L | 20 | 9.612 | 5.447 | 56.671% | 5.0 | 20.00 | 0.73 | 0.5528 |
| BOD | mg/L | 20 | 5.442 | 2.849 | 52.356% | 2.0 | 9.7 | 0.06 | 0.9770 |
| COD | mg/L | 20 | 28.325 | 14.746 | 52.061% | 14.4 | 54.4 | 0.05 | 0.9855 |
| TN | mg/L | 20 | 0.4466 | 0.1647 | 36.876% | 0.1154 | 0.7576 | 0.85 | 0.4913 |
| TP | mg/L | 20 | 0.0782 | 0.0376 | 48.140% | 0.04 | 0.1618 | 0.77 | 0.5323 |
| FC | MPN/100 mL | 20 | 765.25 | 1210.02 | 158.12% | 1.0 | 3255.0 | 0.01 | 0.9991 |
| Parameter | Units | n | Average | SD | Variation Coefficient | Minimum | Maximum | ANOVA | |
|---|---|---|---|---|---|---|---|---|---|
| Reason-F | Value-P | ||||||||
| pH | unitless | 20 | 7.821 | 0.377 | 4.82% | 7.28 | 8.7 | 0.11 | 0.9553 |
| DO | mg/L | 20 | 6.373 | 2.216 | 34.77% | 2.93 | 9.6 | 0.22 | 0.8839 |
| Turbidity | NTU | 20 | 8.825 | 17.829 | 202.032% | 1.0 | 74.0 | 0.46 | 0.7118 |
| EC | µS/cm | 20 | 171.41 | 42.530 | 24.811% | 110.0 | 245.0 | 0.02 | 0.9948 |
| TSS | mg/L | 20 | 10.2835 | 5.708 | 55.5152% | 5.0 | 20.67 | 0.54 | 0.6633 |
| BOD | mg/L | 20 | 4.3625 | 1.984 | 45.484% | 2.0 | 7.16 | 0.19 | 0.9002 |
| COD | mg/L | 20 | 22.091 | 8.959 | 40.555% | 10.66 | 43.85 | 0.41 | 0.7511 |
| TN | mg/L | 20 | 0.7441 | 0.2398 | 32.223% | 0.2835 | 1.1491 | 0.13 | 0.9387 |
| TP | mg/L | 20 | 0.1554 | 0.1598 | 102.815% | 0.0541 | 0.5232 | 0.05 | 0.9845 |
| FC | MPN/100mL | 20 | 457.56 | 692.81 | 151.41% | 20.0 | 2143.0 | 0.41 | 0.7488 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quevedo-Castro, A.; Bandala, E.R.; Rangel-Peraza, J.G.; Amábilis-Sosa, L.E.; Sanhouse-García, A.; Bustos-Terrones, Y.A. Temporal and Spatial Study of Water Quality and Trophic Evaluation of a Large Tropical Reservoir. Environments 2019, 6, 61. https://doi.org/10.3390/environments6060061
Quevedo-Castro A, Bandala ER, Rangel-Peraza JG, Amábilis-Sosa LE, Sanhouse-García A, Bustos-Terrones YA. Temporal and Spatial Study of Water Quality and Trophic Evaluation of a Large Tropical Reservoir. Environments. 2019; 6(6):61. https://doi.org/10.3390/environments6060061
Chicago/Turabian StyleQuevedo-Castro, Alberto, Erick R. Bandala, Jesús G. Rangel-Peraza, Leonel E. Amábilis-Sosa, Antonio Sanhouse-García, and Yaneth A. Bustos-Terrones. 2019. "Temporal and Spatial Study of Water Quality and Trophic Evaluation of a Large Tropical Reservoir" Environments 6, no. 6: 61. https://doi.org/10.3390/environments6060061







