The By-products and Emissions from Manufacturing Torrefied Solid Fuel Using Waste Bamboo Chopsticks
Abstract
:1. Introduction
2. Experimental Methods
2.1. Procedure of Collecting By-Products from the Torrefaction of WBC
2.2. Liquid Analysis
2.3. Gas Analysis
3. Results and Discussion
3.1. Composition of Solid, Liquid, and Gas Produts from the Torrefaction of WBC at 563 K and 40 min
3.2. Liquid Products from the Torrefaction of WBC at 563 K and 40 min
3.2.1. TOC and Water
3.2.2. pH Value
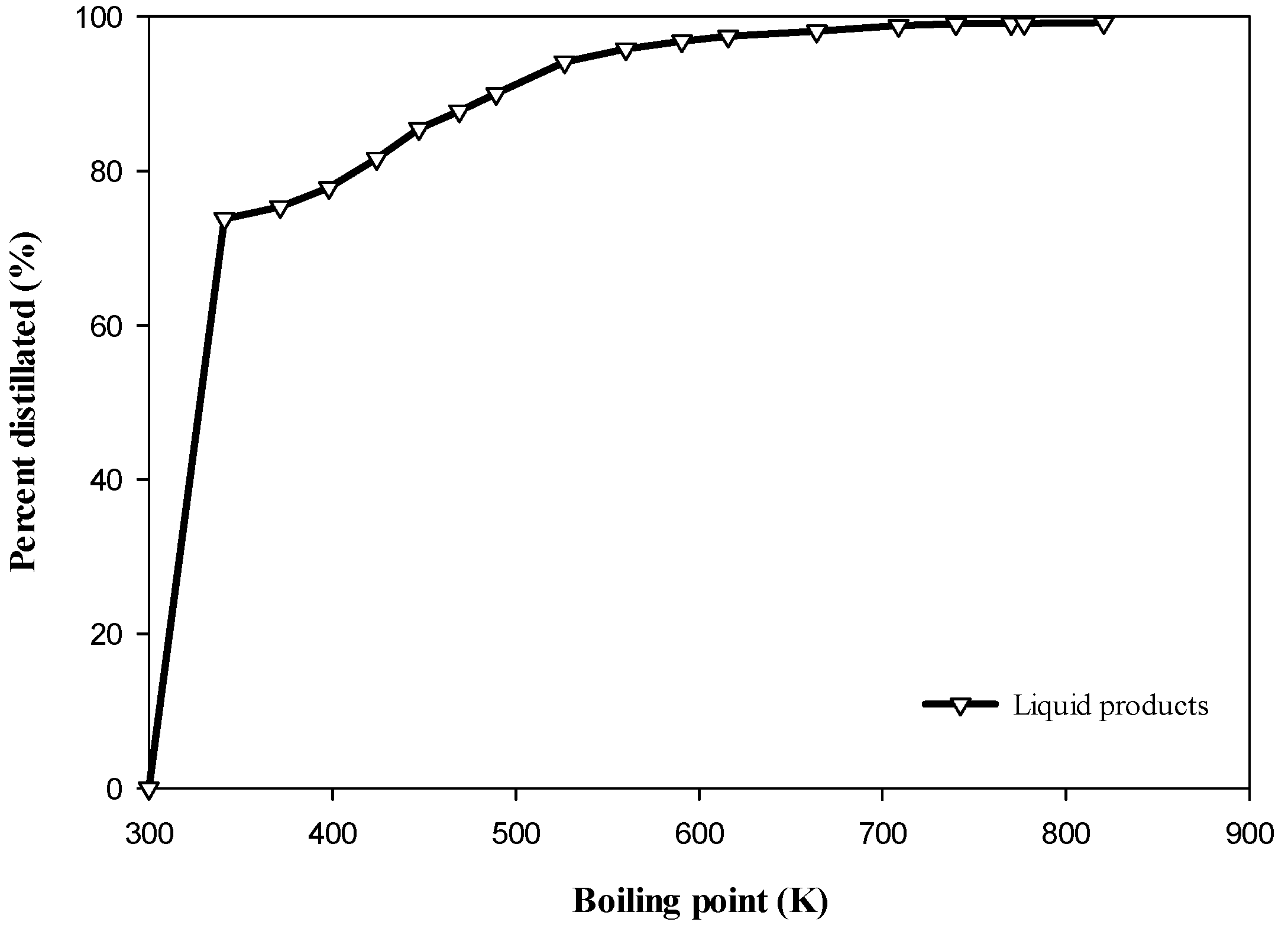
3.2.3. Simulated Distillation
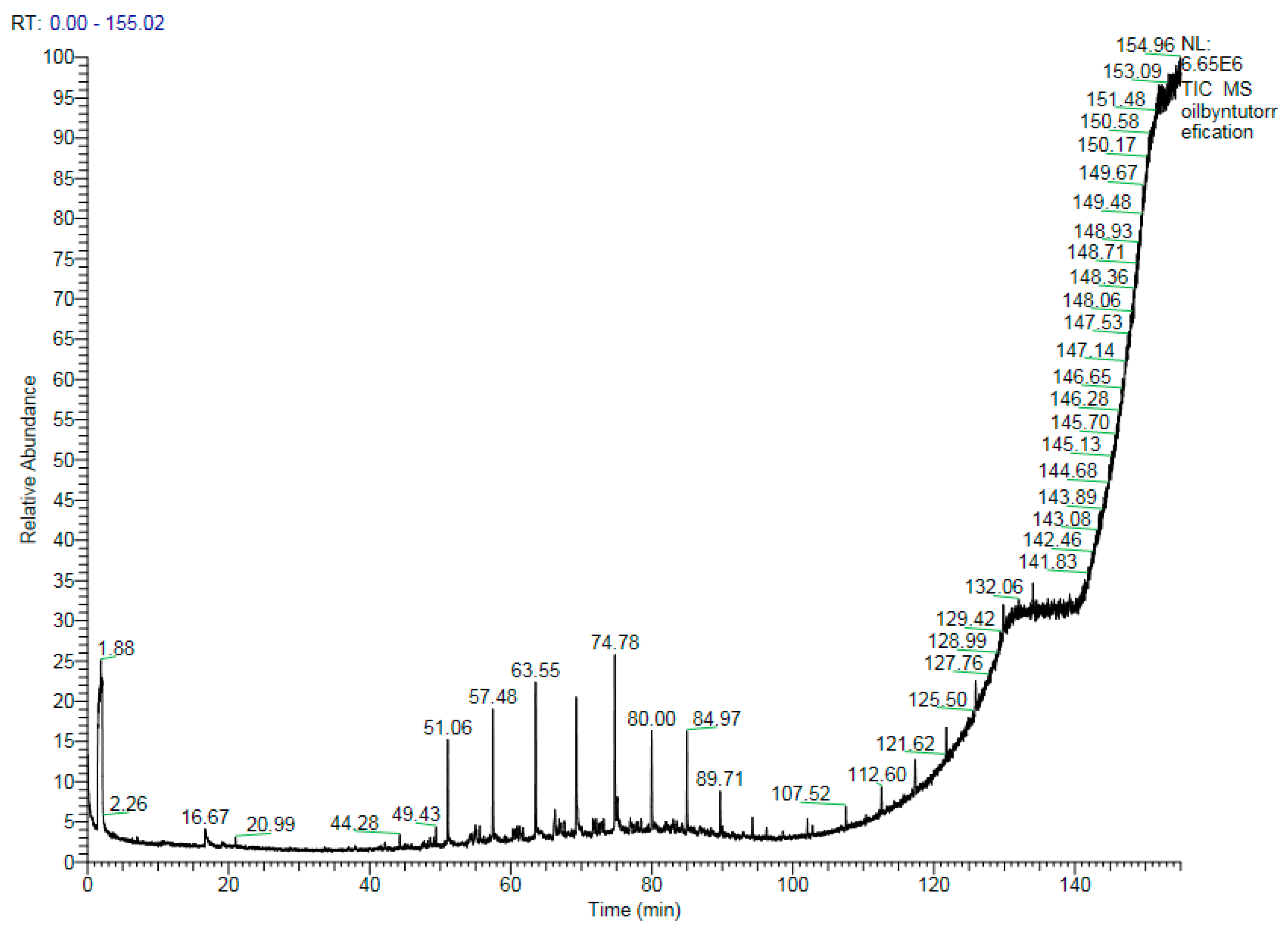
3.2.4. Probable Organic Compounds
3.3. Gas Products from the Torrefaction of WBC at 563 K and 40 min
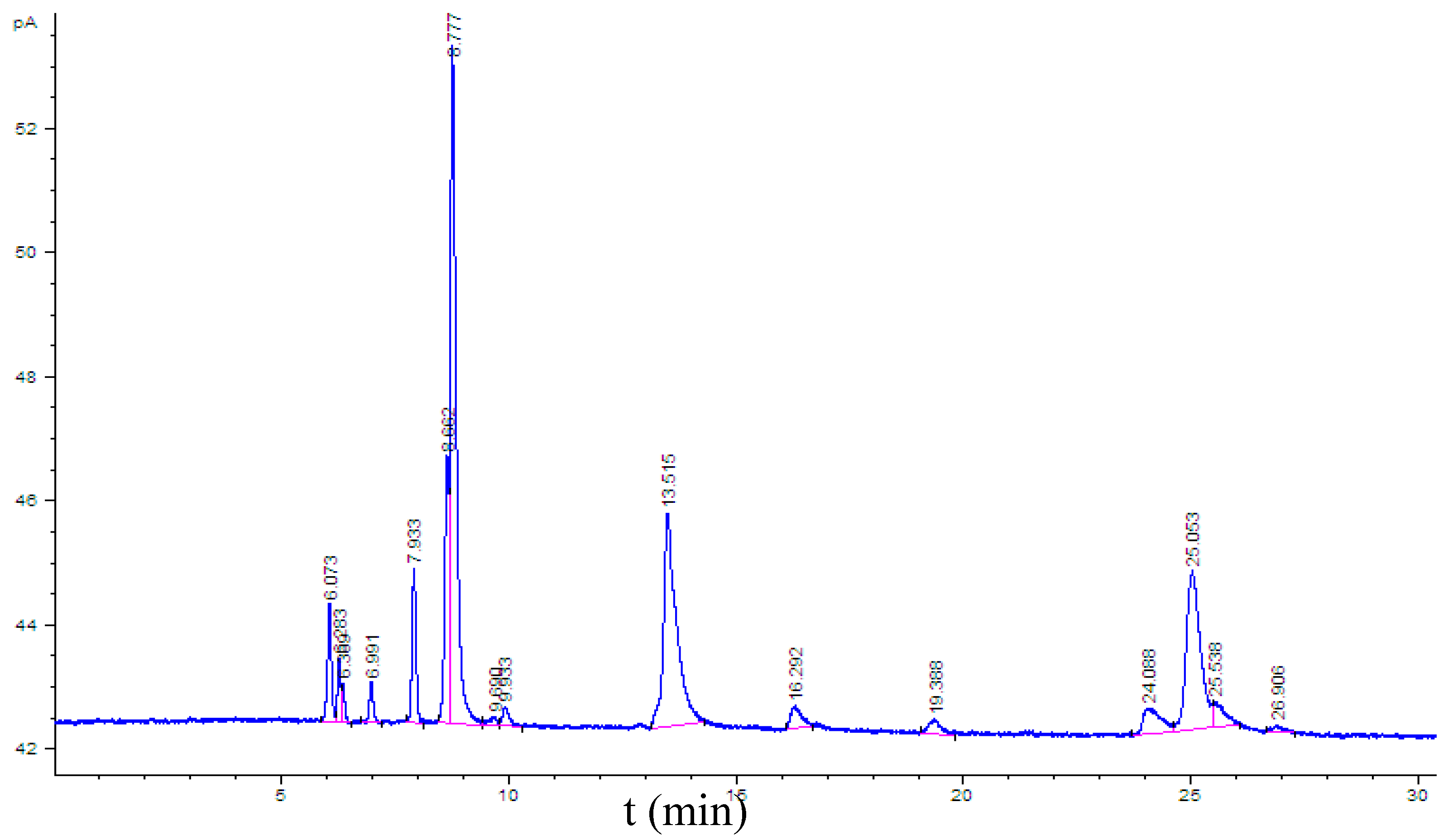
3.3.1. Hydrocarbons
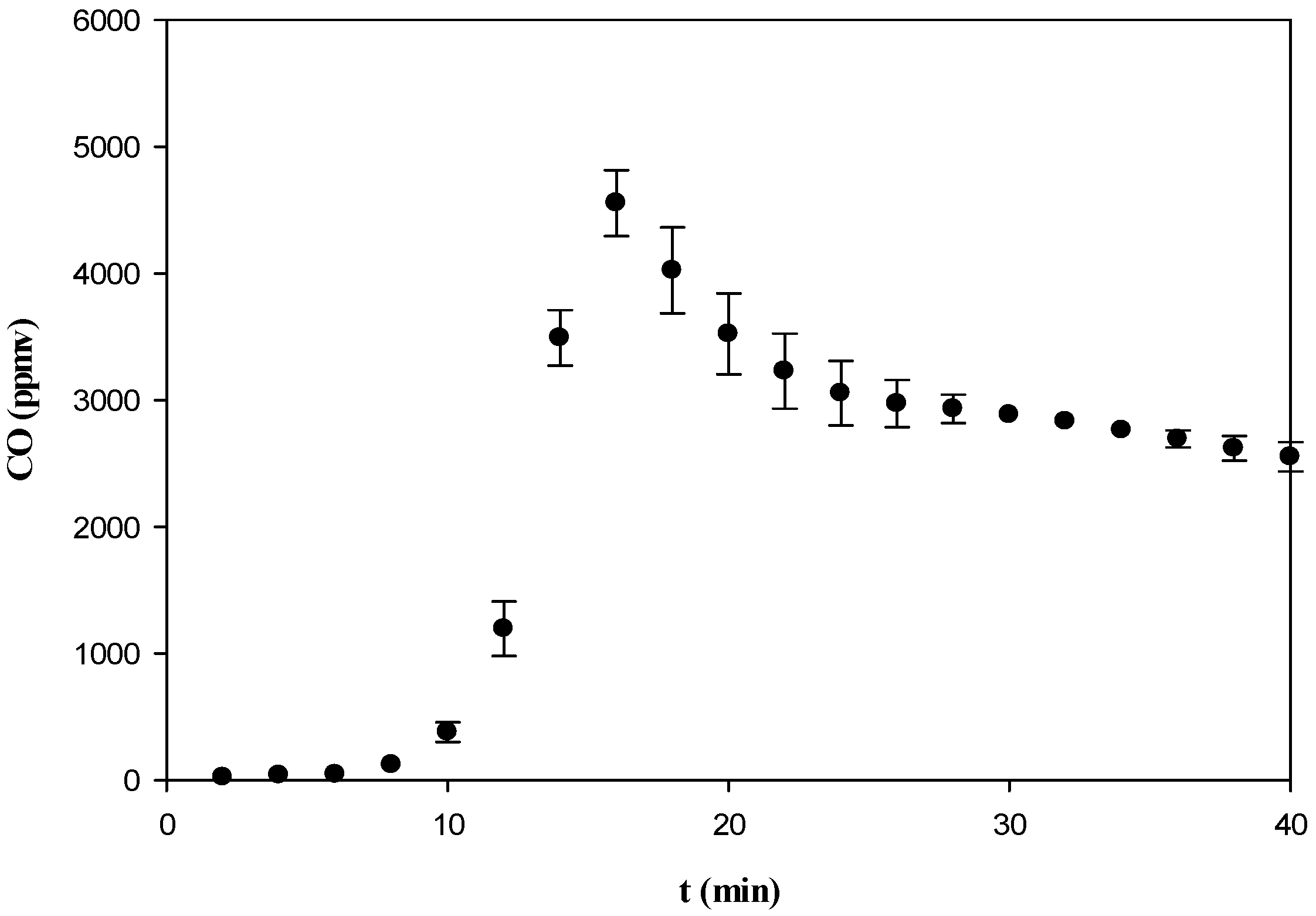
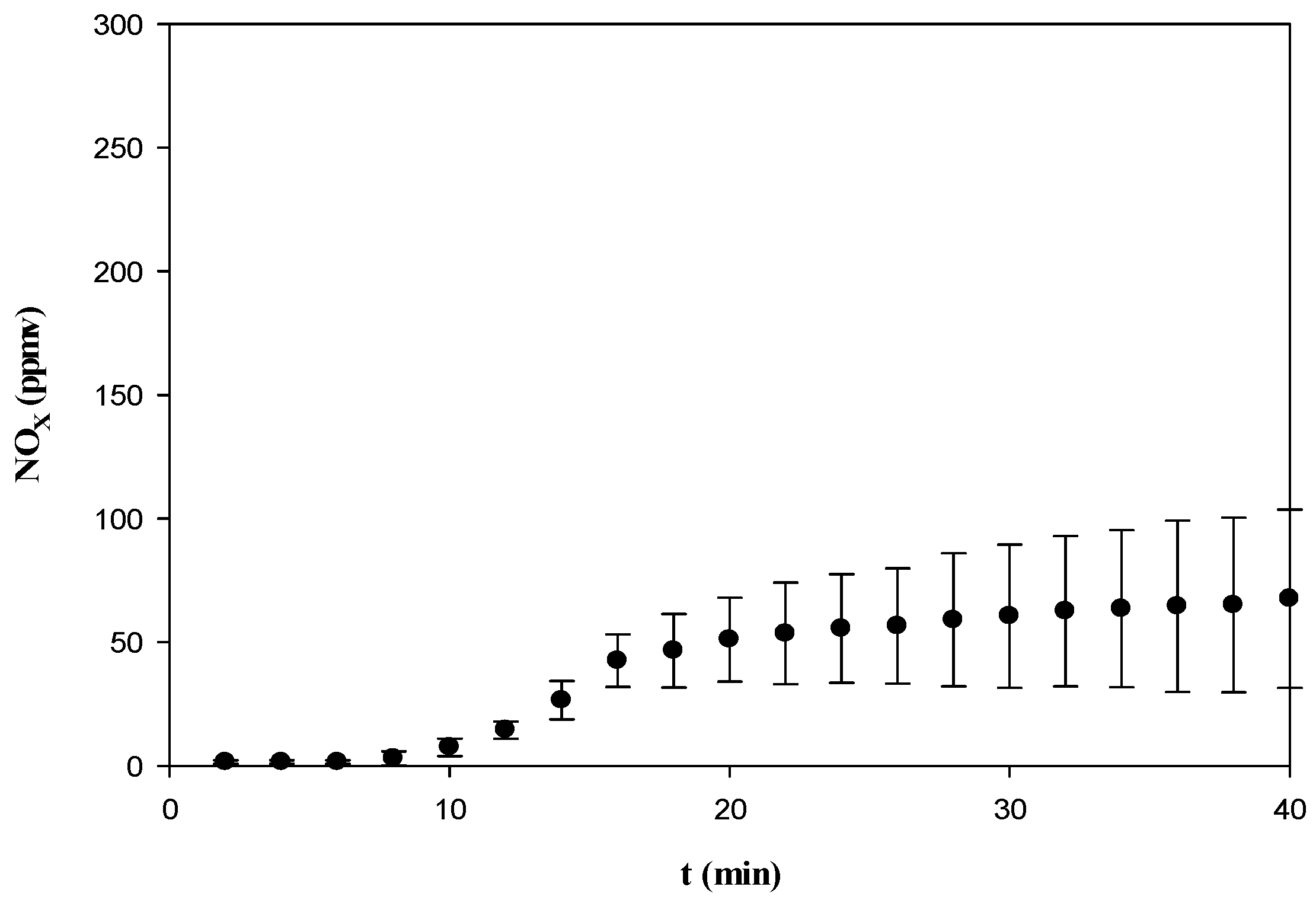
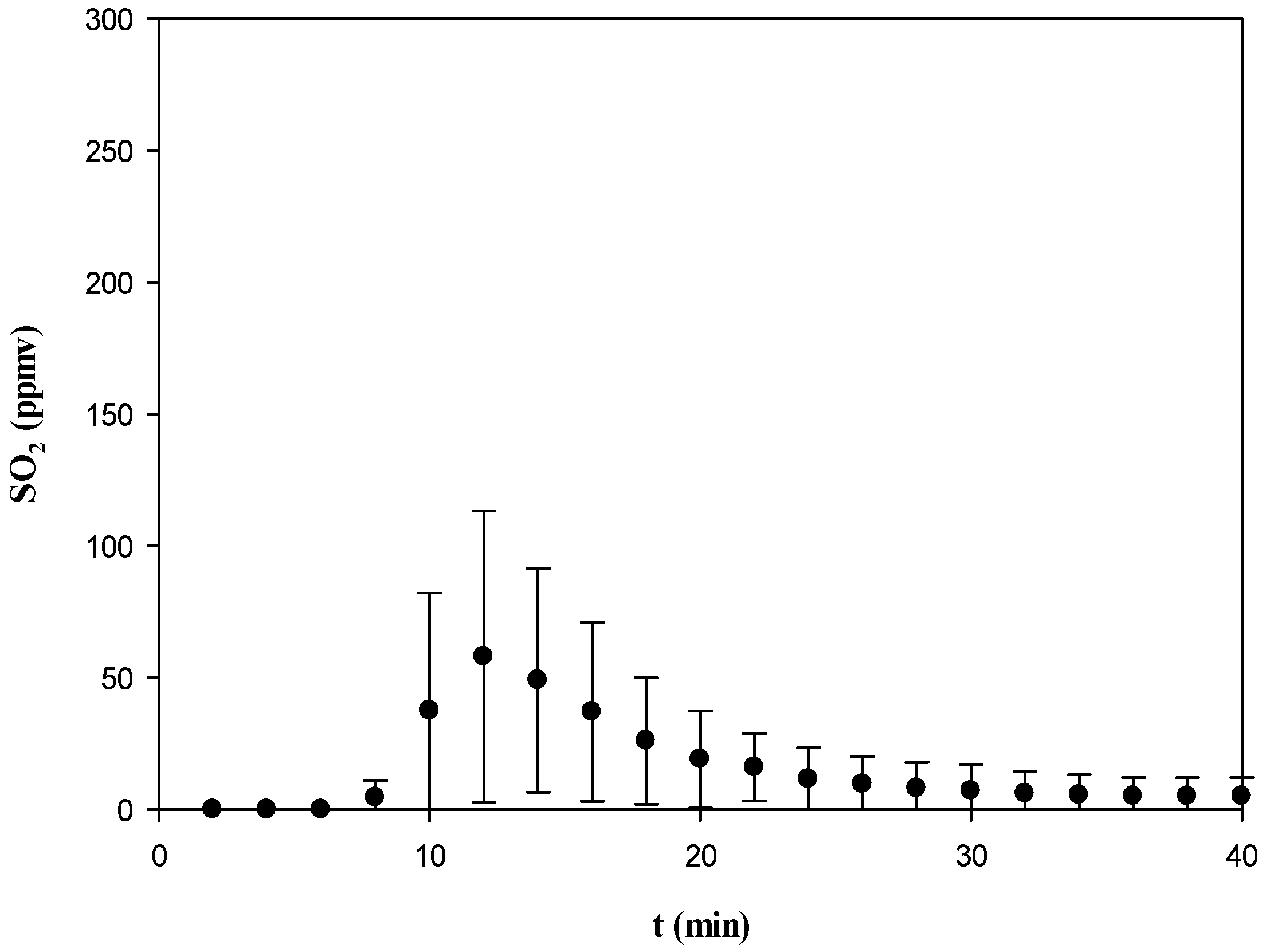
3.3.2. CO, NOx, SO2, and CO2
3.3.3. Gas Balance
3.3.4. Comparison of Gas Emissions with Air Pollution Standards
4. Conclusions
- The pH value of the liquid products is about 0.96, which demonstrates that some preprocesses used for removing acidic components are necessary to more usefully reuse these products.
- With the exception of the water content, the major components of the liquid products are organic matters with a low carbon number below C6.
- There are some useful compounds appearing in the liquid. The main components can be reformed to produced long-chain alkanes, forming diesel fuel (C10–C40) and aviation fuel (C10–C20).
- The liquid contains some useful medicinal components like C23 prednisolone acetate and C22 colchicine.
- Proper air pollution control measures are needed for avoiding air pollution resulting from the torrefaction of WBC.
- Gas products from the large scale torrefaction of WBC represent chance to develop the utilization of biogas (alkanes).
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| bp | Boiling point (K) |
| Cn | Number of carbons (-) |
| CTOC | Concentration of TOC (mg L−1) |
| CVGNi | Volume concentration of component i in total gas including N2 (vol %) |
| CVHCi | Volume concentration of individual hydrocarbon i in total HCs (vol %) |
| ED | Energy densification factor (-) |
| HHD | High heating value in dry basis (kcal kg−1) |
| M | Mass content (wt %) |
| MTOC | Mass percent of TOC (wt %) |
| mL | Mass of liquid product (g) |
| QGe | Average flow rate of exist gas (mL min−1) |
| QNi | Average flow rate of inlet N2 (mL min−1), 100 mL min−1 |
| Tr or Ttor | Torrefaction temperature (K) |
| tr or treaction | Torrefaction time (min) |
| VCn-HC | Volume of Cn HC (mL) |
| VG+N or VT | Volume of total output gas including carrier gas N2 (mL), 4183 mL |
| VGi | Gas volume of component i (mL) |
| VI | Volume of gas products injected into GC-FID (μL), 200 μL |
| VL | Volume of liquid product (mL) |
| VN | Volume of nitrogen (mL) |
| VTHC | Volume of all Cn HC with n = 1 to 6 (mL) |
| YS | Solid yield (wt % or -) |
Abbreviation
| AAQS | Ambient air quality standards |
| DBCs | Disposable bamboo chopsticks |
| DCs | Disposable chopsticks |
| ESSS | Emission standards of stationary sources |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC-MS | GC with mass spectrum |
| HCs | Hydrocarbons |
| TEPA | Taiwan Environmental Protection Administration |
| TOC | Total organic carbon |
| WBCs | Waste bamboo chopsticks |
| WBCT | Torrefied waste bamboo chopsticks |
References
- Arcate, J. New process for torrefied wood manufacturing. Bioenergy Update 2000, 4, 1–4. [Google Scholar]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F. Influence of torrefaction on the grindability and reactivity of wood biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.H.; Bi, X.T.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Reviews 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H.; Pittman, C.U. Current trends in the production and applications of torrefied wood/biomass—A review. BioResources 2015, 10, 8812–8858. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-firing in Existing Coal-fired Power Stations, in “Biocoal”; Report ECN-C-05–013; Energy Research Center of the Netherlands: Petten, The Netherlands, 2005. [Google Scholar]
- Şensöz, S. Slow pyrolysis of wood barks from Pinus brutia Ten and product compositions. Bioresource Technol. 2003, 89, 307–311. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresource Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Saizjimenez, C.; Deleeuw, J.W. Lignin pyrolysis products: Their structures and their significance as biomarkers. Organic Geochem. 1986, 10, 869–876. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, H.C.; Lu, K.M.; Lee, W.J.; Lin, T.C. Thermal pretreatment of wood (Lauan) block by torrefaction and its influence on the properties of the biomass. Energy 2011, 36, 3012–3021. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, S.H.; Juang, T.T.; Tsai, C.M.; Zhuang, Y.Q. Characterization of solid and liquid products from bamboo torrefaction. Appl. Energ. 2015, 160, 829–835. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood—Part 2. Analysis of products. J. Anal. Appl. Pyrol. 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.C.; Chang, C.Y.; Yuan, M.H.; Ji, D.R.; Shie, J.L.; Chen, Y.H.; Chang, W.R.; Yang, T.Y.; Lee, C.H.; et al. Production of Solid Bio-fuel from Waste Bamboo Chopsticks by Torrefaction. J. Anal. Appl. Pyrol. 2017. (Under review). [Google Scholar]
- Biswas, A.; Mahanta, P. Design and experimental analysis of condenser for the production of bamboo vinegar. Int. J. Appl. Res. Mech. Eng. 2012, 2, 79–86. [Google Scholar]
- Hung, Z.S.; Chang, C.C.; Chang, C.F.H.; Lin, Y.S.; Ji, D.R.; Chang, C.Y.; Tseng, J.Y.; Chiang, S.W.; Shie, J.S.; Chen, Y.H.; et al. Autoclaving treatment of wasted disposable bamboo chopsticks. J. Taiwan Inst. Chem. Eng. 2013, 44, 1010–1015. [Google Scholar] [CrossRef]
- Lê Thànha, K.; Commandréa, J.M.; Valettea, J.; Vollea, G.; Meyerb, M. Detailed identification and quantification of the condensable species released during torrefaction of lignocellulosic biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Yang, C.S.; Chen, Y.H.; Huang, M.; Chang, C.Y.; Shie, J.L.; Yuan, M.H.; Chen, Y.H.; Ho, C.; et al. Conversion of waste bamboo chopsticks to bio-oil via catalytic hydrothermal liquefaction using K2CO3. Sustain. Environ. Res. 2016, 26, 262–267. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, W.; Song, W.; Yao, J. Preliminary investigation on concentrating of acetol from wood vinegar. Energy Convers. Manag. 2010, 51, 346–349. [Google Scholar] [CrossRef]
- Sanders, J.; Scott, E.; Weusthuis, R.A.; Mooibroek, H. Bio-refinery as the bio-inspired process to bulk chemicals. Macromol. Biosci. 2007, 7, 105–117. [Google Scholar] [CrossRef] [PubMed]
- TEPA (Taiwan Environmental Protection Administration). Emission Standards of Stationary Sources (ESSS). Available online: http://ivy5.epa.gov.tw/docfile/040070.pdf (accessed on 24 June 2016).




| Tr-tr (K-min) | Mass a (g) | Mass Yield (-) | Volume (mL) | |||||
|---|---|---|---|---|---|---|---|---|
| 563-40 | Solid b1 | Liquid | Gas c | Solid b1 | Liquid | Gas c | Liquid | Gas c |
| 2.07 b2 | 0.43 | 0.51 | 0.69 b2 | 0.14 | 0.17 | 0.41 (0.03) e | 183 d (27.1) e | |
| Time (min) | Temperature (°C) | Possible Compound | Probability (%) | Formula |
|---|---|---|---|---|
| 51.1 | 142.1 | Tetradecane | 51.34 | C14H30 |
| Hexadecane | 13.16 | C16H34 | ||
| Pentadecane | 12.65 | C15H32 | ||
| 57.5 | 155.0 | Pentadecane | 46.03 | C15H32 |
| Hexadecane | 13.29 | C16H34 | ||
| Eicosane | 9.91 | C20H42 | ||
| 63.6 | 167.1 | Hexadecane | 46.33 | C16H34 |
| Heptadecane | 10.22 | C17H36 | ||
| Eicosane | 8.03 | C20H42 | ||
| 69.3 | 178.6 | Heptadecane | 45.90 | C17H36 |
| Hexadecane | 11.34 | C16H34 | ||
| Pentadecane, 2-methyl- | 9.14 | C16H34 | ||
| 74.8 | 189.6 | Octadecane | 26.91 | C18H38 |
| Heneicosane | 13.88 | C21H44 | ||
| Eicosane | 12.08 | C20H42 | ||
| 80.0 | 200.0 | Nonadecane | 19.17 | C19H40 |
| Tetracosane | 16.94 | C24H50 | ||
| Heneicosane | 12.63 | C21H44 | ||
| 85.0 | 209.9 | Heneicosane | 27.05 | C21H44 |
| Tetracosane | 26.00 | C24H50 | ||
| Eicosane | 7.08 | C20H42 | ||
| 89.7 | 219.4 | Heneicosane | 32.52 | C21H44 |
| Pentadecane, 2-methyl- | 9.91 | C16H34 | ||
| Octacosane | 8.37 | C28H58 | ||
| 94.3 | 228.6 | Hydrocortisone acetone | 14.96 | C23H32O6 |
| Digitoxin | 14.38 | C41H64O13 | ||
| Docosane | 9.00 | C22H46 | ||
| 102.1 | 244.2 | Prednisolone acetate | 20.37 | C23H30O6 |
| Beclomethasone | 16.41 | C28H37ClO7 | ||
| Colchicine | 15.78 | C22H25NO6 | ||
| 107.5 | 255.0 | Prednisolone acetate | 18.65 | C23H30O6 |
| Colchicine | 17.92 | C22H25NO6 | ||
| Beclomethasone | 11.22 | C28H37ClO7 | ||
| 112.6 | 265.2 | Colchicine | 23.89 | C22H25NO6 |
| Prednisolone acetate | 12.32 | C23H30O6 | ||
| Beclomethasone | 11.34 | C28H37ClO7 | ||
| 117.4 | 274.7 | Prednisolone acetate | 22.65 | C23H30O6 |
| Beclomethasone | 18.25 | C28H37ClO7 | ||
| Colchicine | 12.16 | C22H25NO6 | ||
| 121.8 | 280.0 | Colchicine | 47.95 | C22H25NO6 |
| Colchicine, (+)- | 12.81 | C22H25NO6 | ||
| Pregn-4-ene-3,20-dione, 11-hydroxy-, (11α)- | 8.02 | C21H30O3 | ||
| 125.9 | 281.9 | Prednisolone acetate | 27.13 | C23H30O6 |
| Gamabufotalin | 18.07 | C24H34O5 | ||
| Beclomethasone | 13.84 | C28H37ClO7 | ||
| 134.0 | 298.0 | Prednisolone acetate | 48.90 | C23H30O6 |
| Colchicine | 17.86 | C22H25NO6 | ||
| Colchicine, (+)- | 4.27 | C22H25NO6 |
| Compound | Formula | Structure |
|---|---|---|
| Hexadecane | C16H34 | CH3(CH2)14CH3 |
| Octadecane | C18H38 | CH3(CH2)16CH3 |
| Heneicosane | C21H44 | CH3(CH2)19CH3 |
| Prednisolone acetate | C23H30O6 | 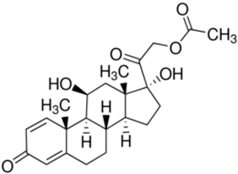 |
| Colchicine | C22H25NO6 | 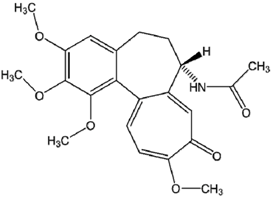 |
| Beclomethasone | C28H37ClO7 | 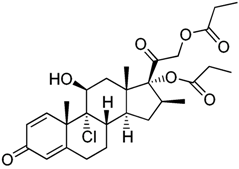 |
| Compound | Volume (mL) |
|---|---|
| O2 a | 79.5 |
| CO b | 16.2 |
| C1–C6 HCs | 1.74 |
| C1 HC | 0.19 |
| C2–C6 HCs | 1.55 |
| CO2 c | 41.4 |
| H2O d | 51.2 |
| Total | 190 |
| Total gases measured from flowmeter e | 183 |
| Item | Emission standards a (ppmv) | Maximum concentration through torrefaction (ppmv) |
|---|---|---|
| SO2 | 650 | 58.0 |
| NO2 | 250 | 67.5 b |
| CO | 2000 | 4555.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Chang, C.-C.; Chang, C.-Y.; Yuan, M.-H.; Ji, D.-R.; Ho, C.; Chiou, C.-S.; Shie, J.-L.; Chen, Y.-H.; Chang, W.-R.; et al. The By-products and Emissions from Manufacturing Torrefied Solid Fuel Using Waste Bamboo Chopsticks. Environments 2017, 4, 36. https://doi.org/10.3390/environments4020036
Chen Y-H, Chang C-C, Chang C-Y, Yuan M-H, Ji D-R, Ho C, Chiou C-S, Shie J-L, Chen Y-H, Chang W-R, et al. The By-products and Emissions from Manufacturing Torrefied Solid Fuel Using Waste Bamboo Chopsticks. Environments. 2017; 4(2):36. https://doi.org/10.3390/environments4020036
Chicago/Turabian StyleChen, Yen-Hau, Chia-Chi Chang, Ching-Yuan Chang, Min-Hao Yuan, Dar-Ren Ji, Chungfang Ho, Chyow-San Chiou, Je-Lueng Shie, Yi-Hung Chen, Wei-Ren Chang, and et al. 2017. "The By-products and Emissions from Manufacturing Torrefied Solid Fuel Using Waste Bamboo Chopsticks" Environments 4, no. 2: 36. https://doi.org/10.3390/environments4020036
APA StyleChen, Y.-H., Chang, C.-C., Chang, C.-Y., Yuan, M.-H., Ji, D.-R., Ho, C., Chiou, C.-S., Shie, J.-L., Chen, Y.-H., Chang, W.-R., Yang, T.-Y., Hsu, T.-C., Huang, M., Wu, C.-H., & Lin, F.-C. (2017). The By-products and Emissions from Manufacturing Torrefied Solid Fuel Using Waste Bamboo Chopsticks. Environments, 4(2), 36. https://doi.org/10.3390/environments4020036







