Abstract
In this work the biosorption of cationic dyes thioflavin T (TT) and methylene blue (MB) from single and binary solutions on dried biomass of freshwater moss Vesicularia dubyana as a function of contact time, pH, and biomass or sorbate concentration has been investigated. The prediction of maximum sorption capacities using adsorption isotherm models were also realized. Biosorption of TT and MB is a rapid process strongly affected by solution pH. Maximum sorption capacities Qmax calculated from Langmuir isotherm were 119 ± 11 mg/g for TT and 229 ± 9 mg/g for MB. In binary mixture, the presence of MB caused significant decrease of TT sorption, advocating the competitive sorption between TT and MB. Results revealed that V. dubyana biomass exhibited significantly higher affinity to thiazine dye MB in comparison with benzothiazole dye TT from both single and binary solutions. Based on the obtained results, the competitive effects in binary system can substantially influence the sorption process and should be thoroughly evaluated before application of selected adsorbents for removal of basic dyes from colored effluents.
1. Introduction
Dyes are the important class of synthetic organic compounds used in many industries such as manufacture of pulp and paper, leather tanning or textile dying and in manufacture of dyestuffs. Ghaly et al. [1] pointed out that the textile industry is one of the major industries in the world and it plays a major role in the economy of many countries. The textile industry utilizes various chemicals (particularly synthetic dyes) and large amounts of water during the production process. The global textile dyes market was estimated to reach USD 4.7 billion in 2015, and is projected to reach USD 6.4 billion by 2019 and USD 8.75 billion by 2023 [2,3].
Industrial application of synthetic dyes is associated with the release of a huge amount of more or less colored effluents into the environment [4,5]. Wastewaters from the textile industry contain a large amount of dyes and chemicals containing trace metals such as Cr, As, Cu and Zn which are capable of harming the environment and human health [1]. During textile dyeing, a wide range of various dyes in a short time period are used, therefore effluents are extremely variable in composition and require an unspecific treatment processes [5]. Conventional physico-chemical (dilution, adsorption, coagulation and flocculation, oxidation, reverse osmosis and ultrafiltration) and biological (aerobic activated sludge and anaerobic processes) treatment technologies presently employed for color removal have several disadvantages such as long operational time, low specificity, formation and disposal of sludge and high cost (see e.g., critical reviews [1,6,7]. Consequently, the development of efficient clean-up technologies is of major interest.
Highly efficient removal of synthetic dyes from effluents has been attempted by bioadsorption and non-conventional biosorbents have been searched in recent years that are easy available, renewable and environmentally friendly that can successfully replace the classical adsorbents [8]. To date, diverse algae, macroalgae, plant biomasses, agricultural residues as well as other biomaterials have been explored as adsorbents for removal of acidic, basic and reactive dyes from aqueous solutions [9,10,11,12,13,14,15,16,17]. Authors have reported that the biosorption capacity is highly dependent on solution pH, biosorbent dosage, temperature and concentration of other solutes present in solution. Since industrial effluents may contain several synthetic dyes, it is necessary to study the simultaneous sorption of two or more dyes and to quantify the mutual effect of one dye on the other. However, contradictory findings related to biosorption of dyes in multi-dye sorption systems were recently published. Albadarin et al. [18] who studied the simultaneous sorption of methylene blue and alizarin red S by olive stone biomass revealed only the limited competition between dyes. Remenárová et al. [19] confirmed that thioflavin T (TT) significantly affected biosorption of malachite green (MG) by moss R. squarrosus in binary system TT + MG. The competitive effect of MG on TT was less pronounced. On the contrary, Giwa et al. [20] found that the presence of rhodamine B and methylene blue had a synergetic effect on the maximum monolayer capacity of the sawdust of Parkia biglobosa for Acid Blue 161 dye in binary and ternary systems.
To ensure the applicability of biosorption technology for colored effluents, more works are still needed for the sorption of a mixture of dyes at various operating conditions. Considering the above mentioned aspects, this study concerns the biosorption of cationic dyes thioflavin T and methylene blue from aqueous solutions by dried biomass of freshwater moss Vesicularia dubyana. The influence of variables (contact time, pH, biomass dosage) controlling the biosorption process has been considered in both single and binary dye systems.
2. Materials and Methods
2.1. Moss Biomass
The biomass of freshwater moss Vesicularia dubyana was used as a sorbent of cationic dyes thioflavin T (TT) and methylene blue (MB). Vital moss biomass was cultivated in diluted Hoagland medium [21] under artificial illumination (2000 lx) at 22 ± 2 °C. Before use in sorption experiments, the biomass was thoroughly rinsed in deionized water (3 times) and dried at 60 °C for 48 h.
2.2. Reagents and Instruments
Thioflavin T (C.I. 49005, Mr(C17H19ClN2S) 318.86, CAS 2390-54-7) and methylene blue (C.I. 52015, Mr (C16H18N3SCl) 319.86; CAS 61-73-4) were purchased from Fluka (USA). All chemicals were of analytical grades. Cationic dyes solutions were prepared in deionized water (conductivity 0.054 µS/cm; Millipore Simplicity). An UV-VIS spectrophotometer Cary 50 (Varian, Australia) was used for determination of TT and MB concentrations in solutions and for establishing calibration curves for TT and MB at maximum absorbances λTT = 412 nm and λMB = 650 nm. The pH effect on MB and TT concentration determination was taken into account.
2.3. Sorption Kinetics in Single and Binary Solutions in Batch System
Dried biomass of V. dubyana (0.5 g/L) was added to 20 mL of solutions containing defined concentrations of thioflavin T (TT), methylene blue (MB) or their mixtures, respectively. Solution pH was adjusted to 6.0. The flasks were incubated on a rotary shaker (250 rpm) at 25 °C. At the time intervals 10, 20, 40, 60, 120, 240, 360 and 1440 min, aliquot samples were obtained and remaining concentration of TT and MB determined. All experiments were performed in duplicate series. The TT and MB uptake was calculated according to Equation (1).
where Qt represents the amount of TT or BB sorbed by moss biomass (mg/g d.w.) from single or binary solutions at time t; C0 and Ct represent the initial concentration of dyes in solution and the concentration of dyes at time t (mg/g). V is solution volume (L) and M is the amount of moss biomass (g; d.w.).
2.4. Influence of pH and Biomass Dosage
To analyze the influence of pH, dried biomass (0.5 g/L) was shaken in 20 mL of TT and MB (C0 TT = 40 mg/L or C0 MB = 80 mg/L) or TT + MB (C0 TT = 40 mg/L and C0 MB = 40 mg/L) solutions of desired pH for 2 h on a rotary shaker at 250 rpm and 25 °C. In order to eliminate interference of buffer components on cationic dyes biosorption, the non-buffered solutions in deionized water were adjusted to the desired pH values by adding 0.1 M HCl or 0.1 M NaOH.
To analyze the influence of biomass dosage, dried biomass of desired amount (CB = 0.25 to 4.0 g/L) was shaken in 20 mL of TT and MB (C0 TT = 40 mg/L or C0 MB = 80 mg/L) or TT + MB (C0 TT = 40 mg/L and C0 MB = 40 mg/L) solutions for 2 h on a rotary shaker at 250 rpm, pH 6.0 and 25 °C.
At the end of the experiments, aliquot samples were obtained and remaining concentration of TT and MB determined. All experiments were performed in duplicate series. The TT and MB uptake was calculated according to Equation (1).
2.5. Sorption Equilibrium in Single and Binary Solutions in Batch System
Dried biomass of V. dubyana (0.5 g/L) was added to 20 mL of solutions with initial dye concentrations C0 in single system ranging from 20 to 200 mg/L (TT) and 20 to 320 mg/L (MB) and in binary system TT + MB from 40 to 160 mg/L. In both single and binary solutions pH was adjusted to 6.0. Flasks were incubated on a rotary shaker (250 rpm) at 25 °C. After 2 h of exposure, the remaining concentration of TT and MB was determined. All experiments were performed in duplicate series. The TT and MB uptake was calculated according to Equation (1).
Equilibrium data in both single and binary systems were analyzed using adsorption isotherm models according to Langmuir and Freundlich. To calculate the corresponding parameters of isotherms non-linear regression analysis was performed by OriginPro 2016 (OriginLab Corporation, Northampton, MA, USA).
2.6. Potentiometric Titration
Potentiometric titration experiment was carried out according to the modified procedure described by Zhang et al. [22]. Dried biomass of freshwater moss V. dubyana (0.30 g) were protonated with 100 mL of equimolar 0.1 mol/L HCl and NaCl for 2 h on the rotary shaker (200 rpm) at 25 °C. Subsequently, moss biomass was separated by centrifugation and transferred into 100 mL of 0.1 mol/L NaCl and agitated under the same conditions. Potentiometric titration was performed in an Erlenmeyer flask with glass electrode (three-point calibrated with buffers—pH 4.0, 7.0 and 10.0) by dropwise addition of 0.1 mol/L mixture of NaOH and NaCl into the bacterial suspension. The titration was conducted in the pH range of 2.0–11.0 and the potentiometric titration curve was obtained by plotting the volume of 0.l mol/L NaOH addition as titrant solution against the pH values measured. ProtoFit ver. 2.1 was used to analyse the titration data.
3. Results and Discussion
3.1. Biosorption Kinetics in Single and Binary Systems
As expected, the uptake of cationic dyes TT and MB by dried biomass of freshwater moss V. dubyana from both single and binary solutions was a rapid process (Figure 1A,B). At the initial phase of uptake, driving force is high (uptake increased linearly) and available high affinity binding sites on V. dubyana biomass are occupied. Initial phase is followed by slower gradual uptake till equilibrium is reached.
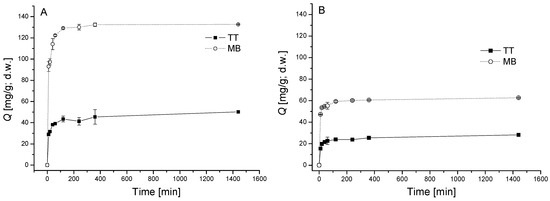
Figure 1.
Kinetics of thioflavin T (TT) and methylene blue (MB) biosorption by dried biomass of moss V. dubyana (CB = 0.5 g/L) from single (A. C0 TT = 40 mg/L or C0 MB =80 mg/L) and binary (B. C0 TT = 40 mg/L and C0 MB = 40 mg/L) solutions at 25 °C and pH 6.0. Error bars represent standard deviation of the mean (±SD, n = 2).
In case of MB, the maximum uptake in single system at initial concentration 80 mg/L was observed after 120 min of exposure (130 ± 5 mg/g; d.w.). Uptake of TT at initial concentration 40 mg/L increased rapidly in the first 90 min and after 120 min reached 43.5 ± 2.7 mg/g. The final equilibrium was reached within the 2 h and after this time, there was no considerable increase in both TT and MB sorption until the end of experiments. Our findings are in agreement with other studies of cationic dyes sorption by various types of biomass. Similar behavior of MB sorption kinetics by green alga Enteromorpha spp. [23] and red (Gracilaria parvispora), brown (Nizamuddinia zanardinii) and green (Ulva fasciata) macroalga [9] was observed. Hameed [24] found that at higher initial concentration of MB longer contact time is needed to reach equilibrium when grass waste was used as sorbent.
Biosorption of TT and MB by V. dubyana biomass from binary solution is shown in Figure 1B. Although the same kinetic profile as in the case of dye sorption in single systems was observed, an evident decrease of TT and MB biosorption capacities in comparison with single systems were recorded as a result of competitive effects (see discussion below). The final equilibrium of both TT and MB was reached within 120 min. At initial dye concentration 40 mg/L, maximum sorption of TT (28.3 ± 0.3 mg/g d.w.) in binary system was significantly lower than maximum sorption of MB (62.7 ± 0.3 mg/g, d.w.). The similar kinetic profile in binary (MB + rhodamine B) and single biosorption system observed Fernandez et al. [25] using Cupressus sempervirens cone chips.
It is evident that V. dubyana biomass exhibited significantly higher affinity to thiazine dye MB in comparison with benzothiazole dye TT from both single and binary solutions. In both dye molecules the positive charge is present on quaternary nitrogen =N+=, however in TT quaternary N is located in position 3, substituted by one methyl group and in MB quaternary N is located in position 3 or 7 and substituted by 2 methyl groups. Therefore, we suppose that among other mechanisms, steric effects in dye molecules can affect dye affinity to moss biomass as well.
3.2. Influence of pH
The pH of dye solution is one of the crucial parameters affecting the sorption process through controlling both the ionization of dye molecules and the degree of ionization of functional groups present on biomass surface.
The influence of solution pH (2.0 to 7.0) on TT and MB sorption by V. dubyana biomass from single solutions is shown in Figure 2A. The lowest sorption capacities Q for both TT (QTT = 12 mg/g d.w.) and MB (QMM = 4 mg/g d.w.) dyes were observed at low initial pH value (pH0 = 2.0) which could be closely related to protonation of binding sites on biomass surface. The low adsorption capacity of MB can also be explained by the fact that MB is mainly found as undissociated species MB0 (99% at pH = 2.0 and 86% at pH = 3.0) [26]. Obtained results indicate that an increase in pH has a positive effect on TT and MB sorption, since the competitions between dye cations and protons for the binding sites decreases and such curves (Figure 2A) represent a typical cationic dye sorption behavior [10,18].
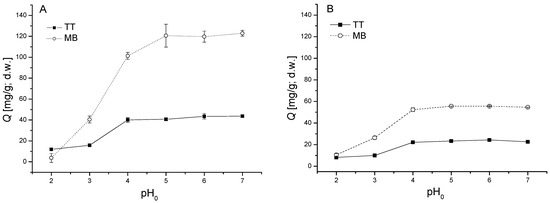
Figure 2.
Influence of initial pH0 on thioflavin T (TT) and methylene blue (MB) biosorption by biomass of moss V. dubyana (CB = 0.5 g/dm3) from single (C0 TT = 40 mg/L or C0 MB 80 mg/L; A) and binary (C0 TT = 40 mg/L and C0 MB = 40 mg/L; B) solutions at 25 °C for 2 h. Error bars represent standard deviation of the mean (±SD, n = 2).
The highest Q values were recorded at pH > 4.0 with maximum QTT = 44 ± 1 mg/g d.w. and QMM = 123 ± 3 mg/g d.w. at pH0 = 7.0. Similarly, Albadarin et al. [23] observed maximum biosorption capacity of methylene blue by olive stone by-products at pH = 7.2 and maximum sorption of thioflavin T by agricultural by-products from the hop (Humulus lupulus L.) was found within the range of initial values of pH 4.0–7.0 [27].
The same behavior was observed in binary sorption system with equimolar initial concentrations of TT and MB. Dye sorption capacities increased from 8.2 ± 0.1 mg/g to 22.7 ± 0.4 mg/g (TT) and 10.5 ± 1.4 mg/g to 54.6 ± 0.1 mg/g (MB) with increasing solution pH from 2.0 to 4.0 and remained almost stable till pH 7.0. From Figure 2B it is evident that the biomass of V. dubyana exhibited significantly higher affinity toward MB.
As was mentioned earlier, the dye sorption capacities are to a great extent affected by the dissociation of functional groups present on moss surface. From the potentiometric titration curve (Figure 3A), it is evident that from pH ~ 4.0 a small addition of NaOH caused a dramatic changes in solution pH what indicates that relevant acidic functional groups (carboxyl, phosphoryl) present on biomass surface are dissociated. To qualitative characterization of functional groups presented on the surface of moss biomass and to determination of binding sites concentration (CAn) the prediction modelling was used. The titration curve shows a relatively unpronounced inflection point, which predicts the existence of several functional groups. According to the ProtoFit prediction and obtained values of residual sum of squares (RSS), it was found that the titration profile was best described by a non-electrostatic model for characterization of the four binding sites. The predicted functional groups with relevant pKa values and concentrations of binding sites (CAn) presented on the surface of moss biomass are listed in Table 1. In connection to these results, we can suppose that MB+ and TT+ adsorbed on surface and balanced a negative charge of biomass. Consequently, one of the possible mechanism of MB and TT biosorption by freshwater moss V. dubyana is the electrostatic attraction.
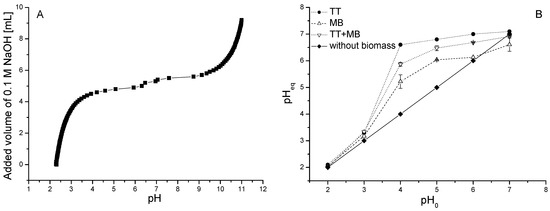
Figure 3.
Potentiometric titration curve for dried biomass of freshwater moss V. dubyana (3.0 g/L) obtained by gradual addition of titrant 0.1 M NaOH at background electrolyte 0.1 M NaCl and reaction temperature 25 °C (A). The change of initial pH0 of dye (single and binary) solutions after biosorption and in control experiments in deionized water and without biomass (B).

Table 1.
Predicted functional groups according to pKa values and concentration of binding sites (CAn) presented on the surface of moss biomass (V. dubyana) using modelling software ProtoFit ver. 2.1.
During and after cationic dyes biosorption, the pH of the dye solutions in both single and binary sorption systems changed (Figure 4B). The solution pH increased from initial pH0 values 4.0, 5.0 and 6.0 to pHeq 5.2, 6.0 and 6.1 (MB), 6.6, 6.8 and 7.0 (TT) and 5.8, 6.4, 6.6 (TT + MB). On the contrary, a slight decrease of solution pH was observed from pH0 = 7.0 to pHeq = ~6.6 probably as a result of a TT–hydrogen and/or MB–hydrogen ion-exchange (Figure 3B). This equilibrium value of pHeq also corresponds to the value of pHzpc = 7.78 predicted within the potentiometric titration and ProtoFit analysis, whereby the pHzpc represents the value of pH at which the biosorbent shows a net zero surface charge.
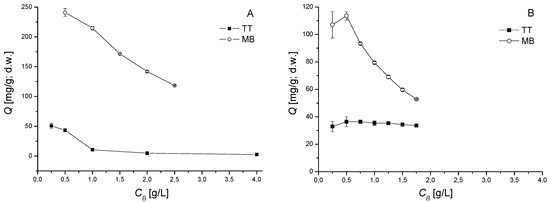
Figure 4.
The effect of biomass concentration CB on thioflavin T (TT) and methylene blue (MB) biosorption by dried biomass of V. dubyana from single (C0 TT = 40 mg/L or C0 MB = 80 mg/L; (A) and binary (C0 TT = 40 mg/L and C0 MB = 40 mg/L; (B) solutions at 25 °C, pH 6.0 for 2 h. Error bars represent standard deviation of the mean (±SD, n = 2).
3.3. Influence of Biomass Dosage
To investigate the biomass dosage on TT and MB removal, various amounts of dried moss biomass were added to both single and binary dye solutions. From Figure 4A it is a clearly seen that the sorption capacity Q of both TT and MB decreased from 241 mg/g to 118 mg/g (MB) and from 50.6 mg/g to 2.6 mg/g (TT) with increasing concentration of moss biomass CB in single solutions. Different relationship between the sorption capacities of dyes Q and the concentration of biomass CB was observed in binary sorption system TT + MB (Figure 4B). The sorption capacity of TT changed only slightly with increasing concentration of moss biomass in solution. On the contrary, the sorption capacity Q of MB decreased significantly (from 107 to 52.8 mg/g) with increasing CB. We suppose that competitive effects between cation dyes and binding sites play an important role and influence the sorption behavior.
Tabaraki and Sadeghinejad [28] pointed out that an increase of biosorbent dose generally increases the overall amount of dye biosorbed (removal efficiency), due to the increased surface area of biosorbent which in turn increases the number of binding sites. Thus, the decrease in the amount of dye sorbed per gram of biosorbent with increase in the biosorbent dose is mainly due to insaturation of binding sites through the sorption process [29]. However, Kumar and Porkodi [30] observed negative aggregation and changes in specific surface area (m2/g) as well as changes in effective mixing of biomass in sorption systems when higher biomass concentrations were used.
3.4. Sorption Equilibrium in Single and Binary Systems
The adsorption equilibrium data of TT and MB from both single and binary sorption systems were described by adsorption isotherms according to Langmuir and Freundlich.
The isotherm parameters obtained by non-linear regression analysis are reported in Table 2. Coefficients of determination (R2) related to the Langmuir model applied to TT and MB biosorption data (R2 = 0.965 for TT; R2 = 0.990 for MB) were higher than those for the Freundlich model (R2 = 0.955 for TT; R2 = 0.938 for MB). In addition, the biosorption of methylene blue and thioflavin T by agricultural by-products from the hop (Humulus lupulus L.) [27] and methylene blue by water hyacinth biomass [31], agro-waste oil tea shell [13], Anethum graveolens biomass [32] and Tremella fuciformis biomass [33] was very well fitted by Langmuir model.

Table 2.
Langmuir and Freundlich equilibrium parameters (±SD) obtained by non-linear regression analysis for TT and MB biosorption by V. dubyana biomass in single systems.
Maximum sorption capacity Qmax and constant b, calculated from Langmuir isotherm model enable to characterize the affinity of V. dubyana biomass towards TT and MB (Table 2) in single sorption systems. Qmax for TT (119 ± 11 mg/g, d.w.) was significantly lower in comparison with Qmax for MB (229 ± 9 mg/g, d.w.). The affinity constant b of the isotherm corresponds to the initial gradient, which indicates the V. dubyana biomass affinity at low concentrations of both TT and MB. Accordingly, a higher initial gradient corresponds to a higher affinity constant b. It is evident, that Langmuir isotherm for MB is steeper at lower equilibrium concentrations than those for TT (Figure 5A,B). The difference in the b values 0.04 ± 0.01 L/mg (TT) and 0.07 ± 0.01 L/mg (MB), confirmed the higher affinity of V. dubyana biomass to MB in comparison with TT. This was also reflected by higher Qmax for MB (Table 2).
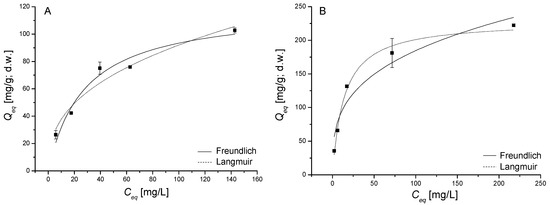
Figure 5.
Fit of the Langmuir and Freundlich isotherms of thioflavin T (TT); (A) and methylene blue (MB); (B) sorption by dried biomass of freshwater moss V. dubyana (CB = 0.5 g/dm3) from single sorption systems after 2 h interactions at 25 °C and pH 6.0.
The comparison of maximal sorption capacities Qmax determined from Langmuir model with those of other authors is reported in Table 3. Although the data about TT (bio)sorption are very limited, our results indicate that V. dubyana biomass exhibited the highest Qmax in comparison with other organic and inorganic sorbents. Similarly, in case of MB biosorption the moss biomass has a high Qmax when compared with different sorbents of plant origin.

Table 3.
Comparison of Qmax values of different sorbents determined from Langmuir isotherm for TT and MB.
In comparison with single sorption systems, in binary or multicomponent mixtures cationic dyes may interact or compete for binding sites of moss biomass. Therefore, the behavior of each species in a multicomponent system depends strongly on the number and properties of other species present. In addition, the solution pH, the physical and chemical properties of both the sorbent and sorbate significantly influenced the sorption process. The simultaneous Langmuir and Freundlich sorption isotherms of MB and TT from binary system MB + TT (concentration ratio C0 TT:C0 MB = 1:1) by V. dubyana biomass are shown in Figure 6A,B. Table 4 presents isotherm parameters calculated by non-linear regression analysis.
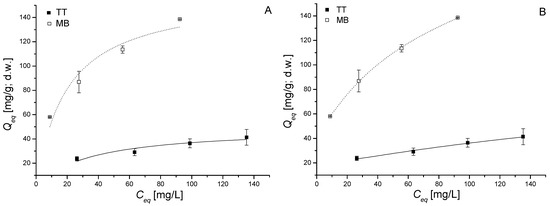
Figure 6.
Fit of the Langmuir (A) and Freundlich (B) isotherms of thioflavin T (TT) and methylene blue (MB) biosorption by dried biomass of freshwater moss V. dubyana (CB = 0.5 g/dm3) from binary sorption system TT + MB (concentration ratio C0 TT:C0 MB = 1:1) after 2 h interactions at 25 °C and pH 6.0.

Table 4.
Langmuir and Freundlich equilibrium parameters (±SD) obtained by non-linear regression analysis for TT and MB biosorption by V. dubyana biomass in binary system TT + MB.
It is evident that sorption of both TT and MB increased with increasing of dyes concentrations in binary mixture. However, the coexistence of MB and TT influenced the sorption capacity of each other, advocating the competitive sorption between TT and MB. The sorption of MB is less affected by the presence of TT than the sorption of TT by the presence of MB in solution.
Maximum sorption capacities Qmax of cationic dyes in binary system TT + MB calculated from Langmuir model reached 49.3 ± 6.2 mg/g (TT) and 160 ± 17 mg/g (MB) and values of affinity parameter b (0.03 ± 0.01 L/mg for TT; 0.05 ± 0.02 L/mg for MB) indicating markedly higher affinity of moss biomass to MB. However, both Qmax values in binary system are significantly lower in comparison with Qmax values obtained in single sorption systems (Table 1). The sorption isotherms of total dye sorption (data calculated as Qeq = Qeq(TT) + Qeq(MB); Ceq = Ceq(TT) + Ceq(MB)) were also constructed and evaluated (not shown). From Table 4 it is clear that maximum sorption capacity Qmax for MB from single sorption system calculated from Langmuir model (Table 2) is almost equal to total Qmax(TT + MB) in binary system. This confirms the hypothesis that both TT and MB in binary mixture TT + MB are sequestered by identical binding sites on biomass surface and observed differences in individual dye sorption capacities Q are due to (i) mutual competitive effects between dyes and (ii) different affinity of biomass to cationic dyes. Remenárová et al. [19] described competitive effects during sorption in binary system TT + MG (malachite green) by moss R. squarrosus. They revealed that TT significantly affected biosorption of MG in binary system TT + MG, the competitive effect of MG on TT was less pronounced. On the contrary, Albadarin et al. [18] observed that the total adsorbed quantity of single dyes is only slightly larger than a mixture of two components with the same concentration in binary system MB and alizarin red S (ARS). This indicating the presence of limited number of active sites by which the two dyes can be sequestrated and for which they will, to some extent, compete for in binary system. Based on obtained data and results of other authors we suppose that variance in affinity in multicomponent dye systems could be attributed to the different chemical structure of dye molecules and physicochemical properties of sorbent used. Tabaraki et al. [28] stated that the chemical structure of dye molecules, the number of sulfonic groups, the basicity and molecular weight of molecules significantly influenced biosorption capacity and affinity.
4. Conclusions
In the present study, dried biomass of freshwater moss V. dubyana has been used as biosorbent for cationic dyes methylene blue and thioflavin T removal from both single and binary systems. Results revealed that an increase in pH has a positive effect on TT and MB sorption and the electrostatic attraction is one of the possible mechanism of MB and TT removal by moss V. dubyana. The experimental equilibrium biosorption data of TT and MB from single systems were well described by Langmuir isotherm and maximum sorption capacities Qmax were 119 ± 11 mg/g for TT and 229 ± 9 mg/g for MB. In binary mixture, the presence of MB caused significant decrease of TT sorption indicating higher affinity of biomass to MB. We conclude that in binary systems the competitive effects can substantially influence the sorption process and should be thoroughly evaluated before application of selected adsorbents for removal of basic dyes from wastewaters containing mixtures of dyes.
Acknowledgments
This work was supported by the project of the Cross-border Co-operation Programme and co-financed with European Regional Development Fund (ERDF), the grant number HUSK/1101/1.2.1/0148 as well as the project of the Operational Program Research and Development and co-financed with European Regional Development Fund (ERDF), the grant number ITMS 26220220191.
Author Contributions
Martin Pipíška and Miroslav Horník conceived and designed the experiments; Denisa Partelová and Martin Valica performed the experiments; Denisa Partelová and Miroslav Horník analyzed the experimental data; Juraj Lesný and Stanislav Hostin contributed materials and analysis tools; Martin Pipíška and Miroslav Horník wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghaly, A.E.; Ananthasankhar, R.; Alhattab, M.; Ramakrishnan, V.V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2014, 5, 182. [Google Scholar] [CrossRef]
- Market Research Future. Global Textile Dyes Market Research Report—Forecast to 2023; ID: MRFR/CnM/2238-HCRR; Market Research Future: Maharashtra, India, 2017. [Google Scholar]
- GosReports. Global Textile Dyes Industry 2015 Market Research Report. 2015. Available online: https://www.prnewswire.com/news-releases/global-textile-dyes-industry-report-2015---forecasts-to-2020-498532981.html (accessed on 2 April 2015).
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2012; pp. 111–162. ISBN 978-94-007-1591-2. [Google Scholar]
- Dias, A.A.; Sampaio, A.; Bezerra, R.M. Environmental Applications of Fungal and Plant Systems: Decolourisation of Textile Wastewater and Related Dyestuffs. In Environmental Bioremediation Technologies; Singh, S.N., Tripathi, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 445–463. ISBN 978-3-540-34790-3. [Google Scholar]
- Sing, K.; Arora, S. Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Paşka, O.M.; Pəcurariu, C.; Muntean, S.G. Kinetic and thermodynamic studies on methylene blue biosorption using corn-husk. RSC Adv. 2014, 4, 62621–62630. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Sillanpää, M.; Bhatnagar, A. A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae—Effect of pretreatment. Chem. Eng. J. 2017, 307, 435–446. [Google Scholar] [CrossRef]
- Liang, J.; Xia, J.; Long, J. Biosorption of methylene blue by nonliving biomass of the brown macroalga Sargassum hemiphyllum. Water Sci. Technol. 2017, 76, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Horník, M.; Šuňovská, A.; Partelová, D.; Pipíška, M.; Augustín, J. Continuous sorption of synthetic dyes on dried biomass of microalga Chlorella pyrenoidosa. Chem. Pap. 2013, 67, 254–264. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, T. Biosorption of methylene blue from wastewater by an extraction residue of Salvia miltiorrhiza Bge. Bioresour. Technol. 2017, 219, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, E.; You, X.; Hu, C.; Hu, Q. Adsorption of methylene blue on an agro-waste oiltea shell with and without fungal treatment. Sci. Rep. 2016, 6, 38450. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.S.; Mittal, A.K. Biosorptive uptake of cationic dyes from aqueous phase using immobilised dead macro fungal biomass. Int. J. Environ. Technol. Manag. 2011, 14, 282–293. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Fini, P.; Semeraro, P.; Cosma, P. An interesting environmental friendly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater. Dyes Pigment. 2017, 140, 480–490. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Gubitosa, J.; Fini, P.; Petrella, A.; Agostiano, A.; Semeraro, P.; Cosma, P. An Alternative use of olive pomace as a wide-ranging bioremediation strategy to adsorb and recover disperse orange and disperse red industrial dyes from wastewater. Separations 2017, 4, 29. [Google Scholar] [CrossRef]
- Semeraro, P.; Rizzi, V.; Fini, P.; Matera, S.; Cosma, P.; Franco, E.; García, R.; Ferrándiz, M.; Núñez, E.; Gabaldón, J.A.; et al. Interaction between industrial textile dyes and cyclodextrins. Dyes Pigment. 2015, 119, 84–94. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C. Mechanism of Alizarin red S and Methylene blue biosorption onto a olive stone by-product: Isotherm study in single and binary systems. J. Environ. Manag. 2015, 164, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Remenárová, L.; Pipíška, M.; Horník, M.; Augustín, J. Biosorption of cationic dyes BY1, BY2 and BG4 by moss Rhytidiadelphus squarrosus from binary solutions. Nova Biotechnol. 2009, 9, 239–247. [Google Scholar]
- Giwa, A.R.A.; Abdulsalam, K.A.; Wewers, F.; Oladipo, M.A. Biosorption of acid dye in single and multidye systems onto sawdust of locust bean (Parkia biglobosa) tree. J. Chem. 2016, 2016, 6436039. [Google Scholar] [CrossRef]
- Hoagland, D.R. Optimum nutrient solution for plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Xu, M.; Zheng, F.; Zhao, M. Study of the mechanisms of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J. Hazard. Mater. 2010, 178, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Ncibi, M.C.; Ben Hamissa, A.M.; Fathallah, A.; Kortas, M.H.; Baklouti, T.; Mahjoub, B.; Seffen, M. Biosorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. J. Hazard. Mater. 2009, 170, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H. Grass waste: A novel sorbent for the removal of basic dye from aqueous solution. J. Hazard. Mater. 2009, 166, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Batch and dynamic biosorption of basic dyes from binary solutions by alkaline-treated cypress cone chips. Bioresour. Technol. 2012, 106, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Partelová, D.; Šuňovská, A.; Marešová, J.; Horník, M.; Pipíška, S.; Hostin, S. Removal of contaminats from aqueous solutions using hop (Humulus lupulus L.) agricultural by-products. Nova Biotechnol. Chim. 2015, 14, 212–227. [Google Scholar] [CrossRef]
- Tabaraki, R.; Sadeghinejad, N. Biosorption of six basic and acidic dyes on brown alga Sargassum ilicifolium: Optimization, kinetics and isotherm studies. Water Sci. Technol. 2017, 75, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Tural, B.; Ertaş, E.; Enez, B.; Fincan, S.A.; Tural, S. Preparation and characterization of a novel magnetic biosorbent functionalized with biomass of Bacillus subtilis: Kinetic and isotherm studies of biosorption processes in the removal of Methylene Blue. J. Environ. Chem. Eng. 2017, 5, 4795–4802. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K. Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J. Hazard. Mater. 2007, 146, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Islam, T.; Das, S. A novel biosorbent, water-hyacinth, uptaking methylene blue from aqueous solution: Kinetics and equilibrium studies. Int. J. Chem. Eng. 2014, 2014, 819536. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhu, F.; You, L.; Shen, X. Evaluation of the biosorption characteristics of Tremella fuciformis for the decolorization of cationic dye from aqueous solution. J. Polym. Environ. 2017, in press. [Google Scholar] [CrossRef]
- Hamitouche, A.; Haffas, M.; Boudjemaa, A.; Benammar, S.; Sehailia, M.; Bachari, K. Efficient biosorption of methylene blue, malachite green and methyl violet organic pollutants on biomass derived from Anethum graveolens: An eco-benign approach for wastewater treatment. Desalin. Water Treat. 2017, 5, 225–236. [Google Scholar] [CrossRef]
- Remenárová, L.; Pipíška, M.; Horník, M.; Augustín, J. Sorption of cationic dyes from aqueous solution by moss Rhytidiadelphus squarrosus: Kinetics and nequilibrium study. Nova Biotechnol. 2009, 9, 75–84. [Google Scholar]
- Shin, W.S. Competitive sorption of anionic and cationic dyes onto cetylpyridinium-modified montmorillonite. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2008, 43, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Venkateswarlu, K.; Thavamani, P.; Lee, Y.B.; Naidu, R.; Megharaj, M. Quercus robur acorn peel as a novel coagulating adsorbent for cationic dye removal from aquatic ecosystems. Ecol. Eng. 2017, 101, 3–8. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus sheathiana bark: Equilibrium, kinetics, thermodynamics and mechanism. Desalin. Water Treat. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]
- Kong, L.; Gong, L.; Wang, J. Removal of methylene blue from wastewater using fallen leaves as an adsorbent. Desalin. Water Treat. 2015, 53, 2489–2500. [Google Scholar] [CrossRef]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Sorption equilibrium and kinetics of basic dye from aqueous solution using banana stalk waste. J. Hazard. Mater. 2008, 158, 499–506. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).