Changes in Microbial Communities After Lettuce Cultivation in Sihwa Reclaimed Soils, Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Analysis of Soil Samples
2.3. Lettuce Used in the Experiment, Cultivation Conditions, and Growth Investigation Method
2.4. Soil DNA Extration and Next Generation Sequencing
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of Sihwa Reclaimed Soil
3.2. Lettuce Growth Results
3.3. Changes in Microbial Communities Before and After Lettuce Cultivation
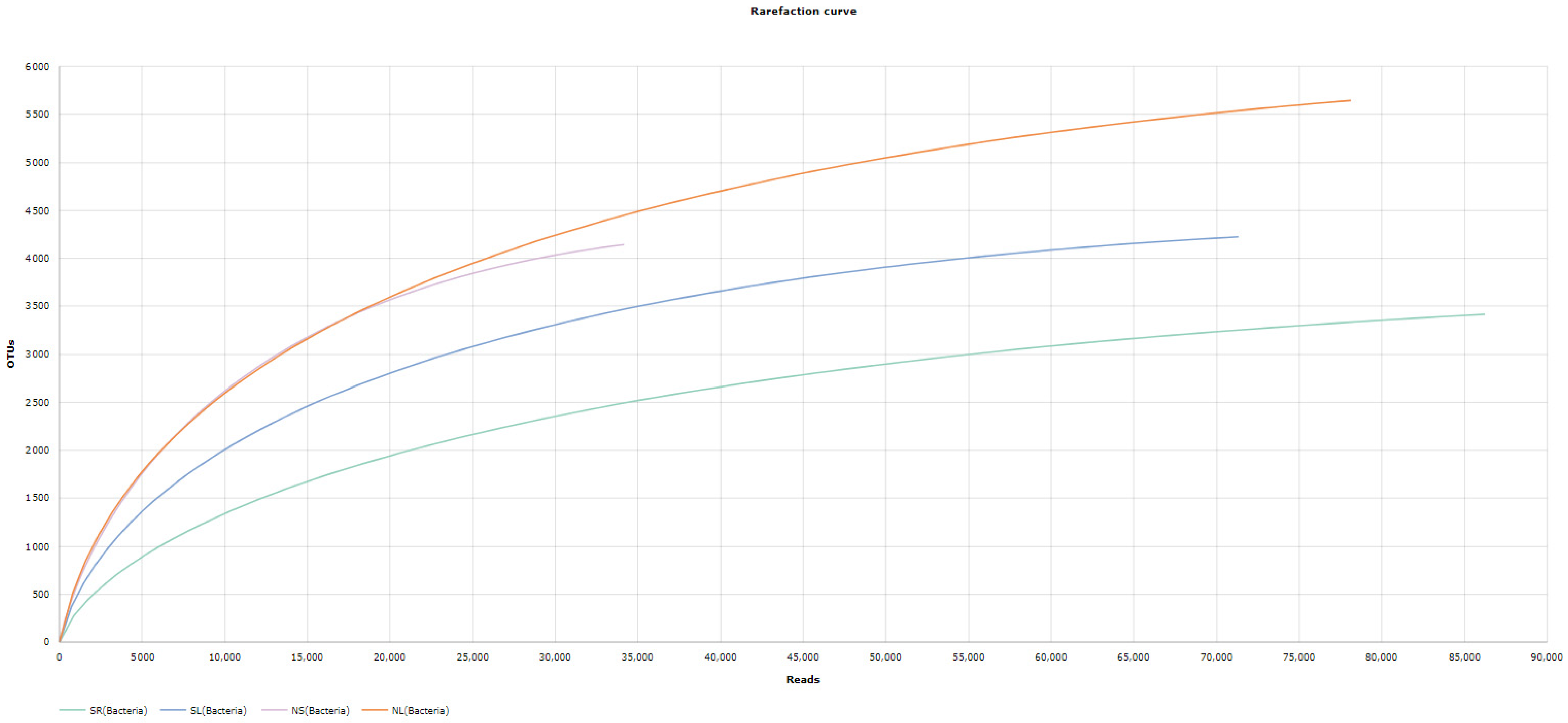
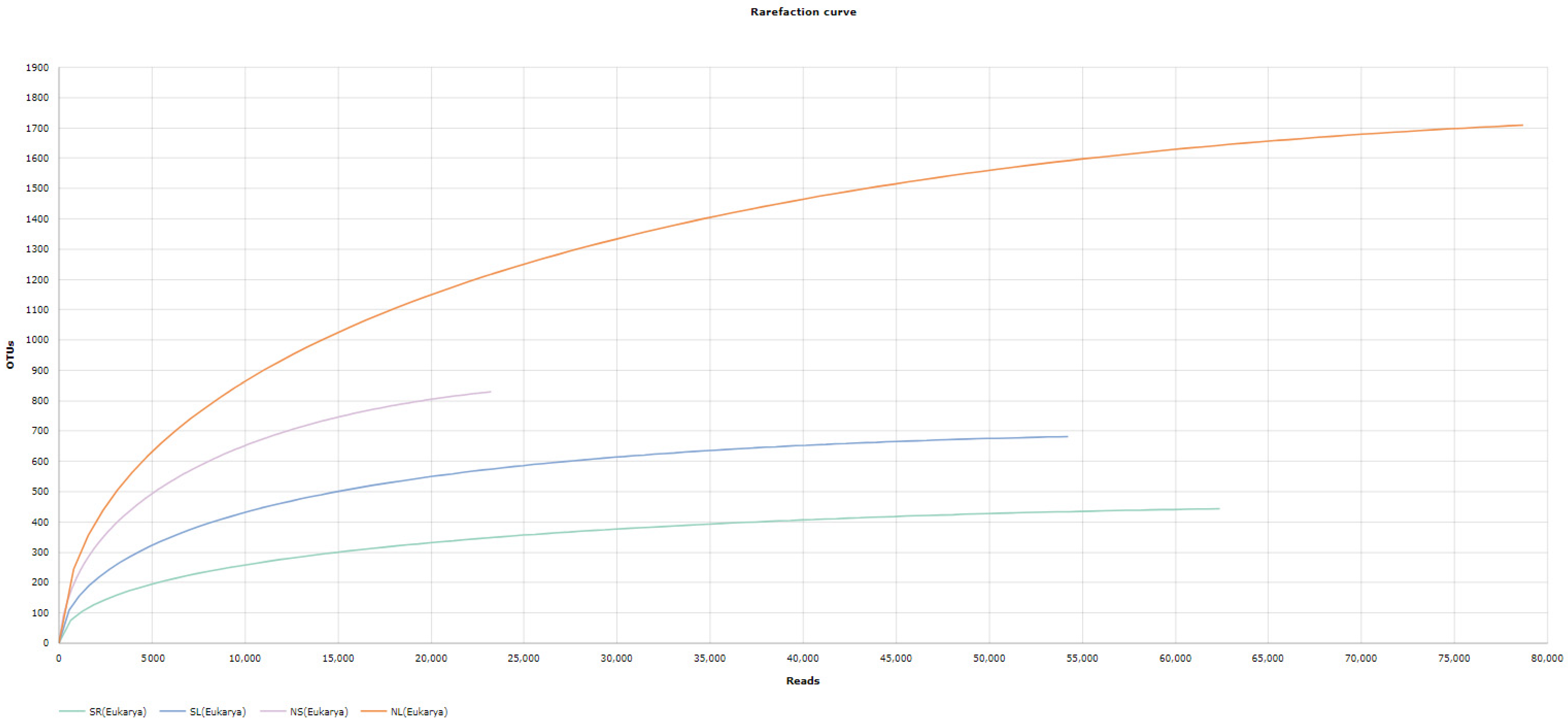
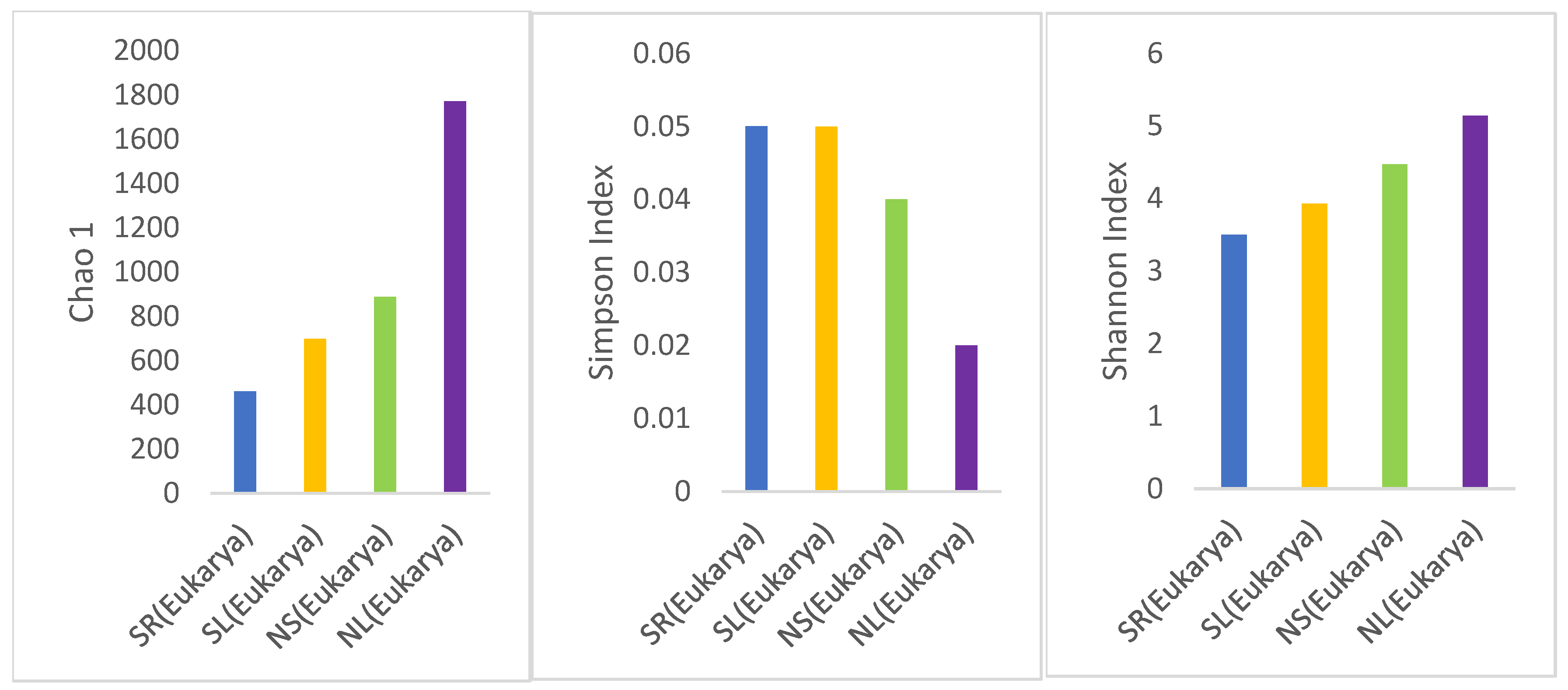
3.3.1. Discovered OTUs
Bacterial and Eukaryotes OTU
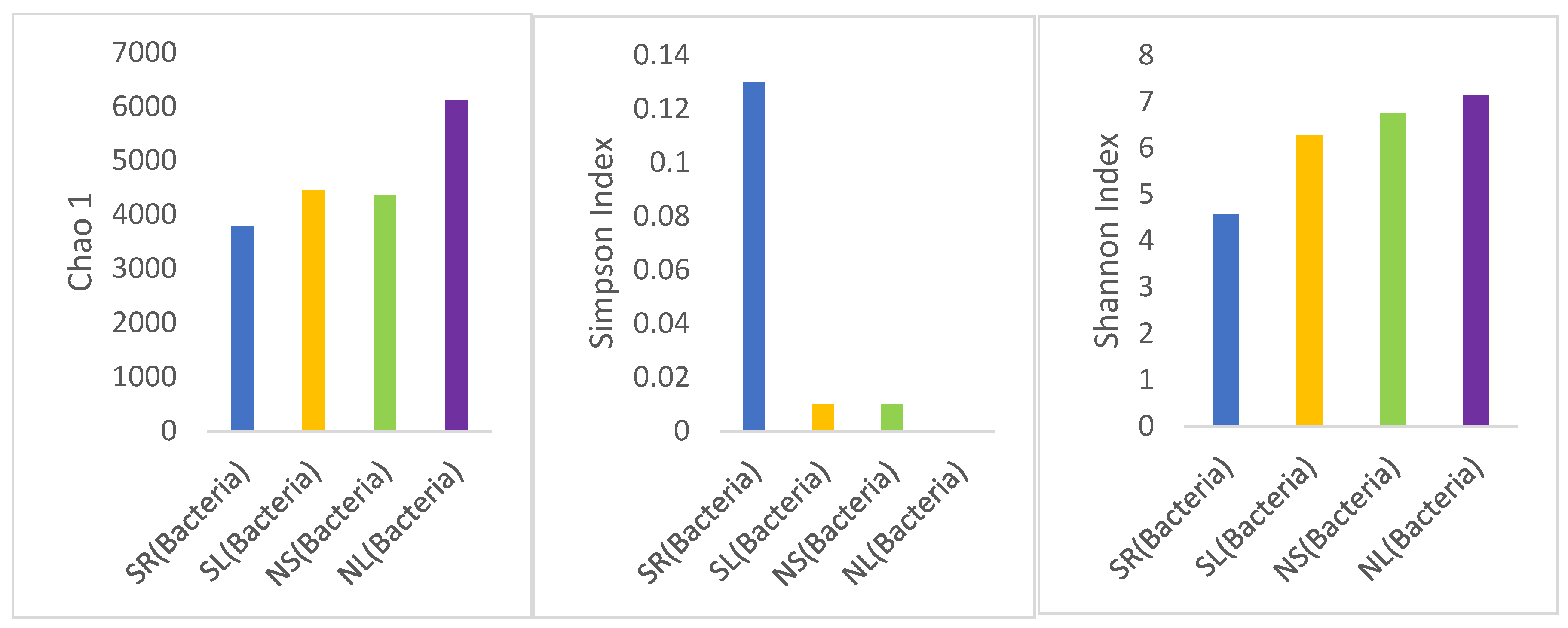
Alpha Diversity Analysis
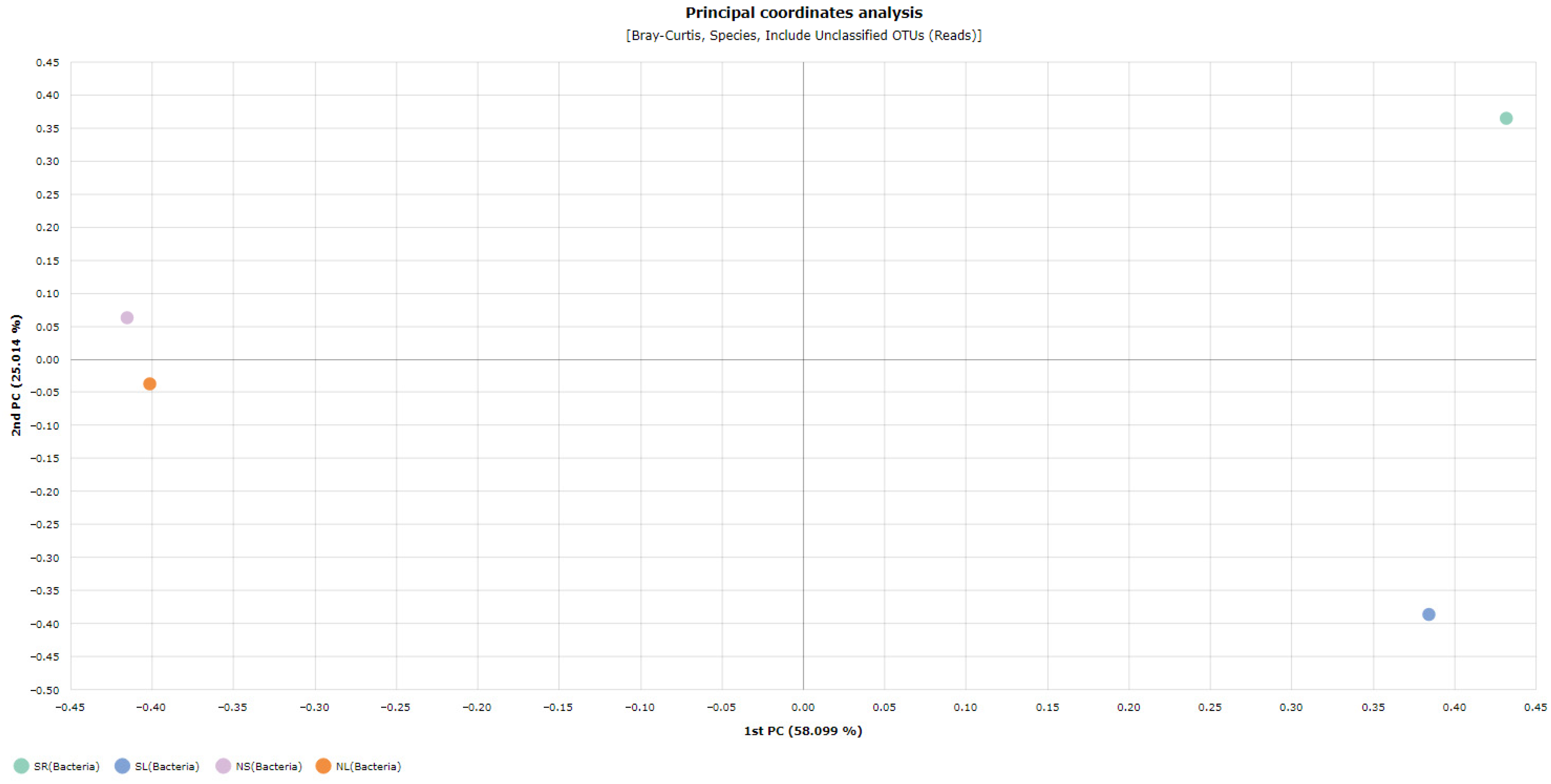
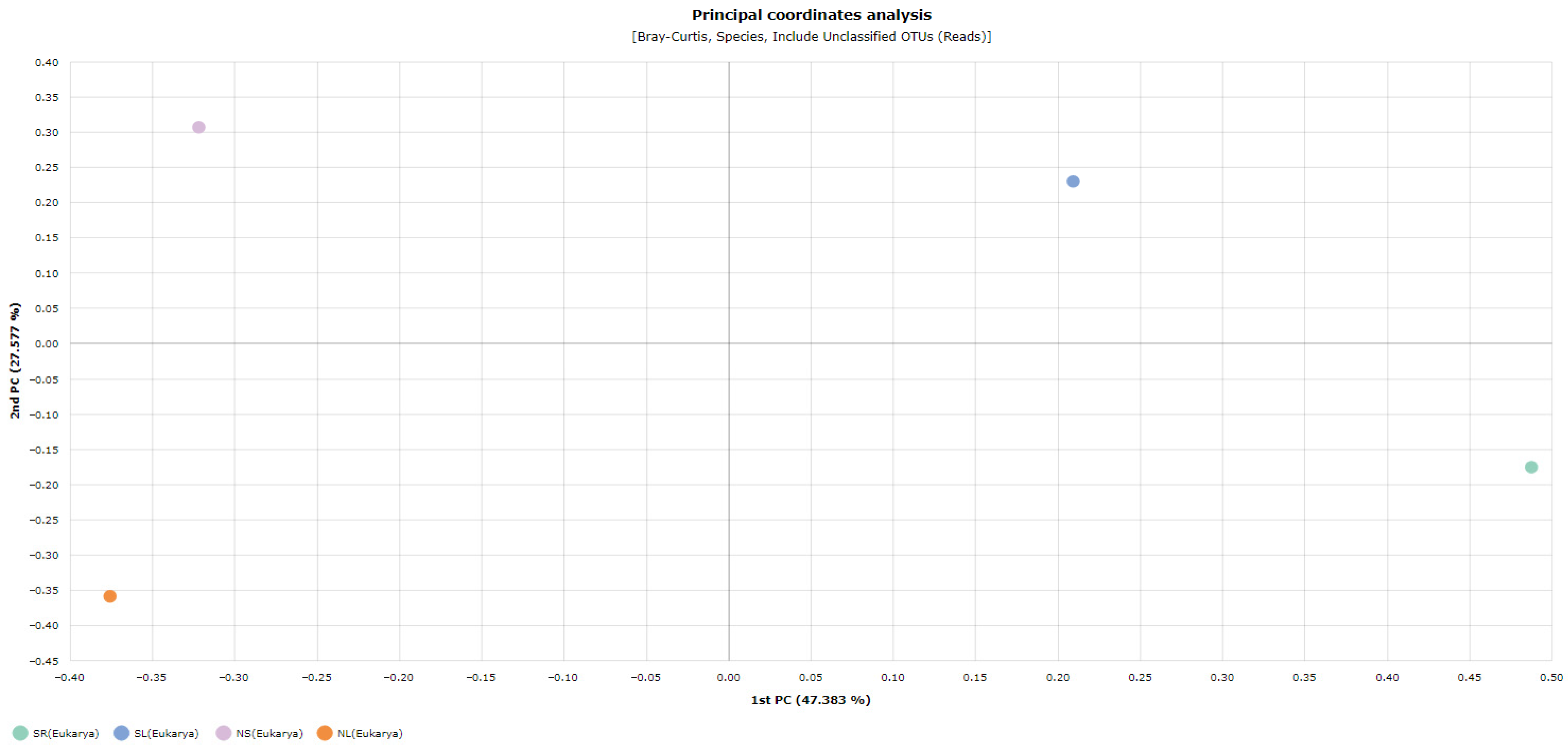
Beta Diversity Analysis
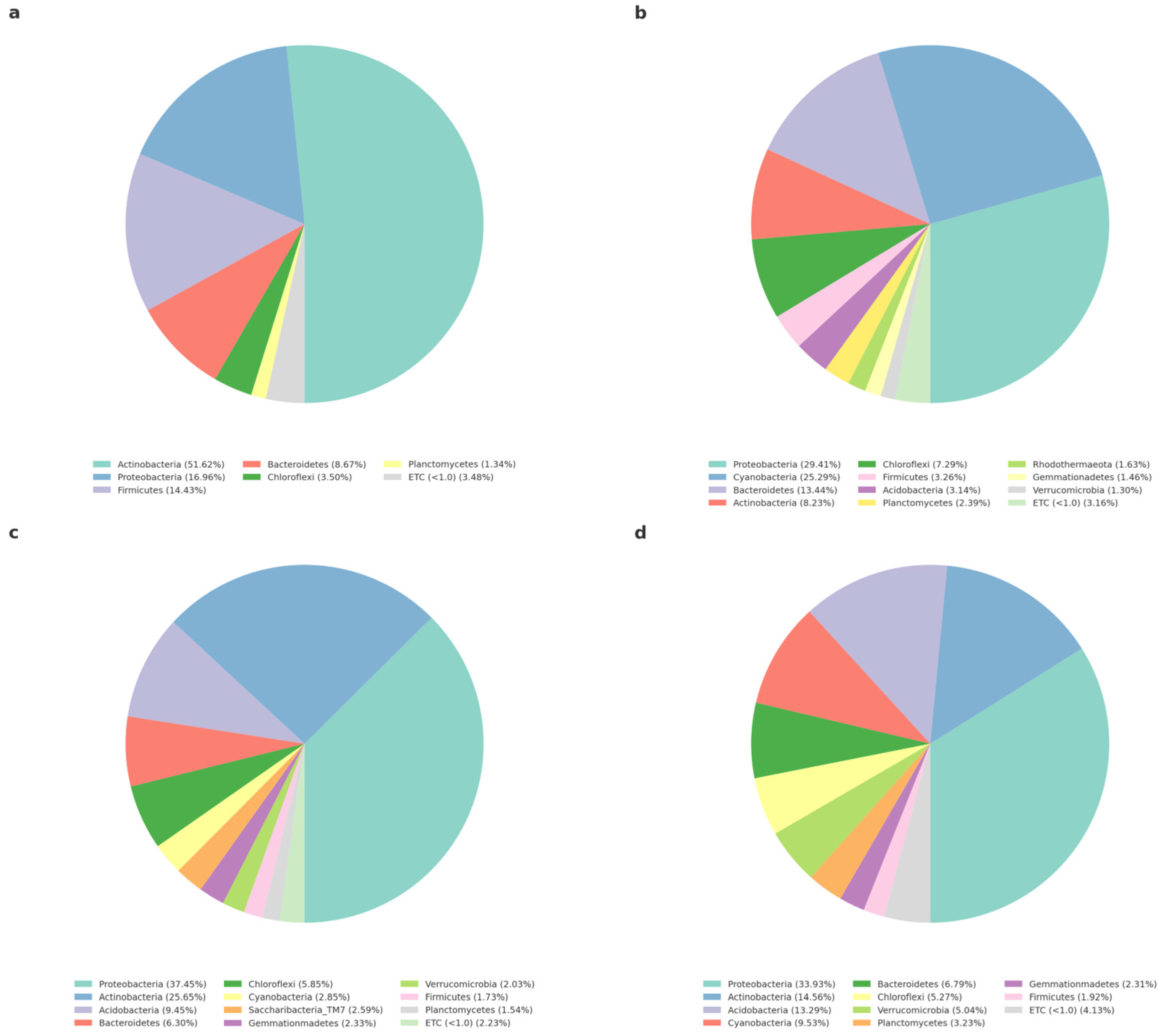
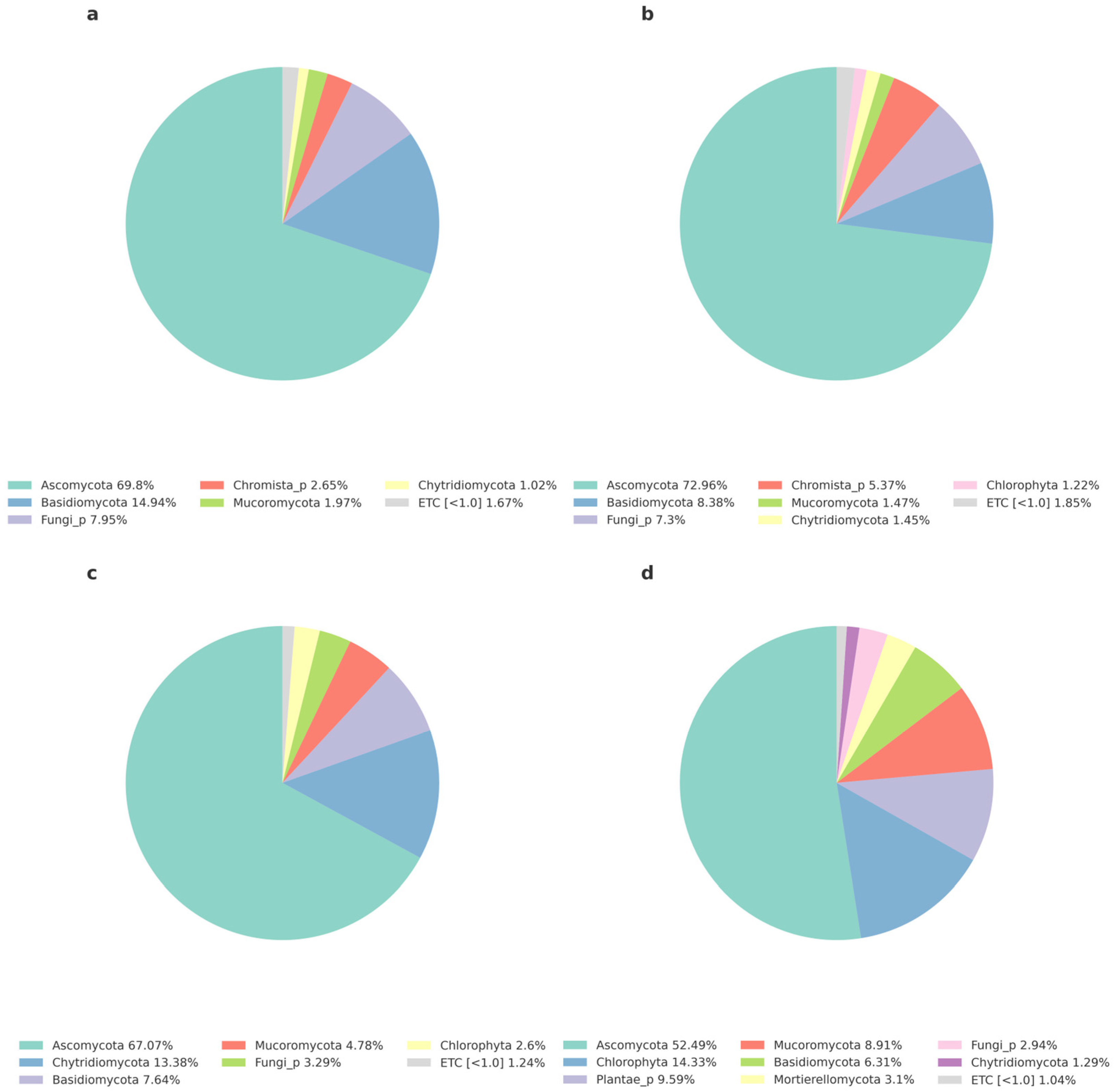
3.3.2. Analysis of Bacteria Community and Eukaryotes Community at the Phylum Level
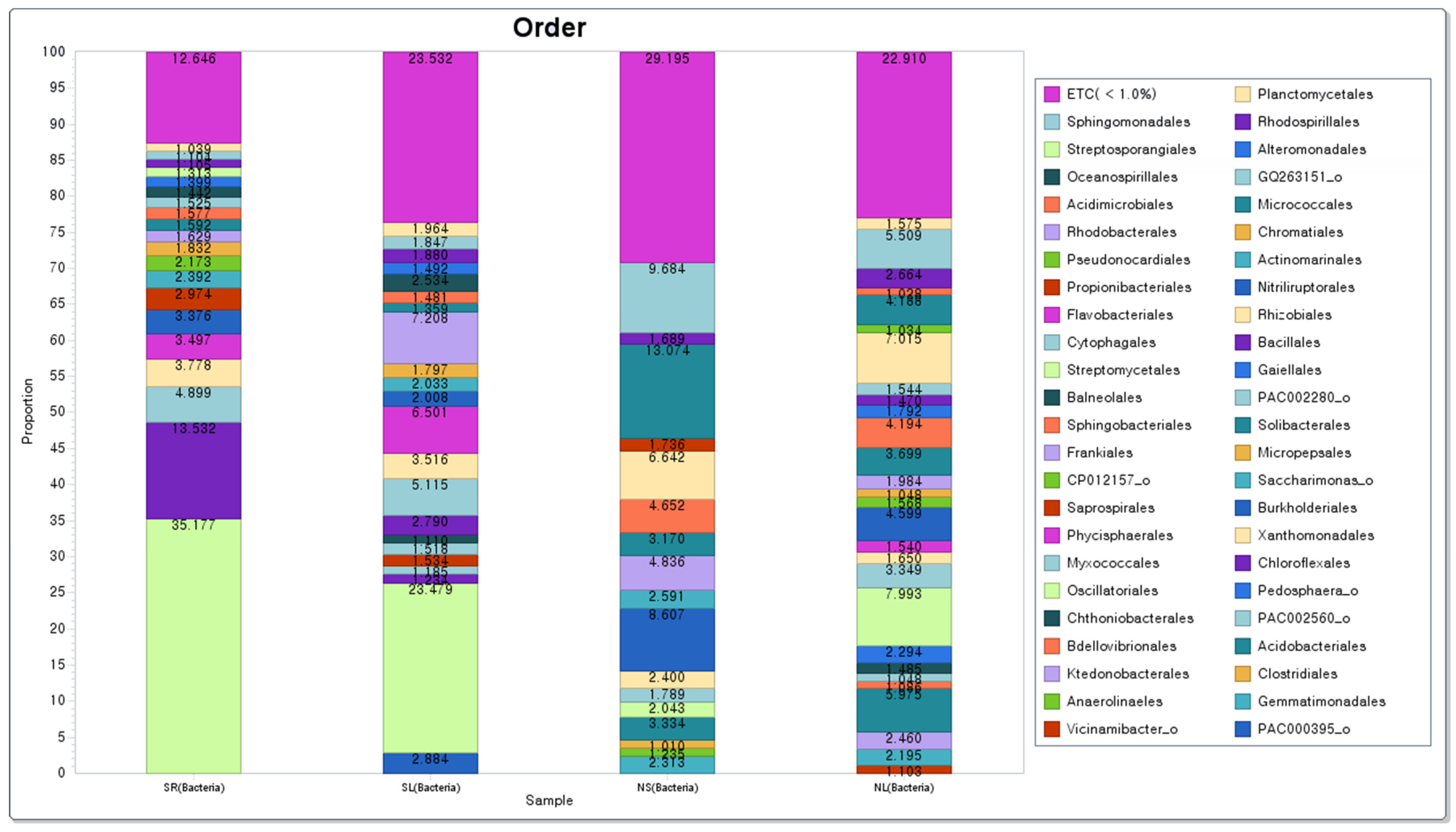
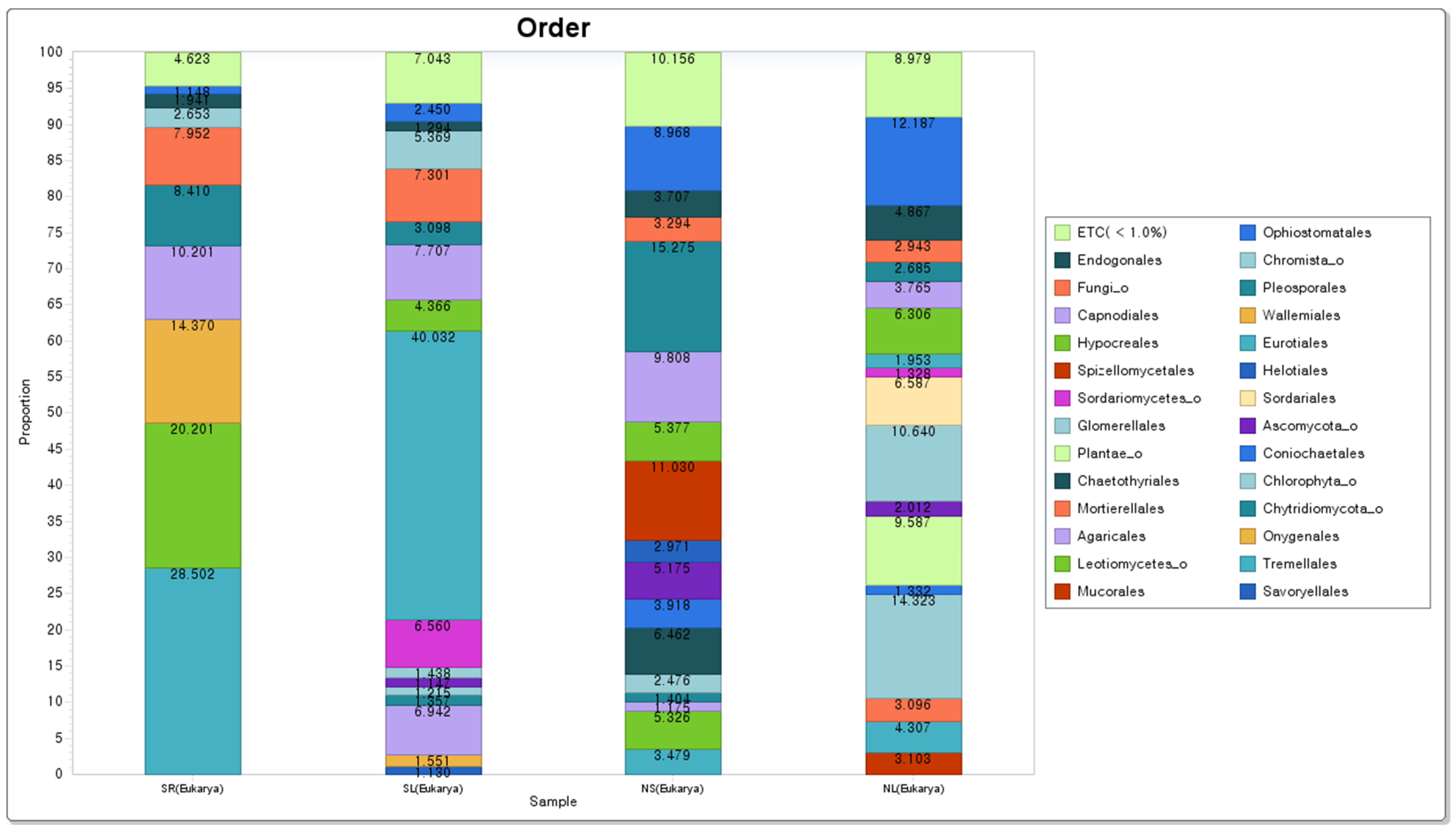
3.3.3. Analysis of Bacteria Community and Eukaryotes Community at Order Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schreiter, S.; Ding, G.C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, B.Y.; Chang, W.K.; Hong, S.; Song, S.J.; Park, J.; Kwon, B.O.; Khim, J.S. Environmental and ecological effects of Lake Shihwa reclamation project in South Korea: A review. Ocean. Coast. Manag. 2014, 102, 545–558. [Google Scholar] [CrossRef]
- Choi, Y.R. Modernization, development and underdevelopment: Reclamation of Korean tidal flats, 1950s–2000s. Ocean. Coast. Manag. 2014, 102, 426–436. [Google Scholar] [CrossRef]
- Chen, Y.; Li, G.; Cui, L.; Li, L.; He, L.; Ma, P. The effects of tidal flat reclamation on the stability of the coastal area in the Jiangsu Province, China, from the perspective of landscape structure. Land 2022, 11, 421. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Jiang, L.; Li, E.; Yang, X.; Yang, J. Salinity affects microbial function genes related to nutrient cycling in arid regions. Front. Microbiol. 2024, 15, 1407760. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, S.H.; Seol, S.I.; An, Y.; Jung, Y.S.; Lee, S.M. Assessment of salt damage for upland-crops in Dae-Ho reclaimed soil. Korean J. Environ. Agric. 2000, 19, 358–363. [Google Scholar]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Almaraz, M.; Foster, M.A.; Hill, A.F.; Huber, D.P.; King, E.K.; Langford, H.; Lowe, M.A.; Mickan, B.S.; Miller, V.S.; et al. Soil salinity and pH drive soil bacterial community composition and diversity along a lateritic slope in the Avon River critical zone observatory, Western Australia. Front. Microbiol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, J.; Li, X.; Zhou, L.; Luo, H.; Wang, L.; Zhang, Y.; Chou, M.; Wei, G. Multiple metabolic phenotypes as screening criteria are correlated with the plant growth-promoting ability of rhizobacterial isolates. Front. Microbiol. 2022, 12, 747982. [Google Scholar] [CrossRef]
- Nazari, M.T.; Schommer, V.A.; Braun, J.C.A.; dos Santos, L.F.; Lopes, S.T.; Simon, V.; Machado, B.S.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Using Streptomyces spp. as plant growth promoters and biocontrol agents. Rhizosphere 2023, 27, 100741. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Saleem, S.; Iqbal, A.; Ahmed, F.; Ahmad, M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021, 28, 5317–5324. [Google Scholar] [CrossRef]
- Windisch, S.; Sommermann, L.; Babin, D.; Chowdhury, S.P.; Grosch, R.; Moradtalab, N.; Walker, F.; Höglinger, B.; El-Hasan, A.; Armbruster, W.; et al. Impact of long-term organic and mineral fertilization on rhizosphere metabolites, root–microbial interactions and plant health of lettuce. Front. Microbiol. 2021, 11, 597745. [Google Scholar] [CrossRef]
- Neumann, G.; Bott, S.; Ohler, M.A.; Mock, H.P.; Lippmann, R.; Grosch, R.; Smalla, K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front. Microbiol. 2014, 5, 2. [Google Scholar] [CrossRef]
- Zhong, J.; Shi, Z.; Zheng, R.; Xiang, H.; Zhang, J. Mitigating acid rain stress on lettuce growth and quality without the root exposure to acid rain. Food Biosci. 2024, 62, 105161. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and physiological analysis of salinity effects on lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef]
- Hartmann, A.; Schmid, M.; Tuinen, D.V.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- National Institute of Crop Science (NICS). Agricultural Status and Soil Explanation of Korean Reclaimed Land; National Institute of Crop Science: Wanju-gun, Republic of Korea, 2009. [Google Scholar]
- Rural Development Administration (RDA). Soil and Plant Analysis Methods; National Institute of Agricultural Science and Technology (NIAST): Suwon, Republic of Korea, 2000. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Kononova, M.M. Soil Organic Matter: Its Nature, Its Role in Soil Formation and in Soil Fertility; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- NAAS. Methods of Soil Chemical Analysis; National Institute of Agricultural Sciences, RDA: Suwon, Republic of Korea, 2010. [Google Scholar]
- Brenner, I. Inductively coupled plasma-atomic emission spectrometry—An atlas of spectral information. Chem. Geol. 1987, 63, 356–357. [Google Scholar] [CrossRef]
- Rural Development Administration (RDA). Manual for Standard Evaluation Method in Agricultural Experiment and Research; RDA: Suwon, Republic of Korea, 2012. [Google Scholar]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils, 4th ed.; Pearson: New York, NY, USA, 2016. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622. [Google Scholar] [CrossRef]
- Kirkby, E.A. Principles of Plant Nutrition; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 1. [Google Scholar]
- Marschner, H.; Römheld, V. In vivo measurement of root-induced pH changes at the soil-root interface: Effect of plant species and nitrogen source. Z. Für Pflanzenphysiol. 1983, 111, 241–251. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M. Vegetable Crops: Improvement of tolerance to adverse chemical soil conditions by grafting. In Improving Crop Resistance to Abiotic Stress; Wiley: Hoboken, NJ, USA, 2012; pp. 979–994. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Wojciechowski, M.F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 2009, 4, e7801. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Garcia-Pichel, F.; López-Cortés, A.; Nubel, U. Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado Plateau. Appl. Environ. Microbiol. 2001, 67, 1902–1910. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Koblížek, M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 2015, 39, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Lenassi, M.; Gunde-Cimerman, N.; Plemenitaš, A. Fungal adaptation to extremely high salt concentrations. Adv. Appl. Microbiol. 2011, 77, 71–96. [Google Scholar] [PubMed]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Lewis, L.A.; McCourt, R.M. Green algae and the origin of land plants. Am. J. Bot. 2004, 91, 1535–1556. [Google Scholar] [CrossRef]
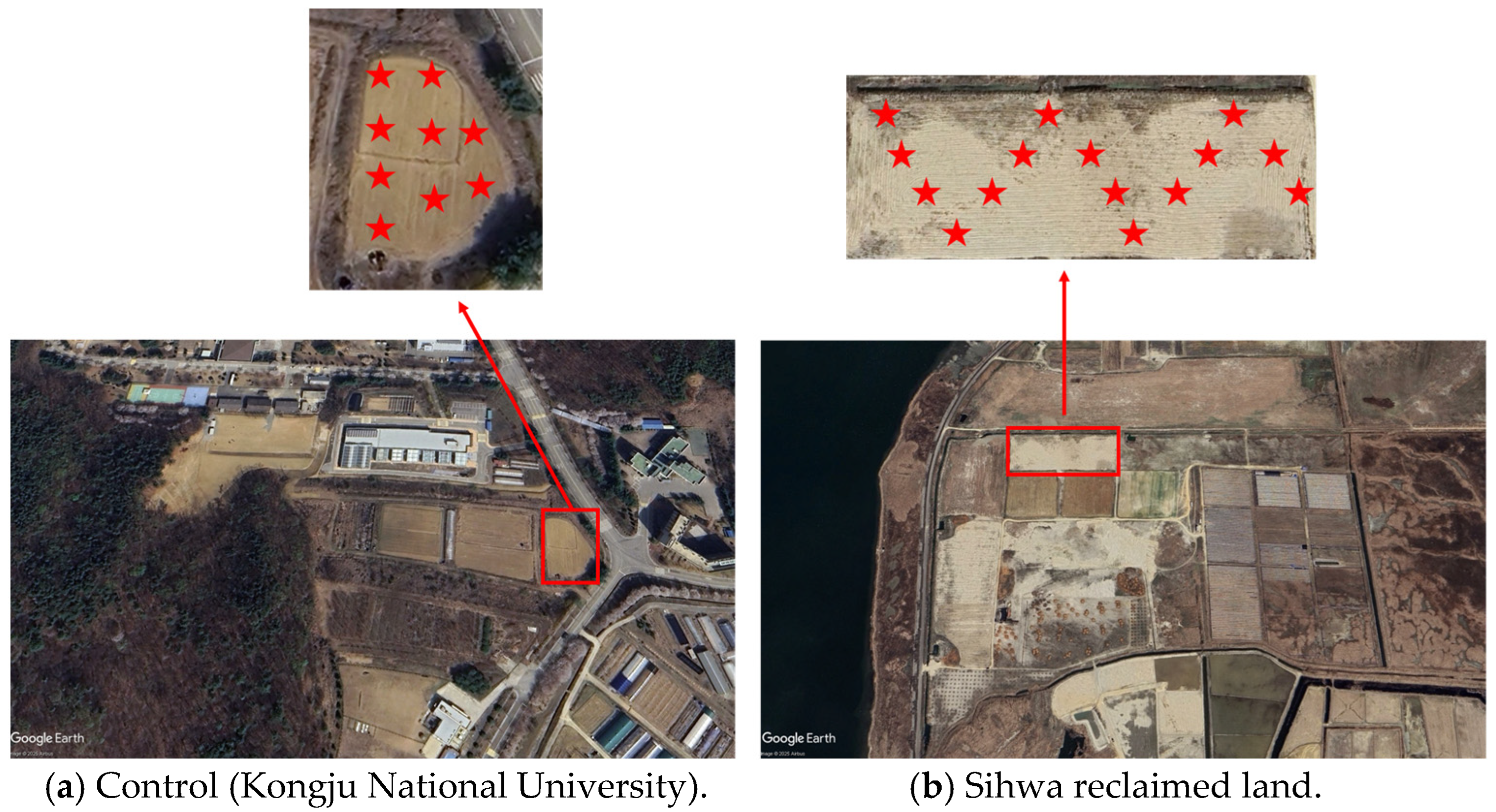
| Sand | Silt | Clay | pH | Ec(25) (ds/m) | OM (mg/kg) | Av. P2O5 (mg/kg) | K | Ca | Mg | Na | Av. SiO2 (mg/kg) | T-N (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmol+/kg | |||||||||||||
| SR | 94.59 a | 2.57 b | 2.85 b | 8.84 a | 7.70 a | 12.84 a | 9.89 b | 10.38 a | 19.45 a | 4.28 a | 12.43 a | 132.58 a | 0.019 b |
| SL | 93.93 a | 3.21 b | 2.85 b | 8.67 a | 7.70 a | 12.47 b | 8.77 b | 9.76 a | 16.97 a | 3.76 a | 11.17 a | 133.18 a | 0.021 b |
| NS | 42.85 b | 45.88 a | 11.27 a | 6.07 b | 1.94 b | 20.13 a | 295.12 a | 0.81 b | 5.47 b | 2.14 b | 1.69 b | 134.11 a | 0.132 a |
| NL | 43.12 b | 45.42 a | 11.47 a | 5.75 b | 1.94 b | 19.12 a | 291.55 b | 0.64 b | 5.18 b | 2.11 b | 1.76 b | 131.14 a | 0.129 a |
| Length (cm) | Stem Width (mm) | Leaf Number (ea) | Leaf Width (cm2) | Weight (g) | Dry Weight (g) | |
|---|---|---|---|---|---|---|
| NL | 14 a | 12 a | 10 a | 580 a | 40.43 a | 0.45 a |
| SL | 10 b | 8 b | 8 b | 460 b | 32.51 b | 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.-R.; Oh, T.S.; Park, Y.J.; Jang, M.-J. Changes in Microbial Communities After Lettuce Cultivation in Sihwa Reclaimed Soils, Korea. Environments 2025, 12, 287. https://doi.org/10.3390/environments12080287
Yu D-R, Oh TS, Park YJ, Jang M-J. Changes in Microbial Communities After Lettuce Cultivation in Sihwa Reclaimed Soils, Korea. Environments. 2025; 12(8):287. https://doi.org/10.3390/environments12080287
Chicago/Turabian StyleYu, Dong-Ryeol, Tae Seok Oh, Youn Jin Park, and Myoung-Jun Jang. 2025. "Changes in Microbial Communities After Lettuce Cultivation in Sihwa Reclaimed Soils, Korea" Environments 12, no. 8: 287. https://doi.org/10.3390/environments12080287
APA StyleYu, D.-R., Oh, T. S., Park, Y. J., & Jang, M.-J. (2025). Changes in Microbial Communities After Lettuce Cultivation in Sihwa Reclaimed Soils, Korea. Environments, 12(8), 287. https://doi.org/10.3390/environments12080287







