A New Method of Environmental Assessment and Monitoring of Cu, Zn, As, and Pb Pollution in Surface Soil Using Terricolous Fruticose Lichens
Abstract
:1. Introduction
2. Materials and Methods
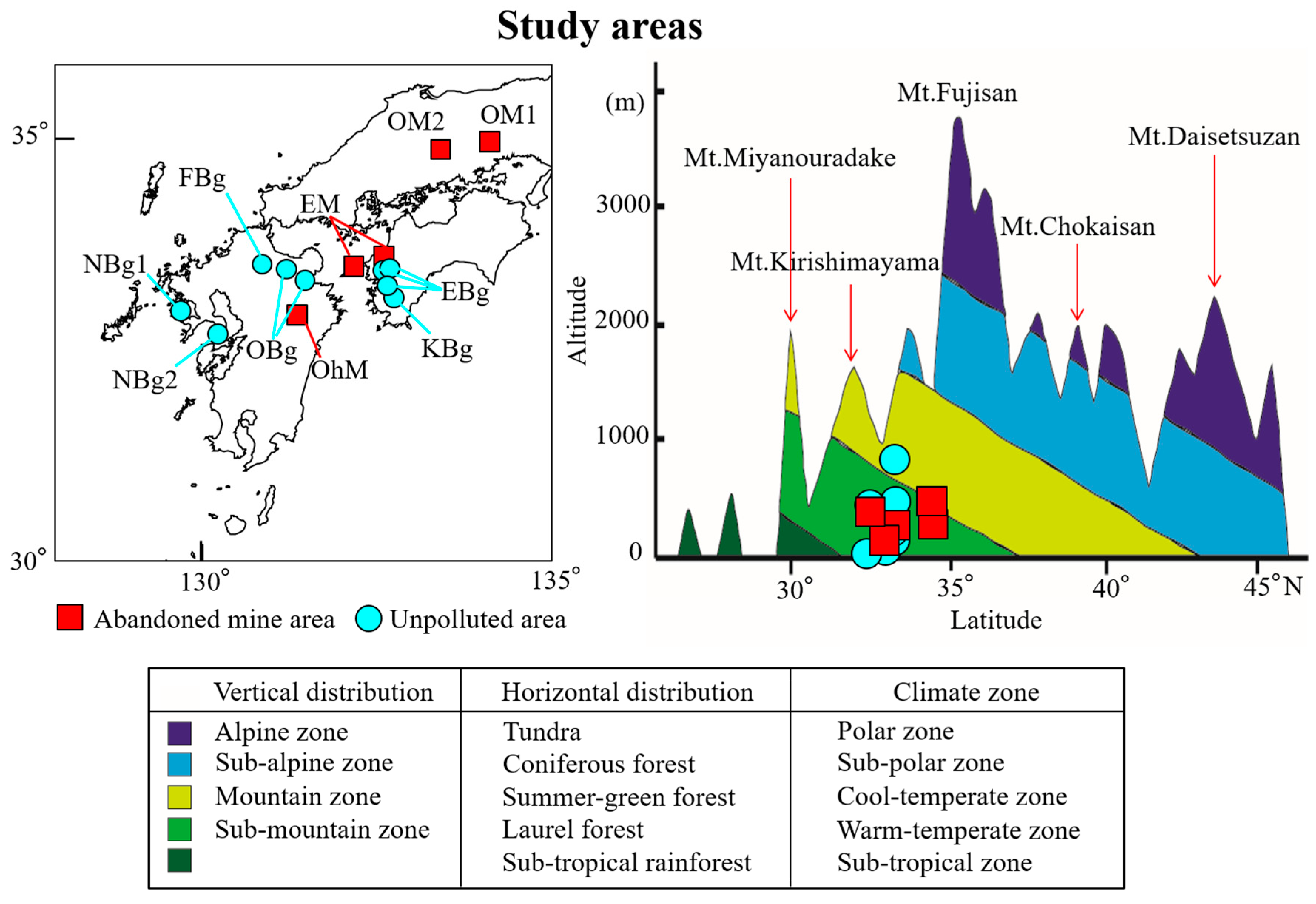
2.1. Study Area
2.2. Sampling and Pulverizing Methods
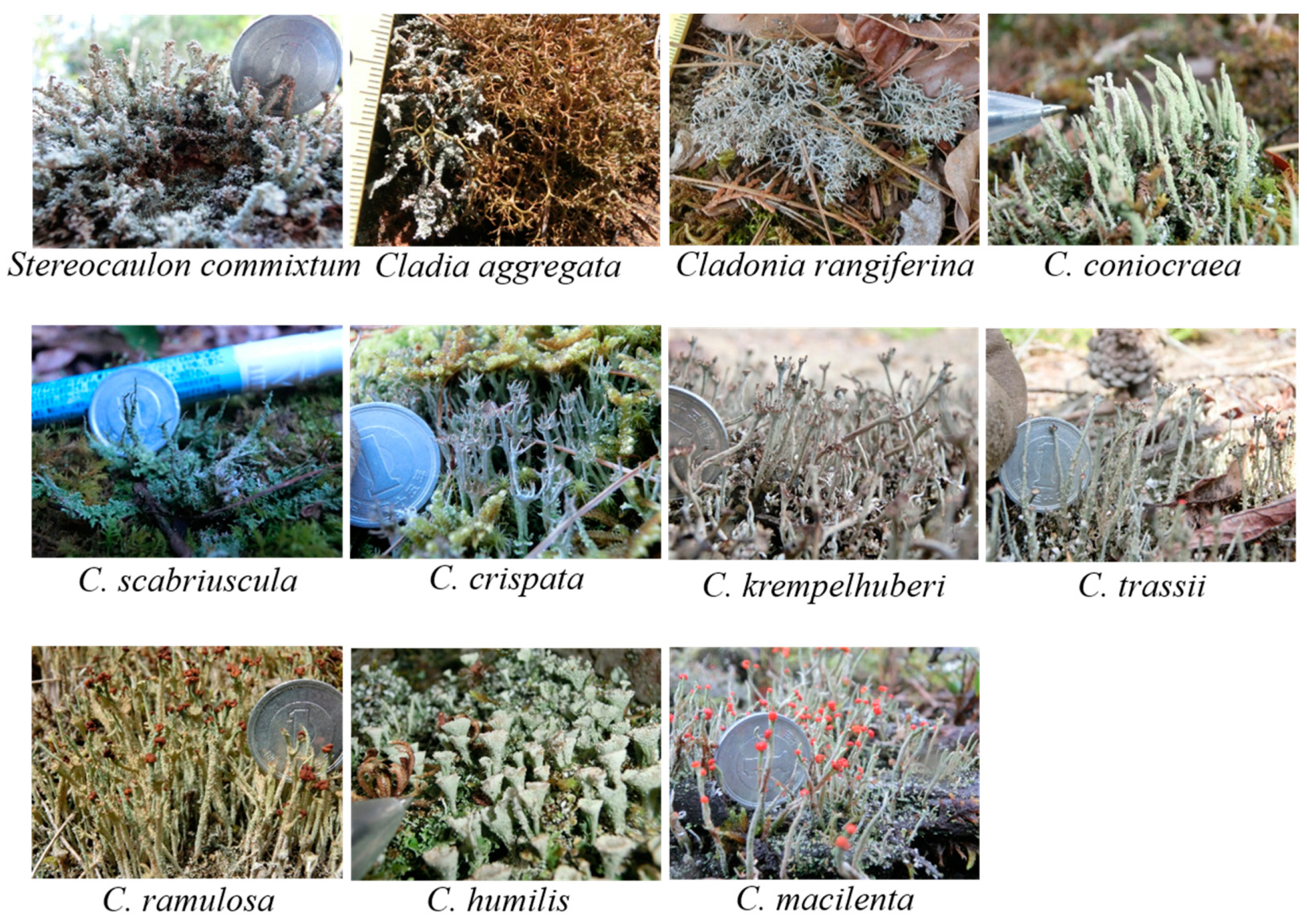
2.3. Identification of Lichens
2.4. Determining Concentrations of Trace Elements in Lichen Materials and Soils
2.4.1. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
2.4.2. X-ray Fluorescence (XRF)
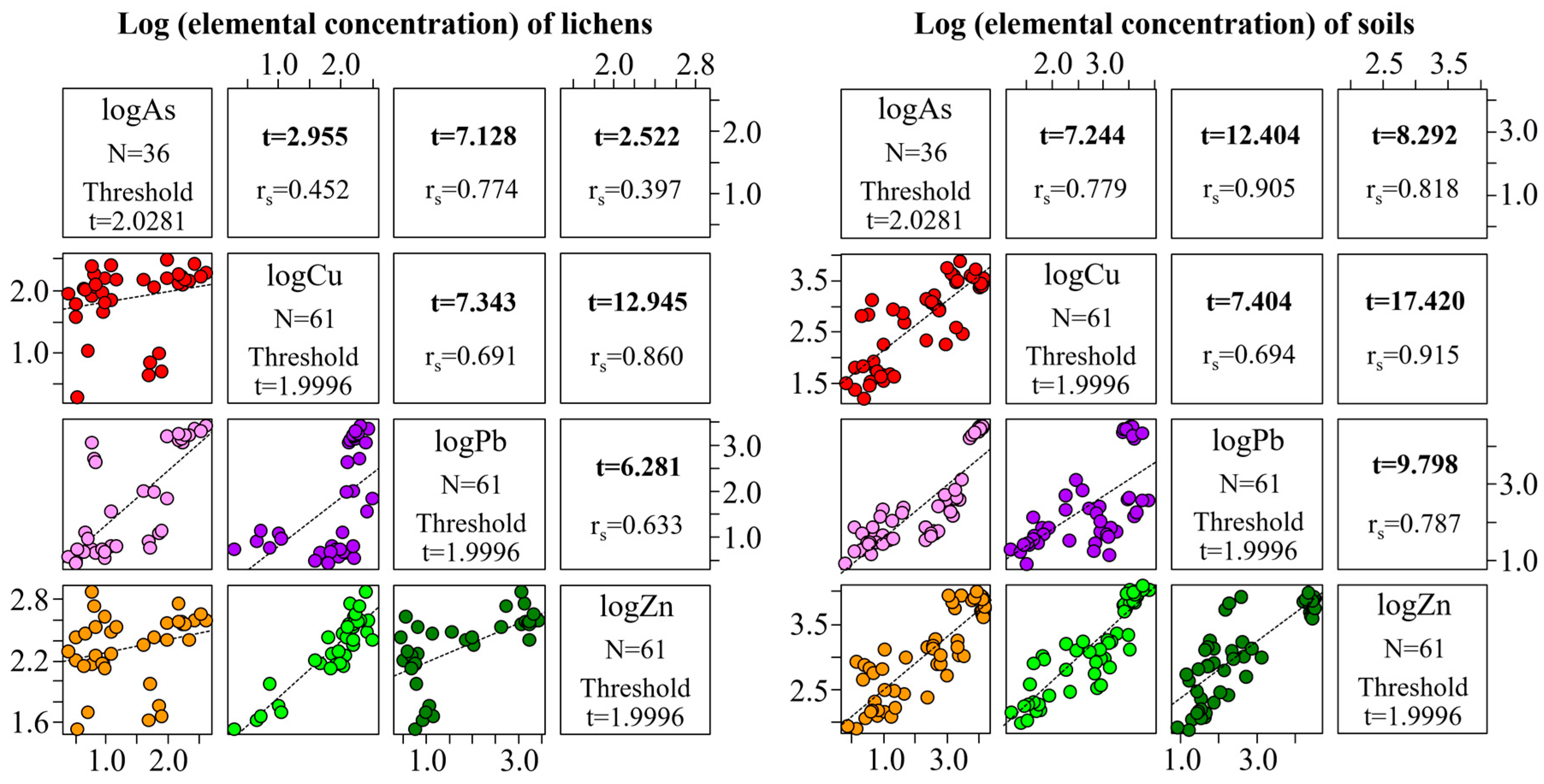
2.5. Statistical Analysis
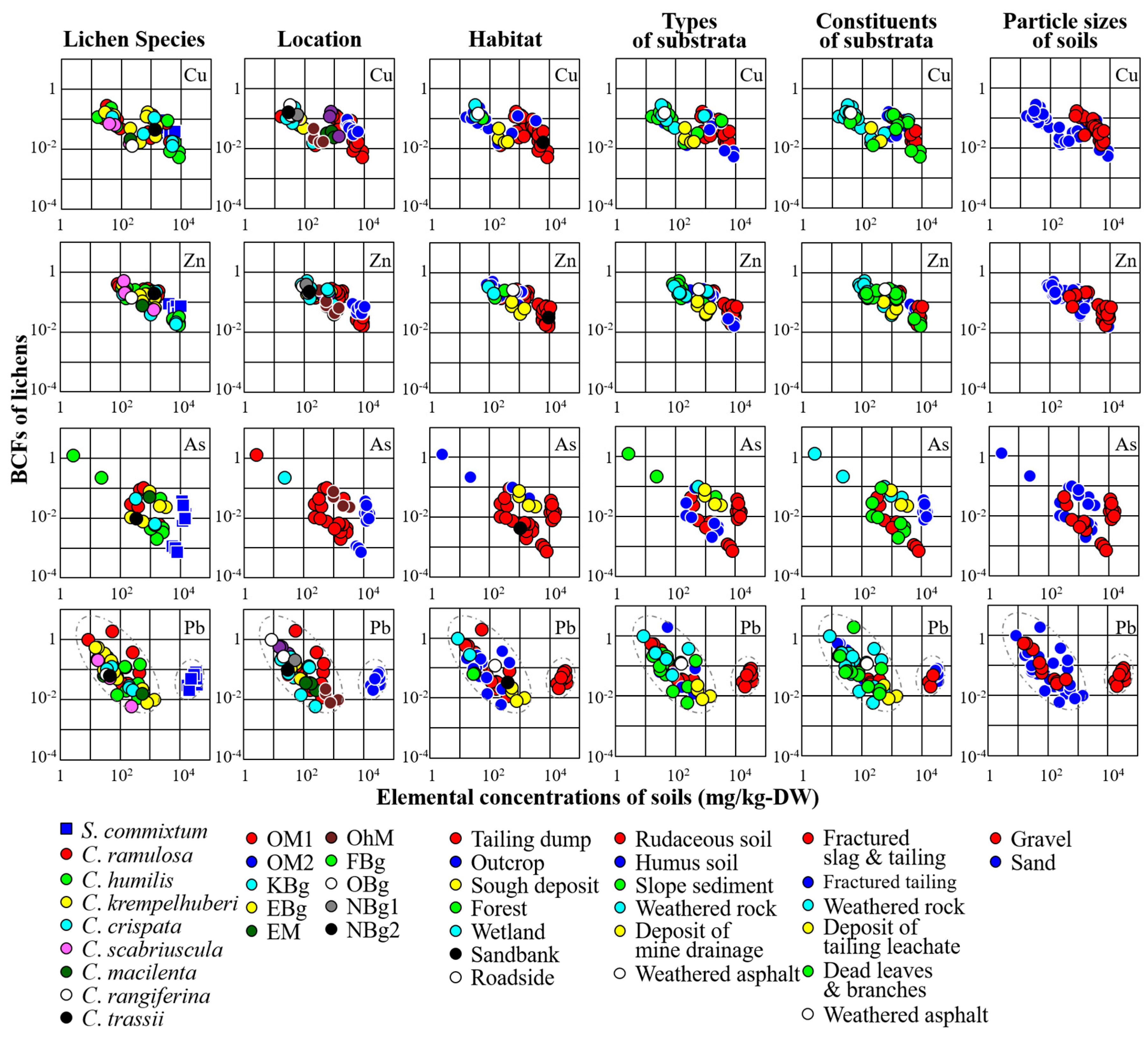
2.6. Bioconcentration Factor
3. Results
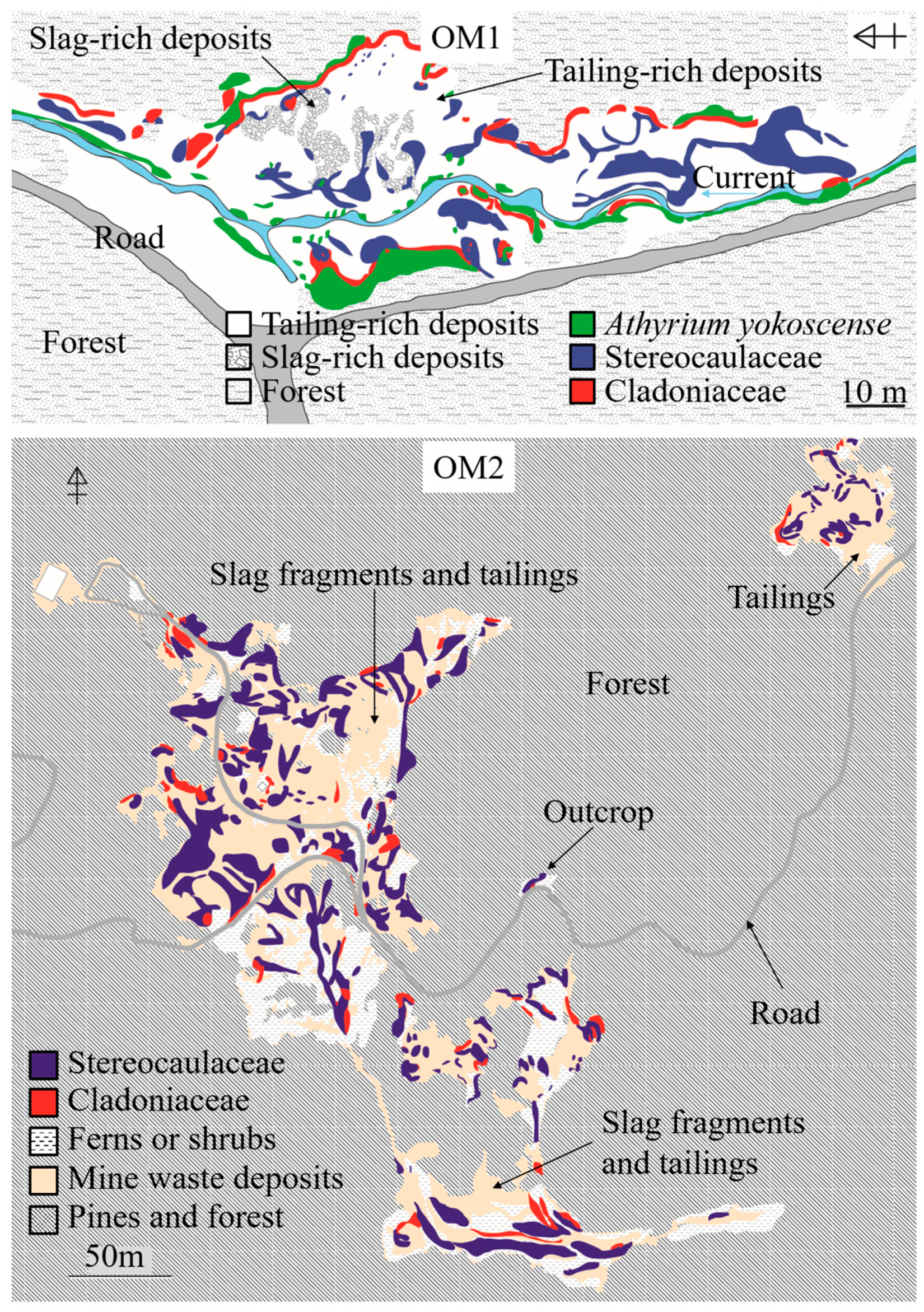
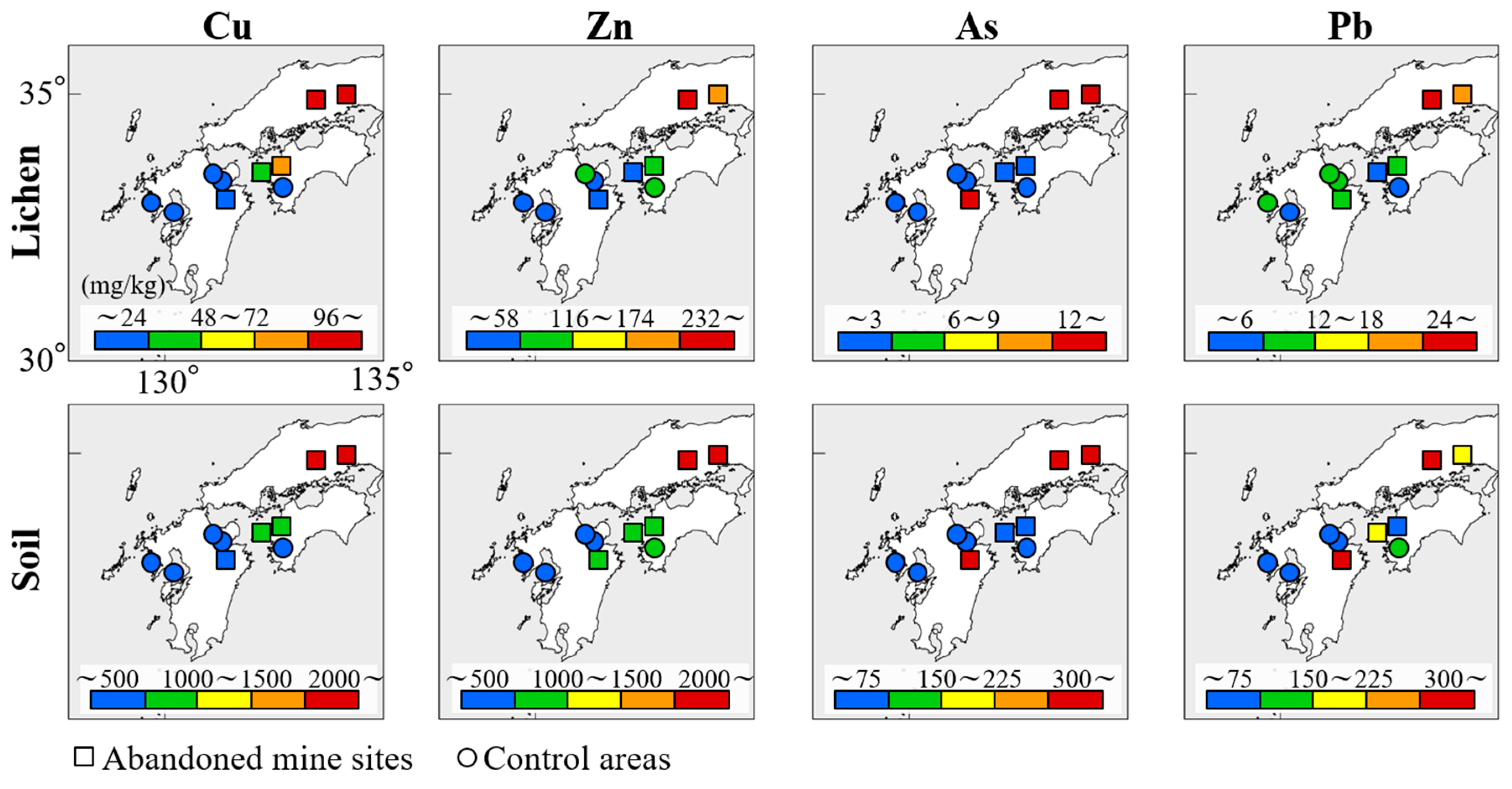
3.1. Distribution of Lichens
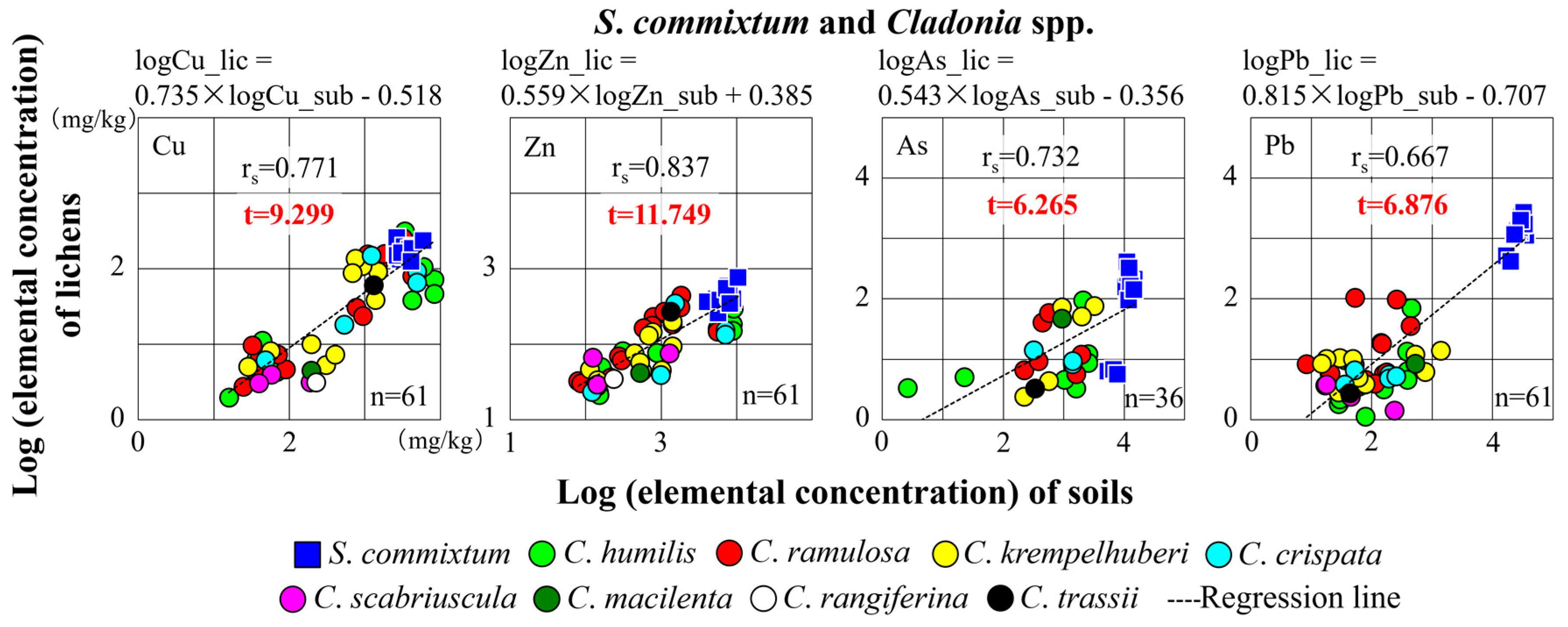
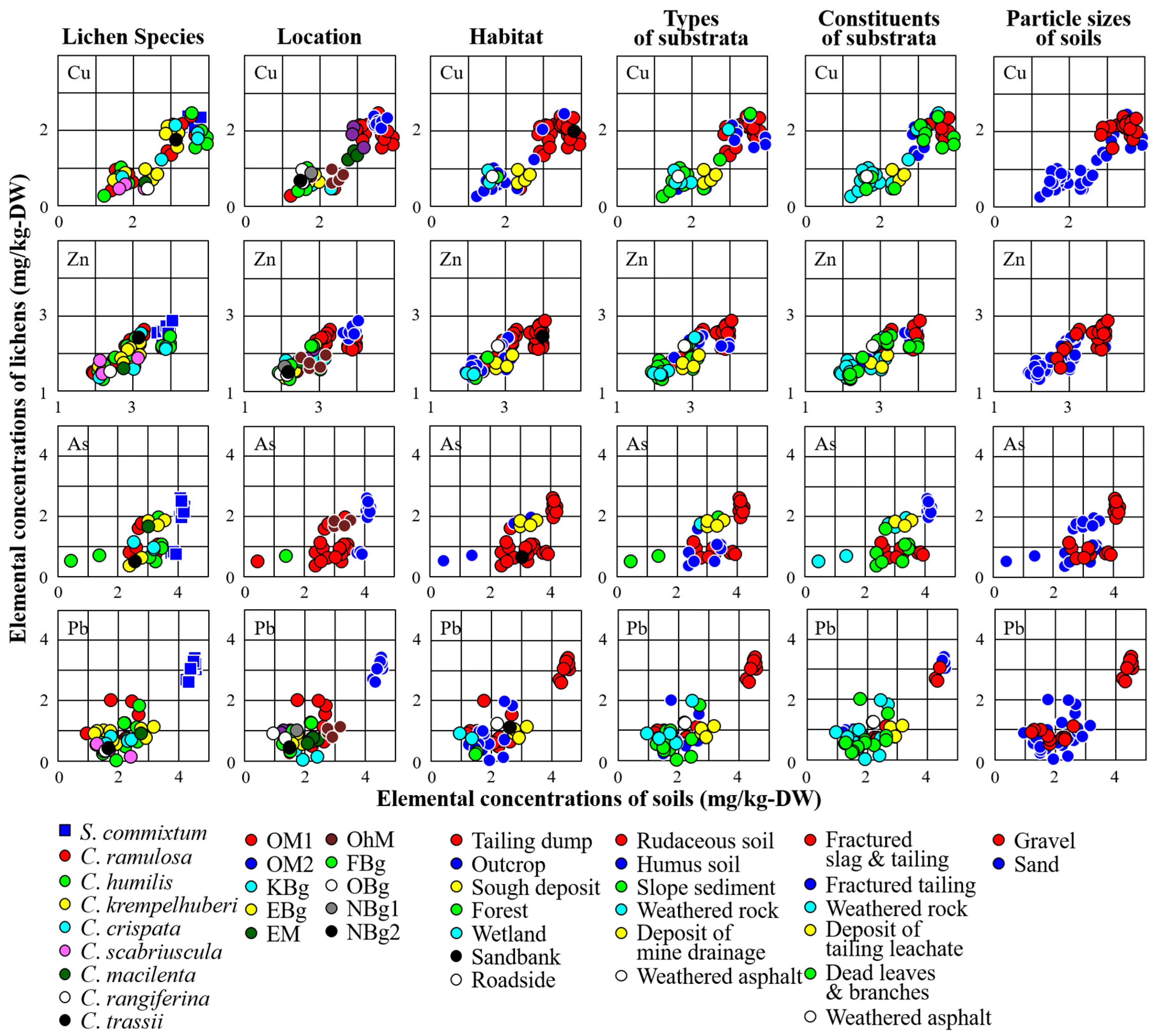
3.2. Relationships between Concentrations of Trace Elements in Lichens and the Corresponding Substrata
4. Discussion
4.1. Trace Element Absorption Properties of Lichens
4.2. Practical Application of Lichens as Biomonitors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TLC | Thin layer chromatography |
| LC/MS | Liquid chromatography-mass spectrometry |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| WD-XRF | Wave dispersive X-ray fluorescence spectrometry |
| BCF | Bioconcentration factor |
References
- Tiwari, M.J.; Bajpai, S.; Dewangan, U.K.; Tamrakar, R.K. Suitability of leaching test methods for fly ash and slag: A review. J. Radiat. Res. Appl. Sci. 2015, 8, 523–537. [Google Scholar] [CrossRef]
- Niemi, G.J.; McDonald, M.E. Application of ecological indicators. Annu. Rev. Ecol. Syst. 2004, 35, 89–111. [Google Scholar] [CrossRef]
- Graney, R.L., Jr.; Cherry, D.S.; Cairns, J., Jr. Heavy metal indicator potential of the Asiatic clam (Corbicula fluminea) in artificial stream systems. Hydrobiologia 1983, 102, 81–88. [Google Scholar] [CrossRef]
- Suter, G.W., II. Applicability of indicator monitoring to ecological risk assessment. Ecol. Indic. 2001, 1, 101–112. [Google Scholar] [CrossRef]
- Demirezen, D.; Aksoy, A. Common hydrophytes as bioindicators of iron and manganese pollutions. Ecol. Indic. 2006, 6, 388–393. [Google Scholar] [CrossRef]
- Birungi, Z.; Masola, B.; Zaranyika, M.F.; Naigaga, I.; Marshall, B. Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo wetland along Lake Victoria. Phys. Chem. Earth 2007, 32, 1350–1358. [Google Scholar]
- Epelde, L.; Becerril, J.M.; Hernández-Allica, J.; Barrutia, O.; Garbisu, C. Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Appl. Soil Ecol. 2008, 39, 299–310. [Google Scholar] [CrossRef]
- Hartley, W.; Uffindell, L.; Plumb, A.; Rawlinson, H.A.; Putwain, P.; Dickinson, N.M. Assessing biological indicators for remediated anthropogenic urban soils. Sci. Total Environ. 2008, 405, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryant, P.; Krystofova, O.; Adam, V.; Galiova, M.; Beklova, M.; Babula, P.; Kaiser, J.; Novotny, K.; Novotny, J.; et al. Multi-instrumental Analysis of Tissues of Sunflower Plants Treated with Silver (I) Ions—Plants as Bioindicators of Environmental Pollution. Sensors 2008, 8, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Yoon, I.H.; Kim, K.W. Heavy metal and arsenic accumulating fern species as potential ecological indicators in As-contaminated abandoned mines. Ecol. Indic. 2009, 9, 1275–1279. [Google Scholar] [CrossRef]
- María-Cervantes, A.; Jiménez-Cárceles, F.J.; Álvarez-Rogel, J. As, Cd, Cu, Mn, Pb, and Zn Contents in Sediments and Mollusks (Hexaplex trunculus and Tapes decussatus) from Coastal Zones of a Mediterranean Lagoon (Mar Menor, SE Spain) Affected by Mining Wastes. Water Air Soil Poll. 2009, 200, 289–304. [Google Scholar] [CrossRef]
- Moss, J.C.; Hardaway, C.J.; Richert, J.C.; Sneddon, J. Determination of cadmium copper, iron, nickel, lead and zinc in crawfish [Procambrus clarkii] by inductively coupled plasma optical emission spectrometry: A study over the 2009 season in Southwest Louisiana. Microchem. J. 2010, 95, 5–10. [Google Scholar] [CrossRef]
- Čeburnis, D.; Steinnes, E. Conifer needles as biomonitors of atmospheric heavy metal deposition: Comparison with mosses and precipitation, role of the canopy. Atmos. Environ. 2000, 34, 4265–4271. [Google Scholar] [CrossRef]
- Gerloff, G.C.; Stout, P.R.; Jones, L.H.P. Molybdenum-Manganese-Iron antagonisms in the nutrition of tomato plants. Plant Physiol. 1959, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Siedlecka, A. Some aspects of interactions between heavy metals and plant mineral nutrients. Acta Soc. Bot. Pol. 1995, 64, 265–272. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A. Epiphytic lichens as sentinels for heavy metal pollution at forest ecosystems (central Italy). Environ. Pollut. 2003, 121, 327–332. [Google Scholar] [CrossRef]
- Goyal, R.; Seaward, M.R.D. Metal uptake in terricolous lichens. III. Translocation in the thallus of Peltigera canina. New Phytol. 1982, 90, 58–98. [Google Scholar]
- Knops, J.M.H.; Nash III, T.H.; Boucher, V.L.; Schlesinger, W.L. Mineral cycling and epiphytic lichens: Implications at the ecosystem level. Lichenologist 1991, 23, 309–321. [Google Scholar] [CrossRef]
- Nash III, T.H. Nutrients, elemental accumulation, and mineral cycling. In Lichen Biology, 2nd ed.; Nash III, T.H., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 234–251. [Google Scholar]
- Nieboer, E.; Richardson, D.H.S.; Tomassini, F.D. Mineral uptake and release by lichens: An overview. Bryologist 1978, 81, 226–246. [Google Scholar] [CrossRef]
- Crittenden, P.D. Nutrient exchange in an Antarctic macrolichen during summer snowfall snow melt events. New Phytol. 1998, 139, 697–707. [Google Scholar] [CrossRef]
- Purvis, O.W.; Pawlik-Skowronska, B.; Cressey, G.; Jones, G.C.; Kearsley, A.; Spratt, J. Mineral phases and element composition of the copper hyperaccumulator lichen Lecanora polytropa. Mineral. Mag. 2008, 72, 607–616. [Google Scholar] [CrossRef]
- Purvis, O.W.; Bennett, J.P.; Spratt, J. Copper localization, elemental content, and thallus color in the copper hyperaccumulator lichen Lecanora sierrae from California. Lichenologist 2011, 43, 165–173. [Google Scholar] [CrossRef]
- Sueoka, Y.; Sakakibara, M.; Sera, K. Heavy Metal Behavior in Lichen-Mine Waste Interactions at an Abandoned Mine Site in Southwest Japan. Metals 2015, 5, 1591–1608. [Google Scholar] [CrossRef]
- Osyczka, P.; Rola, K. Response of the lichen Cladonia rei Schaer. To strong heavy metal contamination of the substrate. Environ. Sci. Pollut. Res. Int. 2013, 20, 5076–5084. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Moody, C.D.; Jorge Villar, S.E.; Wynn-Williams, D.D. Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbiosis in extreme Antarctic habitats: Evaluation for Mars Lander missions. Icarus 2005, 174, 560–571. [Google Scholar] [CrossRef]
- Bačkor, M.; Loppi, S. Interactions of lichens with heavy metals. Biol. Plant. 2009, 53, 214–222. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Isocrono, D.; Piervittori, R. Lichens and ultramafic rocks: A review. Lichenologist 2004, 36, 391–404. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Knudsen, K.; Fryday, A.M.; O’Dell, R.E.; Pore, N.; Olday, F.C.; Woolhouse, S. Investigation of the importance of rock chemistry for saxicolous lichen communities of the New Idria serpentinite mass, San Benito County, California, USA. Lichenologist 2012, 44, 695–714. [Google Scholar] [CrossRef]
- Nakajima, H.; Fujimoto, K.; Yoshitani, A.; Yamamoto, Y.; Sakurai, H.; Itoh, K. Effect of copper stress on cup lichens Cladonia. humilis and C. subconistea growing on copper-hyperaccumulating moss Scopelophila cataractae at copper-polluted sites in Japan. Ecotoxicol. Environ. Saf. 2012, 84, 341–346. [Google Scholar] [PubMed]
- Rajakaruna, N.; Harris, T.B.; Clayden, S.R.; Dibble, A.C.; Olday, F.C. Lichens of the Callahan Mine, a copper- and zinc-enriched superfund site in Brooksville, Maine, U.S.A. Rhodora 2011, 113, 1–31. [Google Scholar] [CrossRef]
- Medeiros, I.D.; Fryday, A.M.; Rajakaruna, N. Additional lichen records and mineralogical data from metal-contaminated sites in Maine. Rhodora 2014, 116, 323–347. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin, Germany, 1996. [Google Scholar]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1996 Compilation of analytical data on nine GSJ geochemical reference samples, “Sedimentary Rock Series”. Geostand. Geoanal. Res. 1996, 20, 165–216. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1998 Compilation of analytical data for five GSJ geochemical reference samples: The “Instrumental Analysis Series”. Geostand. Geoanal. Res. 1998, 23, 223–250. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; DeForest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse relationship between bioconcentration factor and exposure concentration for metals: Implications for hazard assessment of metals in the aquatic environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Nishizono, H.; Suzuki, S.; Ishii, F. Accumulation of heavy metals in the metal-tolerant fern, Athyrium yokoscense, growing on various environments. Plant Soil 1987, 102, 65–70. [Google Scholar] [CrossRef]
- Morishita, T.; Boratyski, J.K. Accumulation of Cadmium and Other Metals in Organs of Plants Growing around Metal Smelters in Japan. Soil Sci. Plant Nutr. 1992, 38, 781–785. [Google Scholar] [CrossRef]
- Yoshihara, T.; Tsunokawa, K.; Miyano, Y.; Arashima, Y.; Hodoshima, H.; Shoji, K.; Shimada, H.; Goto, F. Induction of callus from a metal hypertolerant fern, Athyrium yokoscense, and evaluation of its cadmium tolerance and accumulation capacity. Plant Cell Rep. 2005, 23, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, H.; Komori, I.; Tamura, H.; Sawa, Y.; Karahara, I.; Honma, Y.; Wada, N.; Kawabata, T.; Matsuda, K.; Ikeno, S.; et al. Lead tolerance and accumulation in the gametophytes of the fern Athyrium yokoscense. J. Plant Res. 2005, 118, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term “heavy metals” byabiologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Nakajima, H.; Yamamoto, Y.; Yoshitani, A.; Itoh, K. Effect of metal stress on photosynthetic pigments in the Cu-hyperaccumulating lichens Cladonia humilis and Stereocaulon. japonicum growing in Cu-polluted sites in Japan. Ecotoxicol. Environ. Saf. 2013, 97, 154–159. [Google Scholar] [PubMed]
- Nakajima, H.; Hara, K.; Yamamoto, Y.; Itoh, K. Effects of Cu on the content of chlorophylls and secondary metabolites in the Cu-hyperaccumulator lichen Stereocaulon japonicum. Ecotoxicol. Environ. Saf. 2015, 113, 447–482. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, P.; Rola, K.; Jankowska, K. Vertical concentration gradients of heavy metals in Cladonia lichens across different parts of thalli. Ecol. Indic. 2016, 61, 766–776. [Google Scholar] [CrossRef]
- Beckett, R.P.; Brown, D.H. The control of cadmium uptake in the lichen genus Peltigera. J. Exp. Bot. 1984, 35, 1071–1082. [Google Scholar] [CrossRef]
- Beckett, R.P.; Brown, D.H. The relationship between cadmium uptake and heavy metal uptake tolerance in the lichen genus Peltigera. New Phytol. 1984, 97, 301–311. [Google Scholar] [CrossRef]
- Banfield, J.F.; Baker, W.W.; Welch, S.A.; Taunton, A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sueoka, Y.; Sakakibara, M.; Sano, S.; Yamamoto, Y. A New Method of Environmental Assessment and Monitoring of Cu, Zn, As, and Pb Pollution in Surface Soil Using Terricolous Fruticose Lichens. Environments 2016, 3, 35. https://doi.org/10.3390/environments3040035
Sueoka Y, Sakakibara M, Sano S, Yamamoto Y. A New Method of Environmental Assessment and Monitoring of Cu, Zn, As, and Pb Pollution in Surface Soil Using Terricolous Fruticose Lichens. Environments. 2016; 3(4):35. https://doi.org/10.3390/environments3040035
Chicago/Turabian StyleSueoka, Yuri, Masayuki Sakakibara, Sakae Sano, and Yoshikazu Yamamoto. 2016. "A New Method of Environmental Assessment and Monitoring of Cu, Zn, As, and Pb Pollution in Surface Soil Using Terricolous Fruticose Lichens" Environments 3, no. 4: 35. https://doi.org/10.3390/environments3040035
APA StyleSueoka, Y., Sakakibara, M., Sano, S., & Yamamoto, Y. (2016). A New Method of Environmental Assessment and Monitoring of Cu, Zn, As, and Pb Pollution in Surface Soil Using Terricolous Fruticose Lichens. Environments, 3(4), 35. https://doi.org/10.3390/environments3040035






