Abstract
This paper presents the results of a study of the photocatalytic activity of iron-containing composites based on natural peat and zeolite under external influences: Ultraviolet irradiation (UVI) and UVI + H2O2. It is shown that the optimal method for the photocatalytic destruction of pollutants (oxalic acid and phenol) with composites is to introduce hydrogen peroxide in the system. The composites studied are sources of iron ions in the Ruff-Fenton system; they provide the generation of •OH radicals, which have a high reactivity in the oxidative degradation reactions of organic substances and can be recommended for reuse to purify water drains.
1. Introduction
In view of the growing anthropogenic influence on the ecosystem, close attention has recently been paid to the problems of monitoring both pollutants of natural objects and their regeneration. The pollution of hydrologic systems with wastewaters (WW) represents a special hazard since the processes of self-purification are slower than they are in air, and the sources of pollution of the aqueous medium are more diverse than they are for other natural objects. Besides, getting into underground waters, WW can pollute drinking water reserves of underground waters and, thus, cause harm to the health of the general population.
The pollution of wastewater by different soluble organic compounds—oxalic acid, phenol, formaldehyde, and various dyes, the sources of which are the textile, paint, and varnish industries, the production of building materials, and detergents—creates a serious environmental problem. The pollution of water sources and inefficient water treatment and purification technology are the main causes of poor quality drinking water.
Currently, there are a number of techniques related to advanced oxidation processes (AOPs) that allow for the decomposition of soluble organic substances (SOS) to CO2 and H2O [1]. These include ozonation [2,3], the effect of UV irradiation (UVI) [4], and the use of the Ruff-Fenton system (Fe3+ + H2O2 + UVI), during which there is a continuous photoreduction of salts Fe3+ to Fe2+ and the generation of •OH radicals [5]. In a homogeneous catalysis process, during the disproportionation of hydrogen peroxide by aqua ions, Fe3+ free radicals •OH and HO2• are generated, which have high reactivity in oxidation reactions.
Under conditions of heterogeneous photocatalysis, the photocatalysts based on titanium oxide, bismuth, cobalt, zinc, vanadium, and noble metals, as well as their composites [6,7,8,9,10,11,12] exhibit high activity in the oxidation of soluble organic substances (SOS). Thus, the development of effective and reliable methods of destructing soluble organic substances (SOS) in aqueous media is of great significance. Methods of homogeneous and heterogeneous catalysis using the high oxidative ability of hydroxyl radicals meet such requirements. These methods are related to advanced oxidation processes (AOPs) and allow the destruction of soluble organic substances (SOS) virtually up to СО2 and Н2О [1]. Ozonation [2,3,4,5,6], as well as UV irradiation (UVI) using oxygen-containing catalysts and reagents, contributes to OH-radicals formation. Under UVI conditions, photocatalysts based on bismuth oxide [7], titanium dioxide [9], noble metals [6], and their composites—Bi2O3/TiO2, ZnO/α-Bi2O3 [11], V(IV/V)/TiO2 [12], etc. display high activity in the processes of SOS degradation. Besides, it should be noted that a number of catalysts can participate simultaneously in the processes of both SOS degradation and hydrogen generation [13,14].However, most of these catalysts are synthetic, expensive, and require regeneration.
In the homogeneous catalysis, a large role belongs to the Ruff-Fenton system (Fe3+ + H2O2 + UVI), in which, during the disproportionation of hydrogen peroxide with aqua-ions Fe3+, the continuous photoreduction of salts Fe3+ up to Fe2+ and the generation of •OH radicals occur due to their high reaction ability in SOS oxidation reactions [5]. The drawback of using the Ruff-Fenton system is the presence of a considerable quantity of ions of iron in the solution and the creation of low pH values for the prevention of their hydrolysis. However, the use of heterogeneous iron-containing catalysts in the aqueous medium considerably eliminates the mentioned drawbacks. Thus, to purify waters from phenol, ethanol, and different dyes, some previous studies [15,16,17] used iron-containing synthetic zeolite FeZSM-5; other studies [18,19] used iron-containing metal-ceramic composites. The mentioned catalysts manifested high photocatalytic activity in reactions of oxidative destruction of organic pollutants. Moreover, it can be noted that the regeneration of composites is not complex and the catalysts are cheap.
Thus, ways of obtaining available and cheap photocatalysts/composites are being searched, as well as effective and low-cost methods of purifying industrial wastewaters from soluble organic substances are being developed on their basis, which determines nowadays the novelty and relevance of this study.
There are data in the literature concerning the use of relatively cheap iron-containing composites, in particular synthetic zeolite FeZSM-5, for cleaning. The latter showed high photocatalytic activity in reactions of oxidative destruction of organic substances in water [15,16,17]. However, there are not enough data in the literature on obtaining cheap composites based on natural peat and zeolites with photoactive components for SOS degradation in aqueous media.
Therefore, finding ways to obtain affordable photocatalysts and to develop effective and low-cost methods of industrial wastewater treatment from soluble organic pollutants based on them defines the current novelty and relevance of this research area.
The choice of natural materials (peat and zeolites) for the creation of photocatalysts is determined by their low price and availability (two-thirds of the world reserves of peat are in Western Siberia) as well as their potential for modification. Sorption, kinetic, and photocatalytic characteristics of the obtained composites have been studied. Their high catalytic activity in the process of oxidative destruction of some soluble organic pollutants has been noted [20,21].
Previously, cheap iron-containing composite materials based on natural peat and zeolite were obtained by a simple method of ion-exchange saturation. The composites have been tested as photocatalysts in the process of oxidative destruction of certain soluble organic pollutants, and their high catalytic activity has been observed [22,23,24,25,26].
This paper is a continuation of work begun previously, and it is devoted to studying the possibility of using cheap and available iron-containing natural composites for the photocatalytic destruction of oxalic acid and phenol, which are hazardous pollutants of wastewater, under UVI and the Ruff-Fenton system (Maximum permissible concentration for H2C2O4 in water—0.5 mg/L; for C6H5OH—0.001 mg/L).
Oxalic acid is contained in many cleansing agents and bleaching washing powders, and can be found with the incomplete decomposition of soluble organic substances (SOS) using different methods. Its presence in wastewaters contributes to the development of different chemical chain reactions, leading to the reduction of oxygen in water sources, their pollution, and the death of living organisms. Phenol is a highly toxic compound, having an extremely adverse effect on a living organism. Solubility in water is one of the main causes of the pollution of wastewaters formed during acquisition and reprocessing of phenols, as the harmful effect of phenols manifests itself at very insignificant concentrations (TLV of phenol in water—0.001 mg/L).
To obtain the natural composites, the lowland peat of a Tomsk Region deposit and zeolites (pegasin and hongurin) of the Kemerovo and Yakutsk were used. Features of their structure, the presence of the active centers, the ability to form complexes, and exchange and modification are described in detail in References [20,21,22,23,24,25,26].
2. Materials and Methods
Iron-containing composites were prepared by treating the original form of peat and zeolite with solutions of ferric chloride (III) under static conditions with intensive shaking for 7–10 days until complete saturation. The formation of composites over a long period of time is connected with weak acid-base properties of the initial natural materials (ion exchange equilibrium comes slowly). The optimal concentration of FeCl3 to modify peat and zeolite was found to be 0.500 M. The technique and method of modifying and obtaining the peat composite material is described in Reference [20]. This method can be used also for zeolites. It is similar to method used for zeolites. Natural pegasin and hongurin differ from each other by only exchanging ions. For pegasin, the exchanging ion is magnesium; for hongurin it is natrium. However, when treating natural materials with the salt solution of iron (III), this difference is leveled. The degree of iron (III) absorption by natural sorbents was determined. For peat it was 25%, for pegasin it was 25%, and for hongurin it was 21%. The iron-containing samples of zeolite and peat obtained were used to evaluate their photocatalytic activity in relation to oxalic acid and phenol under UVI and the Ruff-Fenton system. To compare the photocatalytic activity of the iron-containing composites obtained, a synthetic catalyst was selected; FeZSM-5, which is widely used in reactions of photo-oxidative destruction of organic substances used in References [15,16,17] in the photo-oxidative destruction reactions of certain organic substances.
In the experimental procedure, a 100-mg sample was placed in a quartz glass and with 10 mL of oxalic acid solution (c = 0.01 M) or phenol (c = 0.01 g/L). UV irradiation of the samples with and without the addition of hydrogen peroxide was carried out for 30 min. The concentration of hydrogen peroxide in the system was 0.001 mol/L. The required pH value was created with NaOH and HCl solutions and was measured by a pH meter. After the process, an aliquot (15 mL) of the solution was collected, and the residual concentration of SOS was determined. Oxalic acid was determined by permanganometry, and phenol by the spectrophotometric method with 4-aminoantipyrine (Spectrophotometer “Specol 11”). The content of phenol and oxalic acid in the solution was found by the calibration characteristic. The degree of organic substance release from the solution R, % was evaluated by the formula:
where Cfinal and Cinitial are the final and initial concentration of SOS in the solution, respectively.
R, % = 100 − (100 × Cfinal/Сinitial),
The preliminary sorption of oxalic acid and phenol was performed on iron-containing samples under the same conditions, but without external influences. As a source of ultraviolet irradiation, a mercury-quartz lamp type of daytime running lamp (DRL) (240–1100 nm) of 250 watts was used.
To investigate the mechanism of destruction of oxalic acid and phenol, the Infrared radiation (IR) spectroscopy method (Nicolet 6700 FT–IR C Netzsch, Boston, MA, USA with Total internal reflection-fluorescence correlation spectroscopy in the spectral range of 400–4000 cm−1) was used. Simultaneous thermal analysis (STA) was carried out only for the peat composite (thermal analyzer STA 449 C Jupiter, Netzsch). The method allows carrying out both thermographic (TG weight variation) and differential scanning calorimetry (DSC) measurements simultaneously on one sample. The thermal decomposition of the loaded sorbent was carried out in air in an Al2O3 crucible; the heating rate was 10 °/min.
3. Results and Discussion
Comparative study of the destruction of oxalic acid and phenol in the conditions of UVI without additives and with the addition of H2O2 (the Ruff-Fenton system) was carried out by the abovementioned methods. It was assumed that when oxidants are exposed to an iron-containing composite, the photoreduction of Fe3+ to Fe2+ and the generation of •OH radicals would take place, thus determining the effectiveness of the destruction of the organic pollutants studied. The results for the degradation of organic pollutants using iron-containing composites are shown in Table 1.

Table 1.
The degree of degradation of organic pollutants from model solutions under normal conditions and under Ultraviolet irradiation (UVI) and (UVI + H2O2) with natural iron-containing composites. The contact time is 30 min. Observational error 2–3% (P = 0.95, n = 3).
The data from Table 1 show a slight absorption of SOS under normal conditions for 30 min of contact with composites and, at the same time, their high catalytic activity in relation to phenol and oxalic acid using the Ruff-Fenton system. Furthermore, the synthetic catalyst FeZSM-5 shows weaker catalytic properties under the same experimental conditions. That indicates the advantage of the obtained composites in the given experimental conditions.
The weak sorption of oxalic acid and phenol on zeolite composites in a standard environment as compared to the peat sample, in the authors’ opinion, is related to the insufficient amount of time allotted for absorption. On the peat composite, probably owing to its multifunctionality and high ability for complexing, the degree of removal of oxalic acid and phenol is substantially higher. In UVI and UVI + Н2О2 conditions, this difference is insignificant owing to generation of OH-radicals with high reactivity in oxidation reactions.
In the case of zeolite composites, there was almost complete degradation observed for phenol at pH 9 under UVI + H2O2. At low pH values, the destruction degree of phenol was reduced, and at pH 3 with iron-containing pegasin it was 38%; with hongurin it was 20%; at pH 6 it was 80% and 75% for pegasine and hongurin, respectively. Probably, according to Reference [27], with the increase of pH, the self-dissolution of oxidants is accelerated, which contributes to the destruction of phenol in water.
Furthermore, according to References [17,28], iron (III) may be absorbed by the zeolite due to ion exchange and retained in the pores and channels due to electrostatic interaction, and at the same time it has two different surroundings in the zeolite, for example: [HO–Fe–O–Fe–OH]2+. Migrating from one position to another, the iron ions in the zeolite form its catalytic activity and have a positive impact on the degradation of SOS.
There was an attempt conducted to evaluate the catalytic activity of the obtained iron-containing composites under conditions of the Ruff-Fenton system during their six-fold use for the destruction of oxalic acid and phenol under the same experimental conditions. The results revealed the photocatalytic stability of the composites in their multiple uses. The degradation degree of SOS did not change, but was found to be 85–90%. This is probably related to the process of the rapid regeneration of iron (III) in the peat and zeolite phase, which reflects the extent of the novelty and practical significance of this work.
Since the photocatalytic decomposition of oxalic acid and phenol can occur due to sorption by natural composites (Table 1) as well as by the chemical oxidation of SOS by hydrogen peroxide and UV irradiation, it is necessary to determine the contribution of iron-containing samples to the process, that is, to consider a “blank experiment”. For this purpose, the same experiments as described above with oxalic acid and phenol were conducted, but without the composites. The “blank experiment” showed that 13% of oxalic acid was removed due to the chemical interaction of hydrogen peroxide with the acid and exposure of UV irradiation. For the phenol, this value was 6%. Thus, the main part of SOS degradation, as expected, was carried out through the continuous photoreduction of Fe3+ to Fe2+ and the generation of •OH radicals with iron-containing composites.
To investigate the mechanism of the degradation of oxalic acid (H2C2O4 and peat composite were selected for the study), methods of Simultaneous thermal analysis (STA) and IR spectroscopy were used. The following samples of peat were analyzed: the initial peat, peat after sorption, and peat after using the Ruff-Fenton system. The iron-containing composite was selected as the initial one. The thermograms are shown in Figure 1, Figure 2 and Figure 3. From the thermograms it can be seen that all samples are characterized by the following thermal effects, which are caused by the nature of peat:
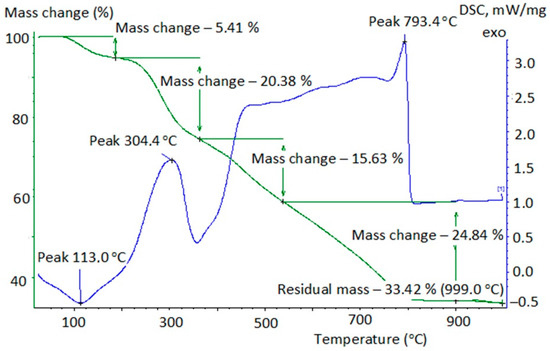
Figure 1.
Thermogram of initial iron-containing peat composite.
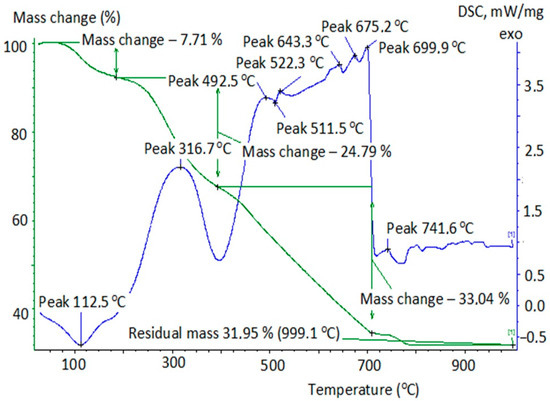
Figure 2.
Thermogram of iron-containing peat composite after the sorption of oxalic acid.
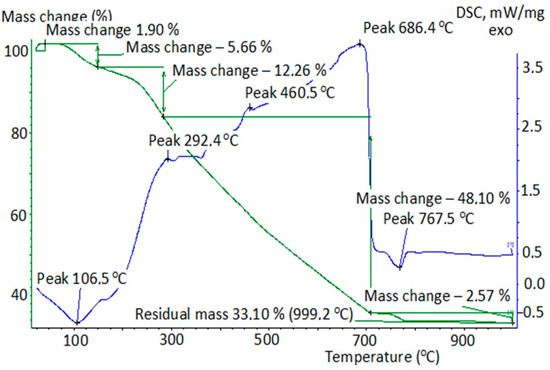
Figure 3.
Thermogram of iron-containing peat composite after the destruction of oxalic acid under UVI + H2O2.
- endothermic effect at 105–115 °C is caused by the removal of adsorbed water;
- exothermic effect at 200–400 °C with a maximum intensity of 290–320 °C corresponds to the thermal destruction of peat, which is associated with the changes and gradual destruction of the humic acid molecules skeleton of peat in the peripheral portion. A further temperature increase leads to the destruction of the nuclear part of the humic acid and the oxidative degradation of its products.
The calculation of the composites’ weight was carried out at their thermal degradation depending on the conditions of the removal of oxalic acid from the model solutions (Table 2). Table 2 shows that the absorption of oxalic acid in normal conditions and in conditions of (H2O2 + UVI) reduced the thermal stability of the peat composite. Its burnout ended at T = 700 °C, in contrast to the initial unmodified peat. Obviously, oxalic acid partially reacted with the components of the peat’s organic part, which was displayed by the displacement of maximum exoeffect into the high-temperature region (316 °C, Figure 2) compared to the initial peat (304 °C, Figure 1). The thermogram in Figure 3 is also of interest. The smooth combustion of the sample and the absence of endoeffect at T = 350 °C at the DSC can be explained, in our opinion, either by the partial destruction of the composite structure due to the oxidant’s effect (H2O2 + UVI), or by the formation of a number of intermediate compounds on the surface of the composite (surface-active complexes, possibly oxalate). The reason for their possible formation could be the interaction of H2C2O4 with the components of the peat’s organic part and the emergence of •OH radicals in the Ruff-Fenton system, which provides process flow at lower activation energies. However, no information was found in the literature to explain the obtained results.

Table 2.
Data on the changing of iron-containing peat composites at their thermal destruction, depending on the conditions of oxalic acid removal from the model solutions.
To clarify this issue, the two versions of the IR spectra of peat composites were taken. In the first and second versions, the same samples as those used for the Simultaneous thermal analysis (STA) method were taken. However, in the second version, the samples have undergone heat treatment in a muffle furnace at 350 °C in closed porcelain crucibles. IR spectra of the first version samples are shown in Figure 4.
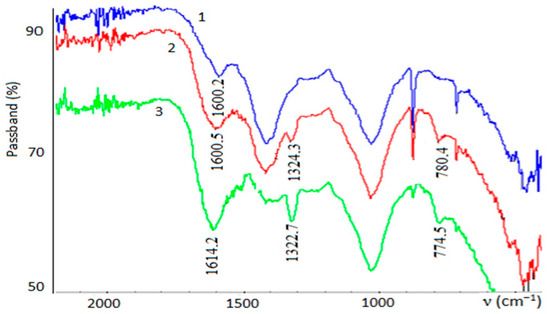
Figure 4.
Infrared (IR) spectra of composites: 1—initial iron-containing composite; 2—iron-containing composite after the sorption of H2C2O4; 3—iron-containing composite after the degradation of H2C2O4 using the Ruff-Fenton system.
Figure 4 shows that the absorption band characterizing the complexation of iron (III) with an oxalate ion appears in the area of 1580–1620 cm−1 (curves 2 and 3). For the initial composite the absorption band is less intense, as in this case there is the complexation of iron ions (III) only with the carboxyl groups of humic substances of peat (curve 1). The shift of the absorption band into the area of shorter wavelengths indicates a strengthening of the M–O bond, as was also observed under sorption and under the condition of (UVR + H2O2). The absorption bands at 1320–1330 and 780 cm−1 in the spectra of the samples are related to the fluctuations of metal oxalates [29]. These bands are not in the initial sample.
The IR spectra of version 2 samples are not given because they were nearly identical to those of version 1. This indicates that the peat sample with oxalic acid degradation in a Ruff-Fenton system is not exposed to destruction, and its smooth combustion (Figure 3) is explained, in the authors’ opinion, by the formation of surface-active complexes on its surface, which may occur as a result of a radical mechanism of reaction flow.
Since studies into revealing the mechanism of oxalic acid degradation in conditions of the Ruff-Fenton system with the participation of an iron-containing peat composite are conducted for the first time, the authors of this paper have not yet found an explanation of the obtained result in the literature.
Based on the above, as well as the results of a comparison of CTA techniques and IR spectroscopy, it can be concluded that the mechanism of releasing oxalic acid from model solutions with iron-containing peat sorbents is complicated and includes both an adsorption step (complexation) and a step of catalytic decomposition of oxalic acid using the Ruff-Fenton system.
Thus, iron-containing composites, obtained by a simple method of treatment using cheap and available natural zeolites and peat with a salt solution of iron (III), are comparable in their photocatalytic activity to synthesized zeolite FeZSM-5 and other well-known metal oxide catalysts in the reaction of oxide destruction in the water of oxalic acid and phenol and do not demand constant regeneration.
4. Conclusions
The possibility of using cheap natural zeolite and peat iron-containing materials in the reaction of oxidative degradation of oxalic acid and phenol under UVI and the Ruff-Fenton system was investigated. It was shown that the optimal method for the photocatalytic destruction of organic pollutants with composites is to introduce hydrogen peroxide. The composites studied are sources of iron ions in the Ruff-Fenton system; they provide the generation of •OH radicals, which have a high reactivity in the oxidative degradation reactions of organic substances and can be reused for cleaning.
Acknowledgments
This research (project No. 8.2.06.2017) was carried out with the support of the Program of increasing the competitiveness of Tomsk State University.
Author Contributions
Ludmila Naumova made an experiment, Nikolay Gorlenko made an experiment and analyzed the experimental data, and Irina Kurzina analyzed the experimental data and wrote the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Munter, R.; Preis, S.; Kallas, J.; Veressinina, Y. Advanced oxidation processes (AOPs): Water treatment technology for the twenty-first century. Kemia Kemi 2001, 28, 354–362. [Google Scholar]
- Dükkancí, M.; Gündüz, G. Ultrasonic degradation of oxalic acid in aqueous solutions. Ultrason. Sonochem. 2006, 13, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Ziolek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Oppenlander, T. Photochemical Purification of Water and Air; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; 383p, ISBN 9783527610884. [Google Scholar]
- Wadley, S.; Waite, T.D. Fenton Processes—Advanced Oxidation Processes for Water and Wastewater Treatment; Parsons, S., Ed.; IWA Publishing: London, UK, 2004; pp. 111–135. ISBN 9781780403076. [Google Scholar]
- Kwak, B.-S.; Chae, J.-H.; Kim, J.-Y.; Kang, M.-S. Enhanced hydrogen production from methanol/water photo-splitting in TiO2 including Pd component. Bull. Korean Chem. Soc. 2009, 30, 1047–1053. (In Russian) [Google Scholar]
- Li, L.; Yan, B. BiVO4/Bi2O3 submicrometer sphere composite: Microstructure and photocatalytic activity under visible-light irradiation. J. Alloys Compd. 2009, 476, 624–628. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Montero-de-Espinosa, R. Ozone-enhanced oxidation of oxalic acid in water with cobalt catalysts. 1. Homogeneous catalytic ozonation. Ind. Eng. Chem. Res. 2003, 42, 3210–3217. [Google Scholar] [CrossRef]
- Fernando, J.; Beltran, F.J.; Rivas, A. ТiO2/А12O3 catalyst to improve the ozonation of oxalic acid in water. Appl. Catal. B 2004, 47, 101–109. [Google Scholar]
- Jing, L.; Wang, J.; Qu, Y.; Luan, Y. Effects of surface-modification with Bi2O3 on the thermal stability and photoinduced charge property of nanocrystalline anatase TiO2 and its enhanced photocatalytic activity. Appl. Surf. Sci. 2009, 156, 657–663. [Google Scholar] [CrossRef]
- Hameed, A.; Gombac, V.; Montini, T.; Felisari, L.; Fornasiero, P. Photocatalytic activity of zinc modified Bi2O3. Chem. Phys. Lett. 2009, 483, 254–261. [Google Scholar] [CrossRef]
- Li, L.; Liu, Ch.; Liu, Y. Study on activities of vanadium (IV/V) doped TiO2(R) nanorods induced by UV and visible light. Mater. Chem. Phys. 2009, 113, 551–557. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Takanabe, K. Solvent-induced deposition of Cu–Ga–In–S nanocrystals onto a titanium dioxide surface for visible-light-driven photocatalytic hydrogen production. Appl. Catal. B 2016, 184, 264–269. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.-A.; Lin, L.; Lin, S.; Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef]
- Kusnetsova, E.V.; Savinov, E.N.; Vostrikova, L.A.; Echevski, G.V. The catalytic and photocatalytic oxidation of organic substances using heterogeneous Fenton-type catalyst. Water Sci. Technol. 2004, 49, 109–115. (In Russian) [Google Scholar]
- Kovban, I.B.; Stolyarova, I.V.; Prihodko, R.V.; TopkinYu, V.; Sychev, M.V.; Goncharuk, V.V. Catalytic activity of zeolite FeZSM-5 in reaction of oxidative destruction of rhodamine G dye. Phys. Chem. Water Treat. Proc. 2006, 28, 319–332. (In Russian) [Google Scholar]
- Stolyarova, I.V.; Kovban, I.B.; Prihodko, R.V.; Kushko, A.O.; Sachev, M.V.; Goncharov, V.V. Interconnection between the catalytic behavior of zeolite FeZSM-5 in reaction of oxidative destruction of dyes and the nature of their active centers. J. Appl. Chem. 2007, 5, 767–773. (In Russian) [Google Scholar]
- Skvortsova, L.N.; Chuklomina, L.N.; Batalova, V.N. Metal–Ceramic Composites Prepared under Combustion Conditions and Their Catalytic Activity in Dye Degradation. Russ. J. Appl. Chem. 2014, 87, 1649–1655. [Google Scholar] [CrossRef]
- Skvortsova, L.N.; Chukhlomina, L.N.; Bolgaru, K.A.; Batalova, V.N.; Shashkina, O.A. Iron-containing metal-ceramic composites for photocatalytic generation of hydrogen from water solutions of organic pollutants. Adv. Mod. Nat. Sci. 2017, 12, 9–15. [Google Scholar]
- Naumova, L.B.; Batalova, V.N.; Mokrousov, G.M.; Didenko, E.A.; Solodkaya, A.A. Investigation of sorption and catalytic activity of the peat based composite material to organic pollutants in water. J. Appl. Chem. 2010, 83, 396–400. (In Russian) [Google Scholar]
- Batalova, V.N.; Skvortsova, L.N.; Mokrousov, G.M.; Naumova, L.B.; Wu Jerry, J. Photocatalytic destruction of organic pollutants of water using Fe-containing natural and synthesized materials. Butl. Messag. 2012, 31, 73–84. [Google Scholar]
- Batalova, V.N.; Mokrousov, G.M.; Skvortsova, L.N.; Naumova, L.B. Formation of Fe-containing functional materials and study of their activity in the process of photocatalytic release of hydrogen. Butl. Messag. 2013, 35, 1–8. [Google Scholar]
- Batalova, V.N.; Naumova, L.B.; Skvortsova, L.N.; Mateyko, I.O. Investigation of the possibility of hydrogen producing in processes of photocatalytic degradation of organic water pollutants using Fe-containing composites. Bull. TSU 2013, 366, 197–200. (In Russian) [Google Scholar]
- Naumova, L.B.; Batalova, V.N.; Gorlenko, N.P.; Sarkisov, Y.S.; Kartashova, A.A. Creation of functional solid-state composites based on black peat. IOP Conf. Ser. Mater. Sci. Eng. 2015, 71, 012038. [Google Scholar] [CrossRef]
- Naumova, L.B.; Batalova, V.N.; Gorlenko, N.P.; Kartashova, A.A. Study of Absorption of Organic Pollutants by Modified Natural Materials. Key Eng. Mater. Submitt. 2016, 683, 275–280. [Google Scholar] [CrossRef]
- Naumova, L.B.; Batalova, V.N.; Alekseenko, K.P. Natural Zeolite-Based Solid Composite Modified by MetalIons for Photodegradation of Dyes in Waters. Key Eng. Mater. 2016, 670, 218–223. [Google Scholar] [CrossRef]
- Plotnikova, A.V. Local treatment of phenol-containing wastewater. Ecol. Ind. Russ. Perm. 2009, 5, 6–10. (In Russian) [Google Scholar]
- Brek, L. Zeolite Molecular Sieves; Mir: Moscow, Russia, 1976; p. 778. (In Russian) [Google Scholar]
- Nakanisi, K. Infraredspectra and Structure of Organic Compounds; Mir: Moscow, Russia, 1965; p. 216. (In Russian) [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).