Ammonia, Hydrogen Sulfide, and Greenhouse Gas Emissions from Lab-Scaled Manure Bedpacks with and without Aluminum Sulfate Additions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Temperature and pH
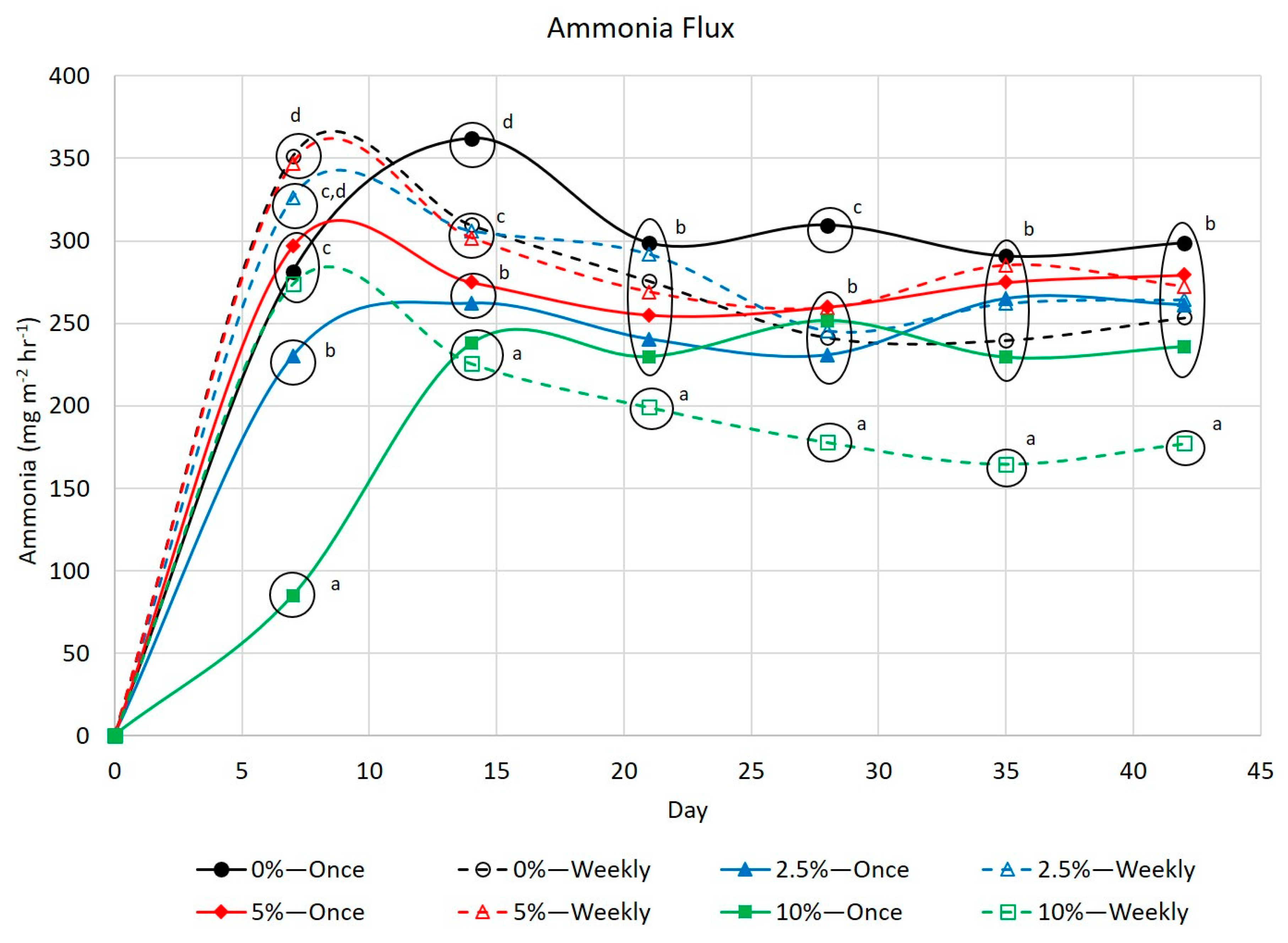
3.2. Ammonia
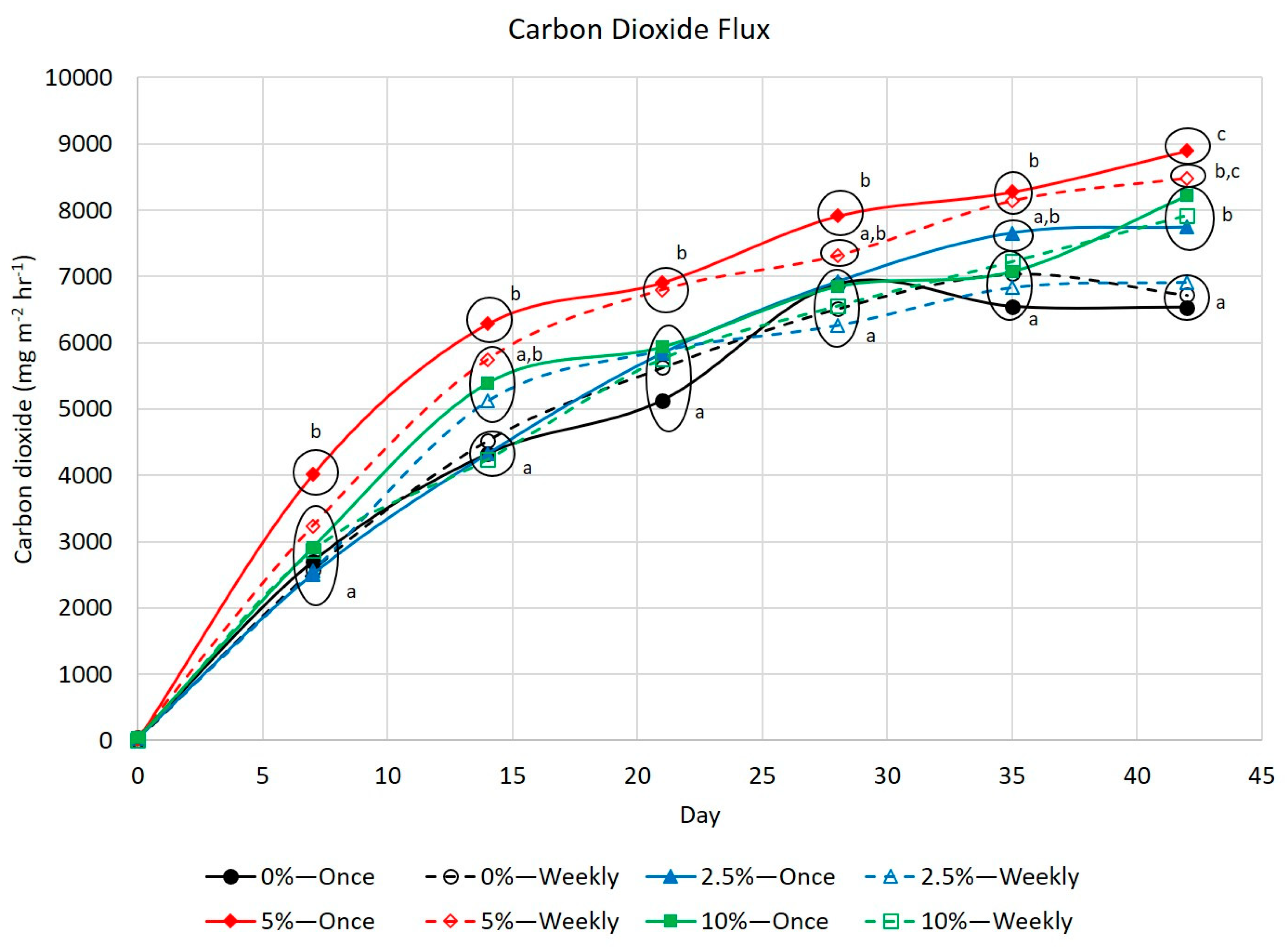
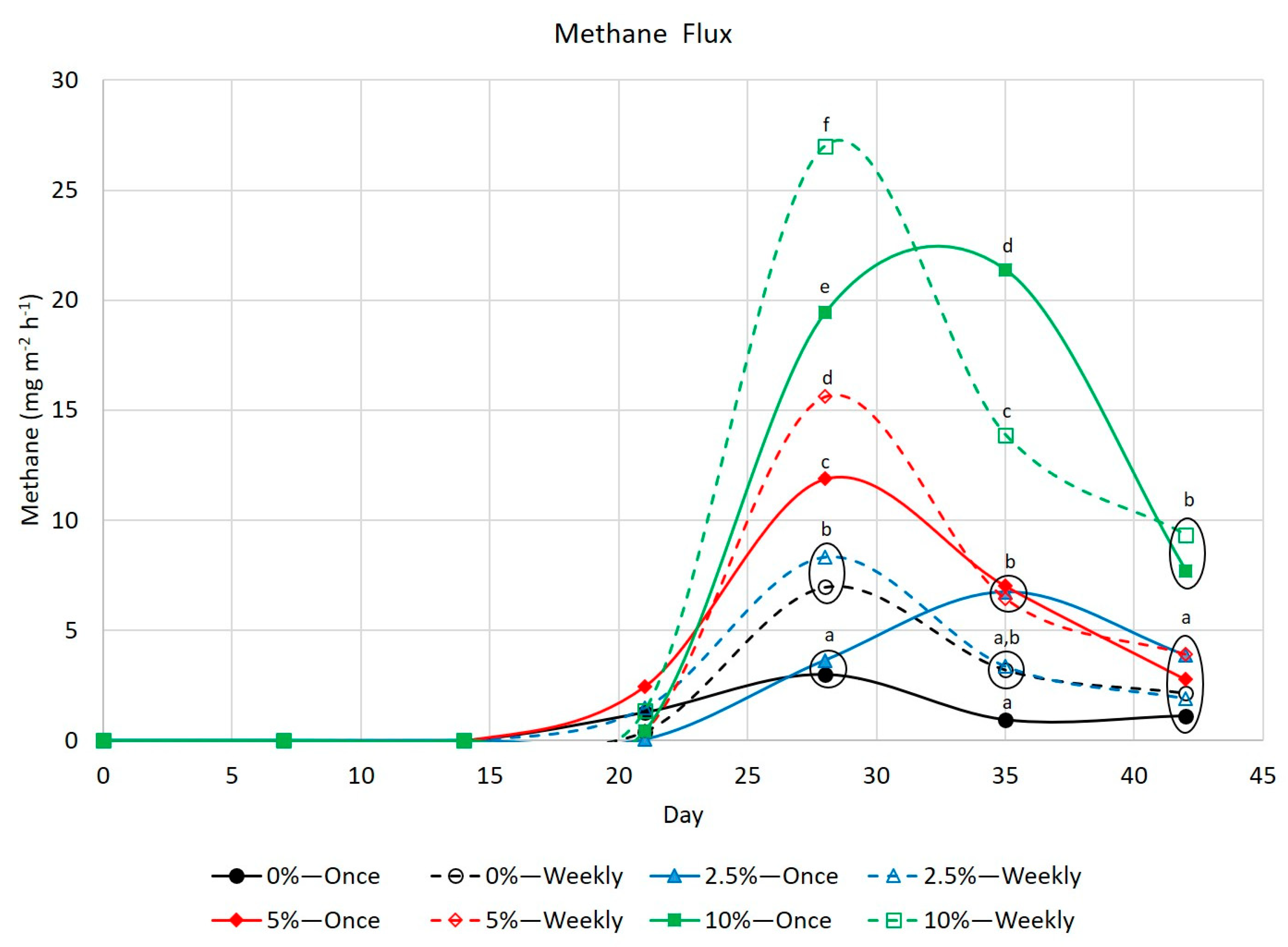
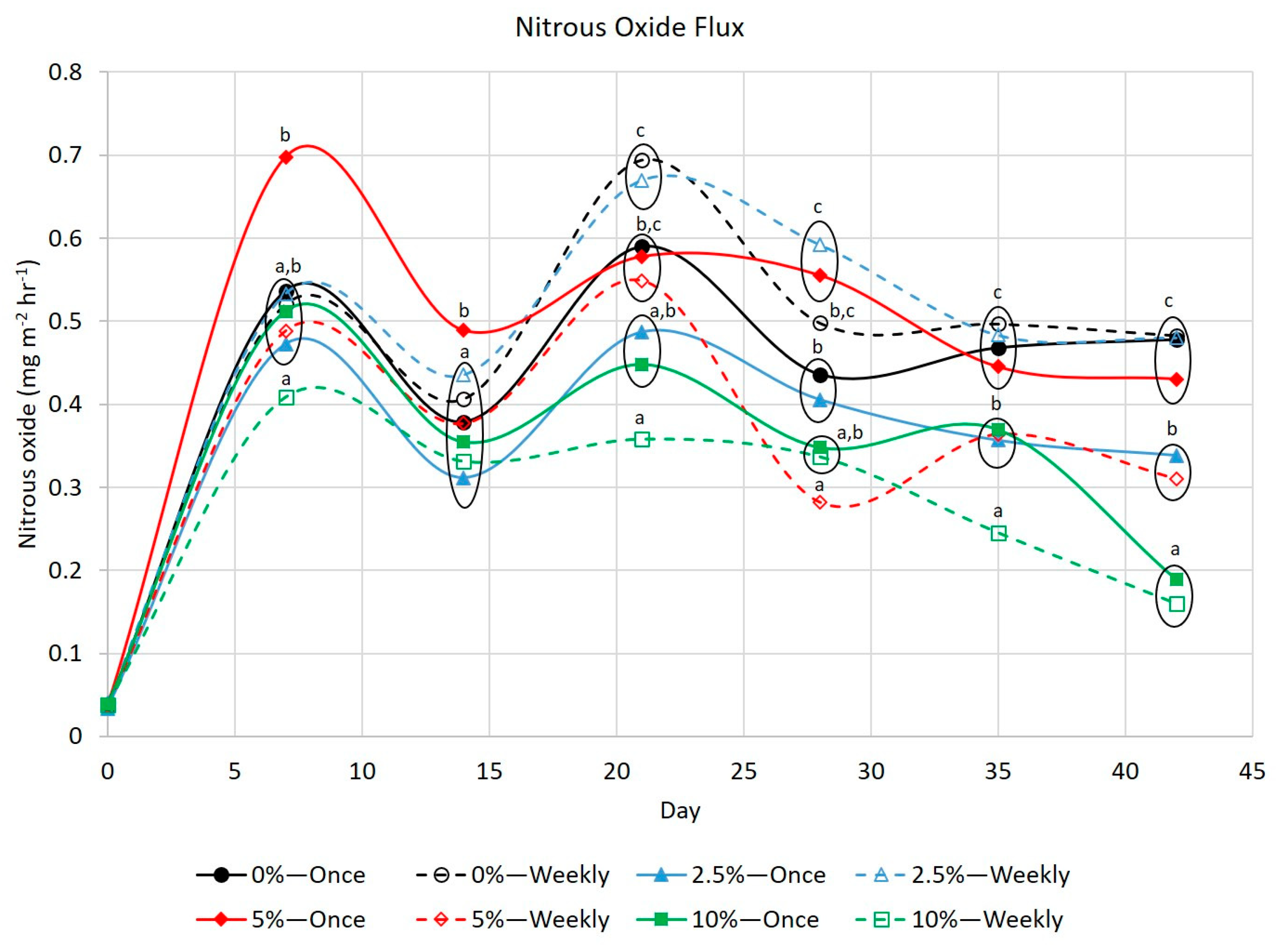
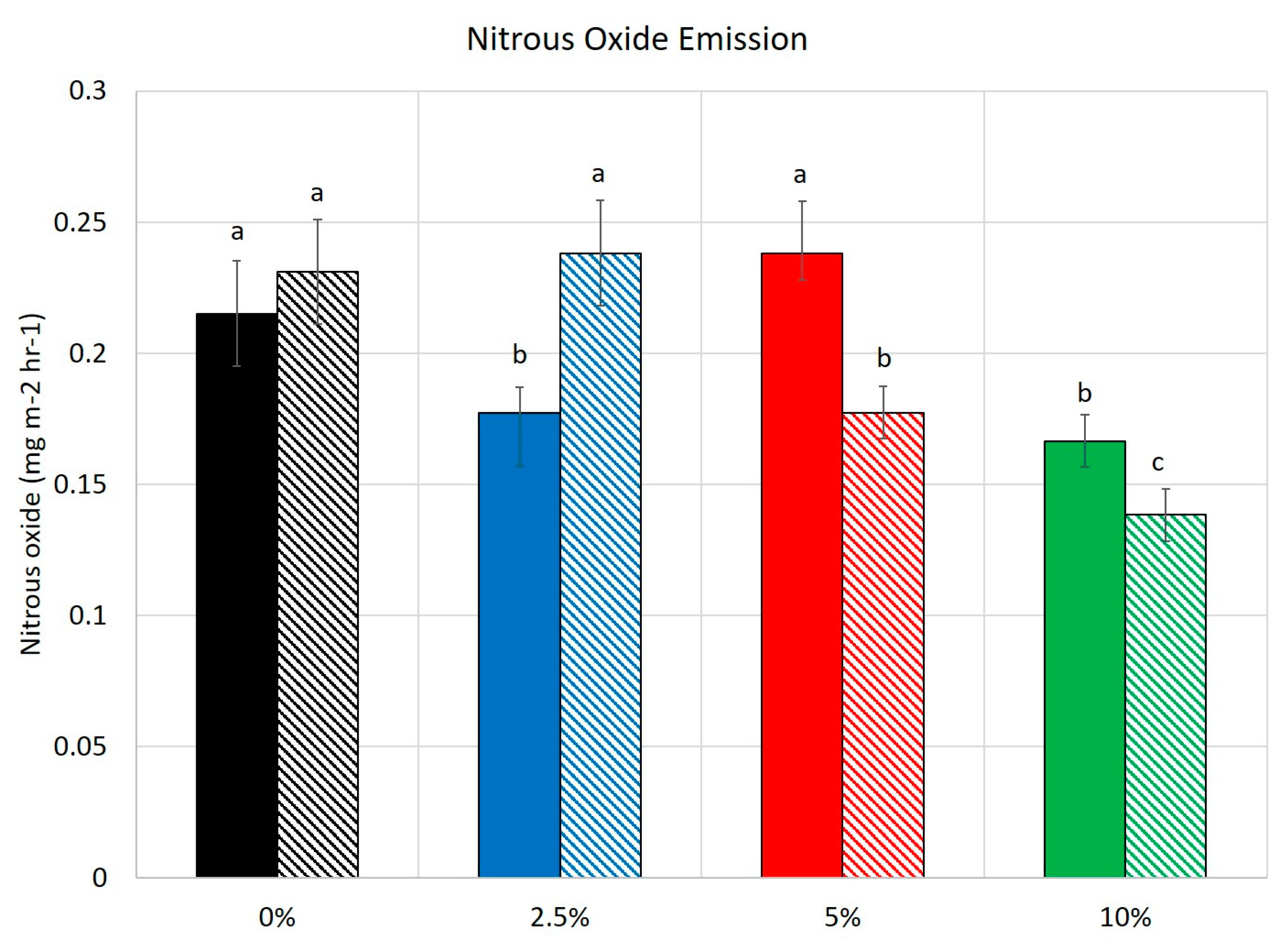
3.3. Greenhouse Gases
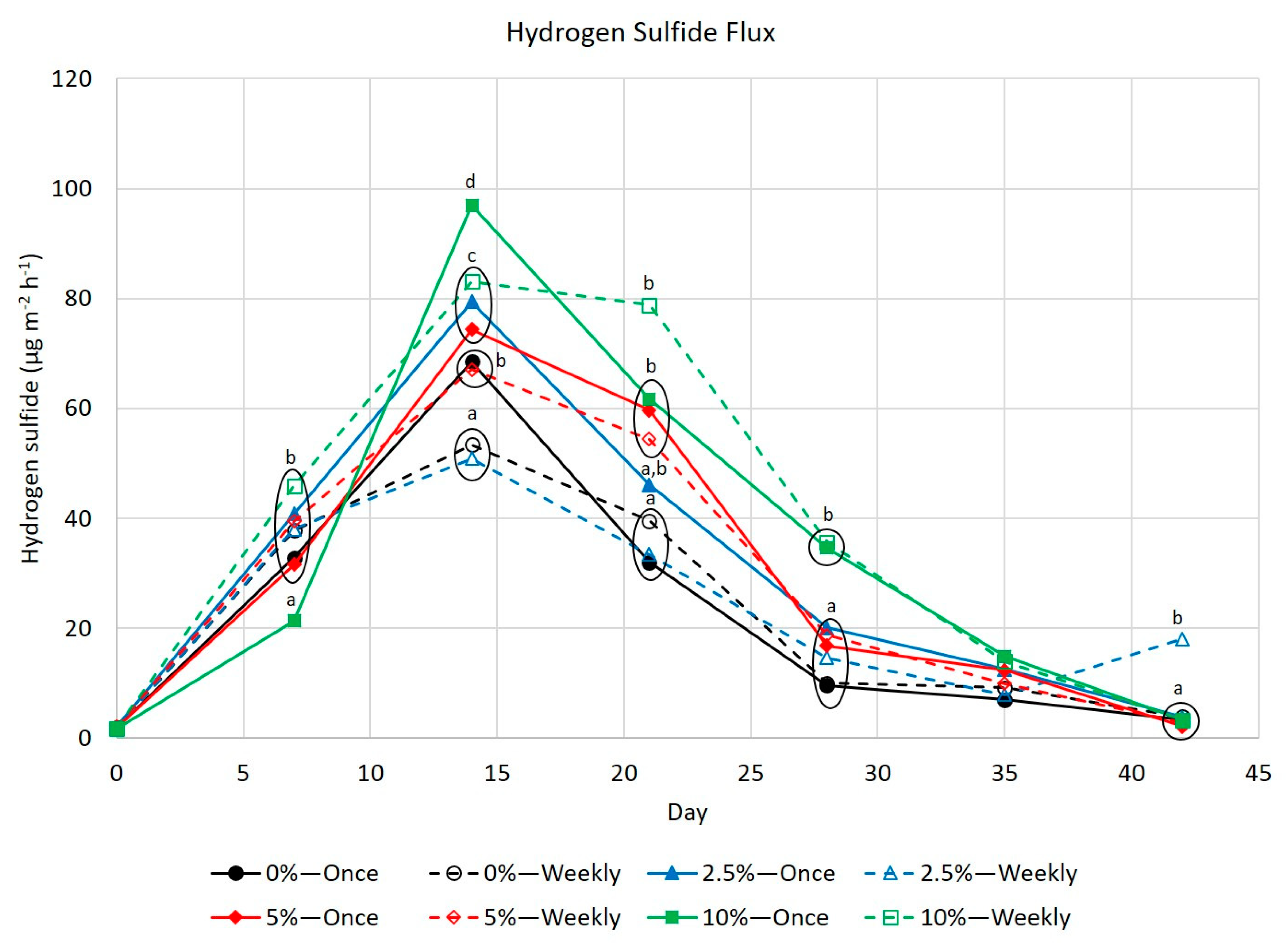
3.4. Hydrogen Sulfide
3.5. Nutrient Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stowell, R. Ammonia loss and emission reporting: Considerations for cattle operations. UNL BeefWatch. 1 February 2018. Available online: http://newsroom.unl.edu/announce/beef/7498/42942 (accessed on 13 June 2018).
- Todd, R.W.; Cole, N.A.; Clark, R.N.; Flesch, T.K. Ammonia emissions from a beef cattle feedyard on the Southern High Plains. Atmos. Environ. 2008, 42, 6797–6805. [Google Scholar] [CrossRef]
- Bierman, S.; Erickson, G.E.; Klopfenstein, T.J.; Stock, R.A.; Shain, D.H. Evaluation of nitrogen and organic matter balance in the feedlot as affected by level and source of dietary fiber. J. Anim. Sci. 1999, 77, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.E.; Milton, C.T.; Klopfenstein, T.J. Dietary protein effects on nitrogen excretion and volatilization in open-dirt feedlots. In Proceedings, Eighth International Symposium on Animal, Agricultural, and Food Processing Waste (ISAAF); American Society of Agricultural Engineers: St. Joseph, MI, USA, 2000. [Google Scholar]
- Koelsch, R.; Erickson, G.; Homolka, M.; Luebbe, M. Predicting Manure Nitrogen, Phosphorus, and Carbon Characteristics of Open Lot Systems; ASABE Paper No. 1800946; ASABE: St. Joseph, MI, USA, 2018. [Google Scholar]
- Bierman, S.; Klopfenstein, T.J.; Stock, R.; Shain, D. Evaluation of Nitrogen, Phosphorus, and Organic Matter Balance in the Feedlot as Affected by Nutrition; Beef Cattle Report MP66-A; University of Nebraska: Lincoln, NE, USA, 1996; pp. 74–76. [Google Scholar]
- Van Horn, H.H.; Newton, G.L.; Kunkle, W.E. Ruminant nutrition from an environmental perspective: Factors affecting whole-farm nutrient balance. J. Anim. Sci. 1996, 74, 3082–3102. [Google Scholar] [CrossRef] [PubMed]
- Hausinger, R.P. Metabolic versatility of prokaryotes for urea deposition. J. Bacteriol. 2004, 186, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.; Phillips, V.R. Control of gaseous emissions from livestock buildings and manure stores. J. Agric. Eng. Res. 1994, 57, 173–189. [Google Scholar] [CrossRef]
- Rhoades, M.B.; Parker, D.B.; Cole, N.A.; Todd, R.W.; Caraway, E.A.; Auvermann, B.W.; Topliff, D.R.; Schuster, G.L. Continuous ammonia emission measurements from a commercial beef feedyard in Texas. Trans. ASABE 2010, 53, 1823–1831. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniels, T.C.; Edwards, D.R. Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminum sulfate. J. Environ. Qual. 2000, 29, 37–49. [Google Scholar] [CrossRef]
- Moore, P.A.; Edwards, D.R. Long-term effects of poultry litter, alum-treated litter, and ammonium nitrate on phosphorus availability in soils. J. Environ. Qual. 2007, 36, 163–174. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniels, T.C.; Edwards, D.R. Reducing phosphorus and improving poultry production with alum. Poult. Sci. 1999, 78, 692–698. [Google Scholar] [CrossRef]
- Sims, J.T.; Luka-McCafferty, N.J. On-farm evaluation of aluminum sulfate (alum) as a poultry litter amendment: Effects on litter properties. J. Environ. Qual. 2002, 31, 2066–2073. [Google Scholar] [CrossRef]
- Penn, C.; Zhang, H. Alum-Treated Poultry Litter as a Fertilizer Source. Oklahoma Cooperative Extension Service PSS-2254. 2017. Available online: http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-5180/PSS-2254web13.pdf (accessed on 11 September 2019).
- Spiehs, M.J.; Brown-Brandl, T.M.; Parker, D.B.; Miller, D.N.; Berry, E.D.; Wells, J.E. Effect of bedding materials on concentration of odorous compounds and Escherichia coli in beef cattle bedded manure packs. J. Environ. Qual. 2013, 42, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Spiehs, M.J.; Brown-Brandl, T.M.; Parker, D.B.; Miller, D.N.; Jaderborg, J.P.; DiCostanzo, A.; Berry, E.D.; Wells, J.E. Use of wood-based materials in beef bedded manure packs: 1. Effect on ammonia, total reduced sulfide, and greenhouse gas concentrations. J. Environ. Qual. 2014, 43, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Spiehs, M.J.; Brown-Brandl, T.M.; Berry, E.D.; Wells, J.E.; Parker, D.B.; Miller, D.N.; Jaderborg, J.P.; DiCostanzo, A. Use of wood-based materials in beef bedded manure packs: 2. Effect on odorous volatile organic compounds, odor activity value, Escherichia coli, and nutrient concentrations. J. Environ. Qual. 2014, 43, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Spiehs, M.J.; Brown-Brandl, T.M.; Parker, D.B.; Miller, D.N.; Berry, E.D.; Wells, J.E. Ammonia, total reduced sulfides, and greenhouse gases of pine chip and corn stover bedding packs. J. Environ. Qual. 2016, 45, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Spiehs, M.J.; Berry, E.D.; Wells, J.; Parker, D.B.; Brown-Brandl, T.M. Odorous volatile organic compounds, Escherichia coli, and nutrient composition when kiln-dried pine chips and corn stover bedding are used in beef bedded manure packs. J. Environ. Qual. 2017, 46, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Spiehs, M.J. Lab-scale model to evaluate odor and gas concentrations emitted by deep bedded pack manure. J. Vis. Exp. 2018, 137, e57332. [Google Scholar] [CrossRef]
- Brown-Brandl, T.M.; Nienaber, J.A.; Eigenberg, R.A. Temperature and humidity control in indirect calorimeter chambers. Trans. ASABE 2011, 54, 685–692. [Google Scholar] [CrossRef]
- Spiehs, M.J.; Woodbury, B.L.; Doran, B.E.; Eigenberg, R.A.; Kohl, K.D.; Varel, V.H.; Berry, E.D.; Wells, J. Environmental conditions in beef deep-bedded mono-slope facilities: A descriptive study. Trans. ASABE 2011, 54, 663–673. [Google Scholar] [CrossRef][Green Version]
- Miller, D.N.; Woodbury, B.L. A solid-phase microextraction chamber method for analysis of manure volatiles. J. Environ. Qual. 2006, 35, 2383–2394. [Google Scholar] [CrossRef]
- Woodbury, B.L.; Miller, D.N.; Eigenberg, R.A.; Nienaber, J.A. An inexpensive laboratory and field chamber for manure volatile gas flux analysis. Trans. ASABE 2006, 49, 767–772. [Google Scholar] [CrossRef]
- Watson, M.; Wolf, A.; Wolf, N. Total nitrogen. In Recommended Methods of Manure Analysis; Publication No. A3769; University of Wisconsin Cooperative Extension: Madison, WI, USA, 2003; pp. 18–24. [Google Scholar]
- Wolf, A.; Watson, M.; Wolf, N. Digestion and dissolution methods for P, K, Ca, Mg, and trace elements. In Recommended Methods of Manure Analysis; Publication No. A3769; University of Wisconsin Cooperative Extension: Madison, WI, USA, 2003; pp. 30–38. [Google Scholar]
- Ayadi, F.Y.; Cortus, E.L.; Spiehs, M.J.; Miller, D.N.; Dijira, G.D. Ammonia and greenhouse gas concentration at surfaces of simulated beef cattle bedded manure packs. Trans. ASABE 2015, 58, 783–795. [Google Scholar]
- Miller, D.N.; Varel, V.H. In vitro study of the biochemical origin and production limits of odorous compounds in cattle feedlots. J. Anim. Sci. 2001, 79, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.N.; Varel, V.H. An in vitro study of manure composition on the biochemical origins, composition, and accumulation of odorous compounds in cattle feedlots. J. Anim. Sci. 2002, 80, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.R.; Blanes-Vidal, V. Emissions of ammonia, carbon dioxide, and hydrogen sulfide from swine wastewater during and after acidification treatment: Effect of pH, mixing, and aeration. J. Environ. Manag. 2013, 115, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hellman, B.; Zelles, L.; Palojarvi, A.; Bai, Q. Emission of climate-relevant trace gases and succession of microbial communities during open-windrow composting. Appl. Environ. Microbiol. 1997, 63, 1011–1018. [Google Scholar]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA anaertobic digestion model no. 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J. Industrial applications of the IWA anaerobic digestions model no. 1 (ADM1). Water Sci. Technol. 2003, 47, 199–206. [Google Scholar] [CrossRef]
- Arikan, O. Effect of temperature on methane production from field-scale anaerobic digesters treating dairy manure. In Waste to Worth: Spreading Science and Solutions; Livestock Poultry Environmental Learning Center: Seattle, WA, USA, 2015. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes; A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Tauseef, S.M.; Premalatha, M.; Abbasi, T.; Abbasi, S.A. Methane capture from livestock manure. J. Environ. Manag. 2013, 117, 187–207. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks 1990–2015. 2017. Available online: https://www.c2es.org/content/internation-emissions (accessed on 18 June 2018).
- Reis, M.A.M.; Almeida, J.S.; Lemos, P.C.; Carrondo, M.J.T. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef] [PubMed]
| pH | ||||||||
| Dose Frequency | Alum | Day | ||||||
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Once | 0% | 7.06 | 8.49 a | 8.18 a | 8.03 a | 7.97 a | 7.81 a | 7.79 b |
| Weekly | 0% | 7.04 | 8.51 a | 8.27 a | 8.10 a | 7.76 a,b | 7.91 a | 7.82 b |
| Once | 2.5% | 7.05 | 8.43 a,b | 8.16 a,b | 8.14 a | 7.84 a,b | 7.98 a | 7.96 a |
| Weekly | 2.5% | 6.95 | 8.44 a | 8.19 a | 8.04 a | 7.77 a,b | 7.85 a | 7.66 b,c |
| Once | 5% | 7.00 | 8.03 c | 8.14 a | 8.00 a | 7.75 a,b | 7.85 a | 7.77 b |
| Weekly | 5% | 7.20 | 8.29 a,b | 8.08 a,b | 8.03 a | 7.75 a,b | 7.83 a | 7.83 b |
| Once | 10% | 7.00 | 6.92 d | 7.93 b | 7.97 a,b | 7.68 b | 7.84 a | 7.70 b,c |
| Weekly | 10% | 7.17 | 8.21 a,b | 8.17 a,b | 7.62 b | 7.64 b | 7.65 b | 7.60 c |
| Temperature | ||||||||
| Dose Frequency | Alum | Day | ||||||
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | ||
| Once | 0% | 20.0 | 19.1 | 20.2 | 21.2 | 21.7 | 21.1 | 21.6 |
| Weekly | 0% | 20.0 | 19.1 | 20.0 | 21.4 | 21.3 | 21.1 | 21.6 |
| Once | 2.5% | 20.0 | 19.1 | 20.0 | 21.3 | 21.4 | 20.9 | 21.6 |
| Weekly | 2.5% | 20.0 | 19.2 | 20.1 | 21.1 | 21.4 | 20.9 | 21.6 |
| Once | 5% | 20.0 | 19.1 | 19.9 | 21.0 | 21.1 | 20.9 | 21.5 |
| Weekly | 5% | 20.0 | 19.2 | 19.7 | 21.1 | 21.1 | 20.7 | 21.5 |
| Once | 10% | 20.0 | 19.3 | 20.0 | 21.0 | 21.1 | 20.8 | 21.7 |
| Weekly | 10% | 20.0 | 19.4 | 20.4 | 21.2 | 21.4 | 21.1 | 21.7 |
| Alum | Phosphorus | Potassium | Sulfur | Nitrogen |
|---|---|---|---|---|
| g kg−1 (DM) | ||||
| 0% | 0.90 | 3.40 | 5.09 | 26.76 |
| 2.5% | 0.91 | 3.63 | 6.56 | 25.84 |
| 5% | 0.93 | 3.55 | 8.01 | 26.28 |
| 10% | 0.91 | 3.47 | 10.73 | 26.31 |
| p-values | ||||
| Alum | 0.9197 | 0.436 | <0.01 | 0.5036 |
| Frequency | 0.4274 | 0.5431 | 0.5338 | 0.8204 |
| Alum × Frequency | 0.4269 | 0.5966 | 0.6204 | 0.6662 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiehs, M.J.; Woodbury, B.L.; Parker, D.B. Ammonia, Hydrogen Sulfide, and Greenhouse Gas Emissions from Lab-Scaled Manure Bedpacks with and without Aluminum Sulfate Additions. Environments 2019, 6, 108. https://doi.org/10.3390/environments6100108
Spiehs MJ, Woodbury BL, Parker DB. Ammonia, Hydrogen Sulfide, and Greenhouse Gas Emissions from Lab-Scaled Manure Bedpacks with and without Aluminum Sulfate Additions. Environments. 2019; 6(10):108. https://doi.org/10.3390/environments6100108
Chicago/Turabian StyleSpiehs, Mindy J., Bryan L. Woodbury, and David B. Parker. 2019. "Ammonia, Hydrogen Sulfide, and Greenhouse Gas Emissions from Lab-Scaled Manure Bedpacks with and without Aluminum Sulfate Additions" Environments 6, no. 10: 108. https://doi.org/10.3390/environments6100108
APA StyleSpiehs, M. J., Woodbury, B. L., & Parker, D. B. (2019). Ammonia, Hydrogen Sulfide, and Greenhouse Gas Emissions from Lab-Scaled Manure Bedpacks with and without Aluminum Sulfate Additions. Environments, 6(10), 108. https://doi.org/10.3390/environments6100108




