A Citizen Science Approach for Documenting Mass Coral Bleaching in the Western Indian Ocean
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Surveys
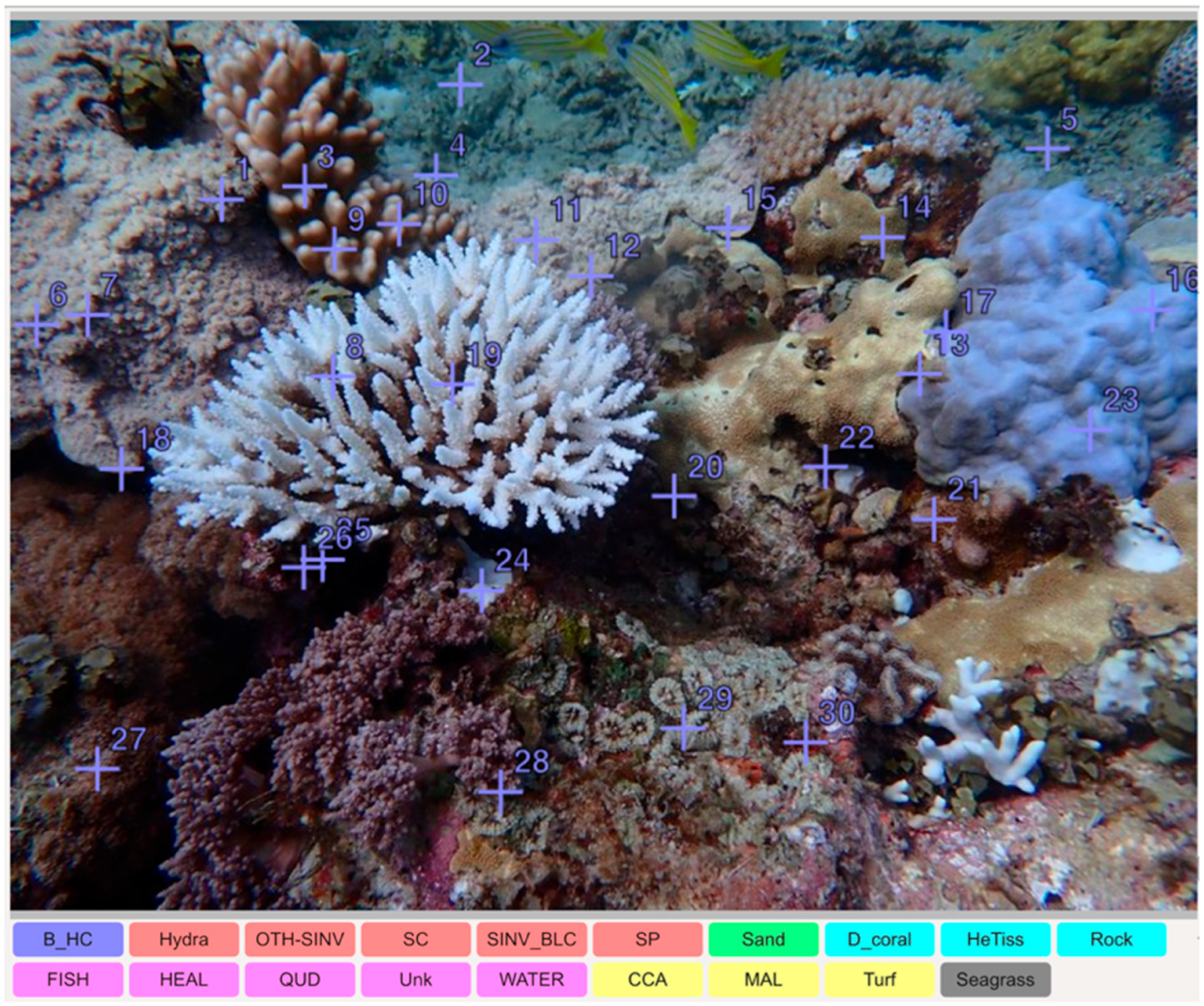
2.2. CoralNet AI Training
2.3. Data Analysis
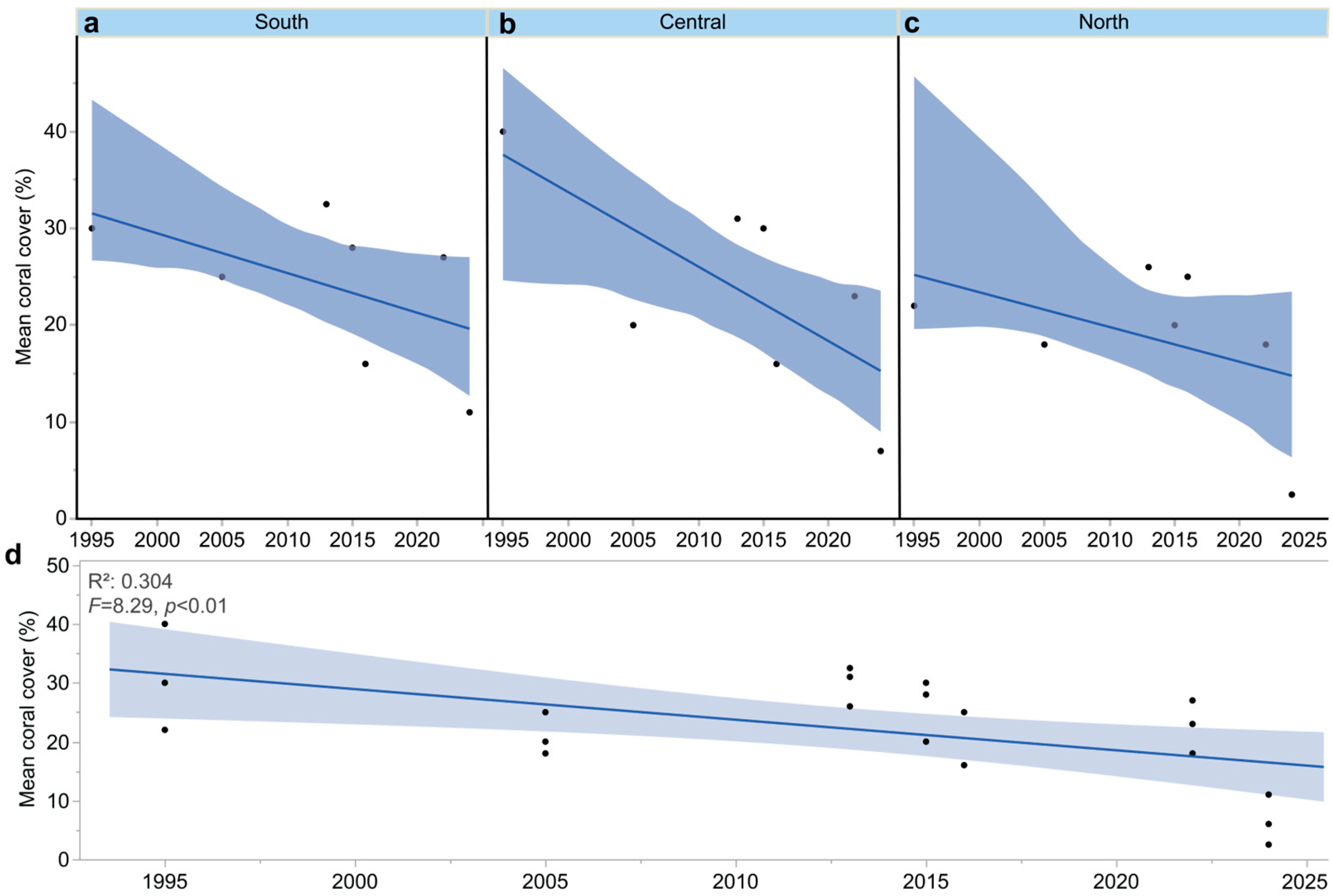
2.4. Meta-Analysis
3. Results and Discussion
3.1. CoralNet Performance
3.2. Temperature
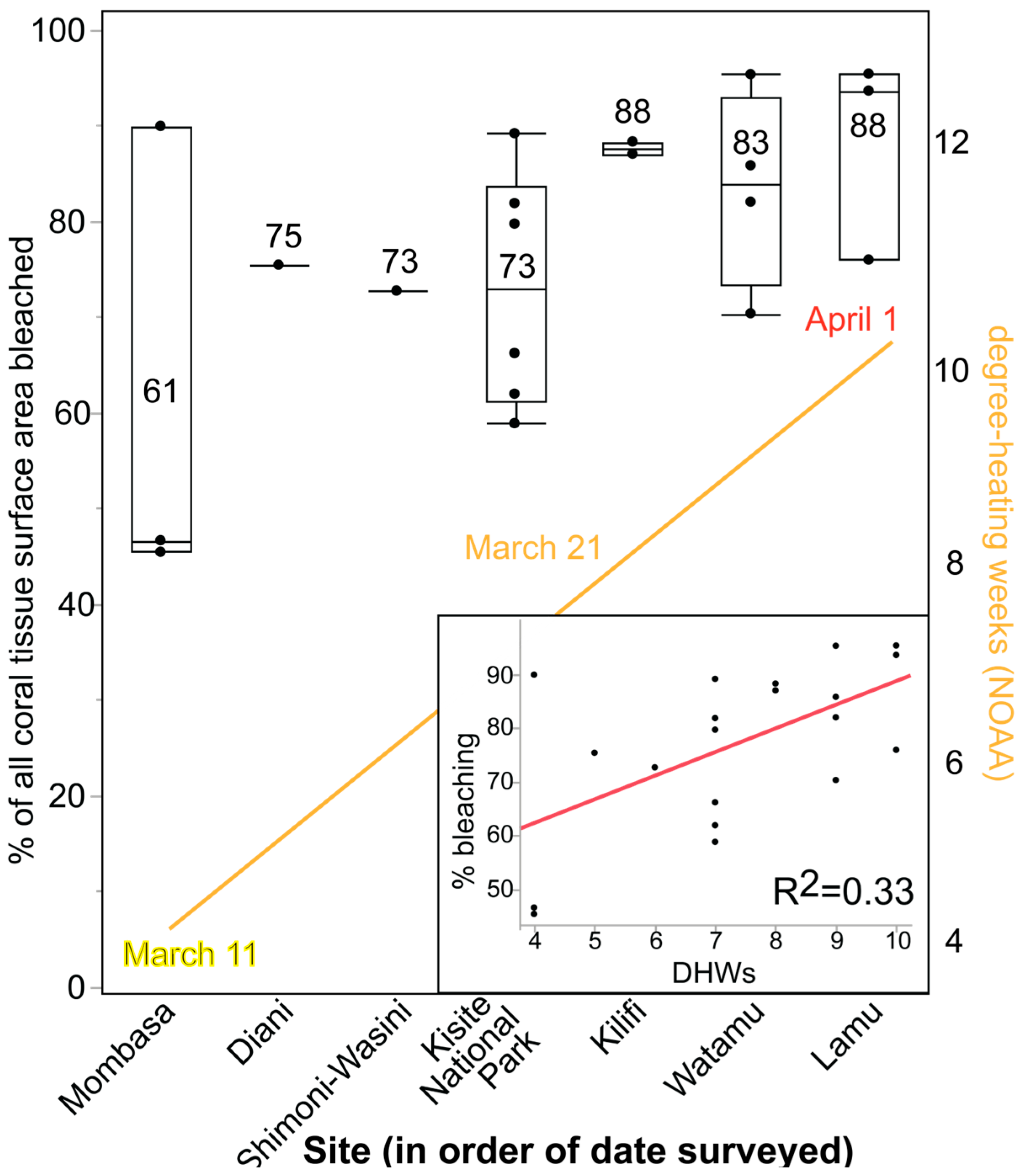
3.3. Coral Bleaching
3.4. Coral Cover
| Region | Geographic Zone | % Bleaching (% of Colonies) | % Change in Coral Cover: Pre- vs. Post-Event | 2024 Bleaching: % of All Coral Tissue Surface Area |
|---|---|---|---|---|
| 1998 event [24,37]: 8–9 DHWs | ||||
| Mombasa | Central | >90% [24] | (−)55% [37] | 61% |
| Diani | South | NR | (−)60% [37] | 75% |
| Kisite National Park | South | NR | (−)55% [37] | 73% |
| Watamu | Central | NR | (−)76% [37] | 83% |
| 2007 (minor) event [38,39]: <1 DHW | ||||
| Mombasa | Central | 27% [38] | NC [38] | See above. |
| Watamu | Central | 20% [39] | (−)33% [39] | See above. |
| 2016 event [33]: 4–5 DHWs | ||||
| Mombasa | Central | NR | (−)11% | See above. |
| Shimoni | South | NR | (+)14% | 73% |
| Kilifi | Central | NR | NC | 88% |
| Watamu | Central | NR | NC | See above. |
| Lamu | North | NR | (−)52% | 88% |
3.5. Sources of Error and Bias
3.6. Depth Effects
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| BIC | Bayesian information criterion |
| DHWs | Degree-heating weeks |
| FNR | False-negative bleaching classification rate |
| FPR | False-positive bleaching classification rate |
| GCRMN | Global Coral Reef Monitoring Network |
| HS | Hot spot |
| NC | No change (detected) |
| NR | Not reported (in manuscript) |
| SST | Sea surface temperatures |
| Temp. | Temperature |
References
- Reimer, J.D.; Peixoto, R.S.; Davies, S.W.; Traylor-Knowles, N.; Short, M.L.; Cabral-Tena, R.A.; Burt, J.A.; Pessoa, I.; Banaszak, A.T.; Winters, R.S.; et al. The Fourth Global Coral Bleaching Event: Where Do We Go from Here? Coral Reefs 2024, 43, 1121–1125. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- McClanahan, T.; Muthiga, N.; Mangi, S. Coral and Algal Changes after the 1998 Coral Bleaching: Interaction with Reef Management and Herbivores on Kenyan Reefs. Coral Reefs 2001, 19, 380–391. [Google Scholar] [CrossRef]
- Obura, D.O. Resilience and climate change: Lessons from coral reefs and bleaching in the Western Indian Ocean. Estuar. Coast. Shelf Sci. 2005, 63, 353–372. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Baird, A.H.; Marshall, P.A.; Toscano, M.A. Comparing Bleaching and Mortality Responses of Hard Corals between Southern Kenya and the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2004, 48, 327–335. [Google Scholar] [CrossRef]
- McClanahan, T.; Maina, J.; Pet-Soede, L. Effects of the 1998 Coral Morality Event on Kenyan Coral Reefs and Fisheries. AMBIO 2002, 31, 543–550. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Ateweberhan, M.; Omukoto, J. Long-Term Changes in Coral Colony Size Distributions on Kenyan Reefs under Different Management Regimes and across the 1998 Bleaching Event. Mar. Biol. 2008, 153, 755–768. [Google Scholar] [CrossRef]
- McClanahan, T.R. Changes in Coral Sensitivity to Thermal Anomalies. Mar. Ecol. Prog. Ser. 2017, 570, 71–85. [Google Scholar] [CrossRef]
- Liu, G.; Heron, S.F.; Eakin, C.M.; Muller-Karger, F.E.; Vega-Rodriguez, M.; Guild, L.S.; Cour, J.L.; Geiger, E.F.; Skirving, W.J.; Burgess, T.F.R.; et al. Reef-Scale Thermal Stress Monitoring of Coral Ecosystems: New 5-Km Global Products from NOAA Coral Reef Watch. Remote Sens. 2014, 6, 11579–11606. [Google Scholar] [CrossRef]
- Marshall, N.J.; Kleine, D.A.; Dean, A.J. CoralWatch: Education, Monitoring, and Sustainability through Citizen Science. Front. Ecol. Environ. 2012, 10, 332–334. [Google Scholar] [CrossRef]
- Licuanan, W.Y.; Mordeno, P.Z.B.; Go, M.V. C30—A Simple, Rapid, Scientifically Valid, and Low-Cost Method for Citizen-Scientists to Monitor Coral Reefs. Reg. Stud. Mar. Sci. 2021, 47, 101961. [Google Scholar] [CrossRef]
- Done, T.; Roelfsema, C.; Harvey, A.; Schuller, L.; Hill, J.; Schläppy, M.-L.; Lea, A.; Bauer-Civiello, A.; Loder, J. Reliability and Utility of Citizen Science Reef Monitoring Data Collected by Reef Check Australia, 2002–2015. Mar. Pollut. Bull. 2017, 117, 148–155. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Dempsey, A.C. Using Tourist Diver Images to Estimate Coral Cover and Bleaching Prevalence in a Remote Indian Ocean Coral Reef System. Biodivers. Conserv. 2025. under review. [Google Scholar]
- Chowdhury, A.; Jahan, M.; Kaisar, S.; Khoda, M.E.; Rajin, S.M.A.K.; Naha, R. Coral Reef Surveillance with Machine Learning: A Review of Datasets, Techniques, and Challenges. Electronics 2024, 13, 5027. [Google Scholar] [CrossRef]
- Zhang, C. Applying Data Fusion Techniques for Benthic Habitat Mapping and Monitoring in a Coral Reef Ecosystem. ISPRS J. Photogramm. Remote Sens. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Lawson, C.L.; Chartrand, K.M.; Roelfsema, C.M.; Kolluru, A.; Mumby, P.J. Broadscale Reconnaissance of Coral Reefs from Citizen Science and Deep Learning. Environ. Monit. Assess. 2025, 197, 814. [Google Scholar] [CrossRef]
- Burns, J.H.R.; Delparte, D.; Gates, R.D.; Takabayashi, M. Integrating Structure-from-Motion Photogrammetry with Geospatial Software as a Novel Technique for Quantifying 3D Ecological Characteristics of Coral Reefs. PeerJ 2015, 3, e1077. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.B. Characterizing the 2024 Red Sea Bleaching Event with Tourist Diver Photos. Environments 2025, 12, 248. [Google Scholar] [CrossRef]
- Wright, S.; Hull, T.; Sivyer, D.B.; Pearce, D.; Pinnegar, J.K.; Sayer, M.D.J.; Mogg, A.O.M.; Azzopardi, E.; Gontarek, S.; Hyder, K. SCUBA Divers as Oceanographic Samplers: The Potential of Dive Computers to Augment Aquatic Temperature Monitoring. Sci. Rep. 2016, 6, 30164. [Google Scholar] [CrossRef]
- Marlowe, C.; Hyder, K.; Sayer, M.D.J.; Kaiser, J. Citizen Scientists’ Dive Computers Resolve Seasonal and Interannual Temperature Variations in the Red Sea. Front. Mar. Sci. 2022, 9, 976771. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.-Y.; Tan, C.-J.; et al. Towards Automated Annotation of Benthic Survey Images: Variability of Human Experts and Operational Modes of Automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef] [PubMed]
- Siebeck, U.; Marshall, N.; Klüter, A.; Hoegh-Guldberg, O. Monitoring Coral Bleaching Using a Colour Reference Card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Mdodo, R.M. Environmental Factors and Coral Bleaching in Kenya. Master’s Thesis, Moi University, Kesses, Kenya, 1999. [Google Scholar]
- Karisa, J.F.; Obura, D.O.; Chen, C.A. Spatial Heterogeneity of Coral Reef Benthic Communities in Kenya. PLoS ONE 2020, 15, e0237397. [Google Scholar] [CrossRef]
- Obura, D.O. Kenya. Mar. Pollut. Bull. 2001, 42, 1264–1278. [Google Scholar] [CrossRef]
- McClanahan, T. Coral Bleaching, Diseases and Mortality in the Western Indian Ocean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 157–176. ISBN 978-3-662-06414-6. [Google Scholar]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Polunin, N.V.C.; Jennings, S.; Chabanet, P.; Clark, S.; Spalding, M.D.; Letourneur, Y.; et al. Climate Warming, Marine Protected Areas and the Ocean-Scale Integrity of Coral Reef Ecosystems. PLoS ONE 2008, 3, e3039. [Google Scholar] [CrossRef]
- Allen, K.A.; Bruno, J.F.; Chong, F.; Clancy, D.; McClanahan, T.R.; Spencer, M.; Żychaluk, K. Among-Site Variability in the Stochastic Dynamics of East African Coral Reefs. PeerJ 2017, 5, e3290. [Google Scholar] [CrossRef]
- UNEP; WIOMSA. The Regional State of the Coast Report: Western Indian Ocean; UNEP: Nairobi, Kenya, 2015; 546p. [Google Scholar]
- McClanahan, T.R.; Darling, E.S.; Maina, J.M.; Campbell, S.J.; Ateweberhan, M.; Muthiga, N.A. Long-Term Changes in Coral Reef Assemblages in Kenya. Mar. Pollut. Bull. 2023, 194, 115210. [Google Scholar] [CrossRef]
- Obura, D.O.; Church, J.E.; Gabrié, C. Assessing Marine World Heritage from an Ecosystem Perspective: The Western Indian Ocean; World Heritage Centre, United Nations Education, Science and Cultural Organization (UNESCO): Paris, France, 2012; 124p. [Google Scholar]
- Gudka, M.; Obura, D.; Mwaura, J.; Porter, S.; Yahya, S.; Mabwa, R. Impact of the 3rd Global Coral Bleaching Event on the Western Indian Ocean in 2016; Global Coral Reef Monitoring Network (GCRMN)/Indian Ocean Commission: London, UK, 2018; p. 67. [Google Scholar]
- Obura, D. East Africa-Summary. In Coral Reef Degradation in the Indian Ocean: Status Report 2005; Souter, D., Linden, O., Eds.; CORDIO: Stockholm, Sweden, 2005; pp. 25–31. [Google Scholar]
- Souter, D.; Planes, S.; Wicquart, J.; Logan, M.; Obura, D.; Staub, F. (Eds.) Status of Coral Reefs of the World: 2020 Report; Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI): London, UK, 2021. [Google Scholar] [CrossRef]
- Gudka, M.; Obura, D.; Treml, E.; Samoilys, M.; Aboud, S.A.; Osuka, K.E.; Mbugua, J.; Mwaura, J.; Karisa, J.; Knoester, E.G.; et al. Leveraging the Red List of Ecosystems for Action on Coral Reefs through the Kunming-Montreal Global Biodiversity Framework. Conserv. Sci. Pract. 2024, 6, e13255. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Ateweberhan, M.; Muhando, C.A.; Maina, J.; Mohammed, M.S. Effects of Climate and Seawater Temperature Variation on Coral Bleaching and Mortality. Ecol. Monogr. 2007, 77, 503–525. [Google Scholar] [CrossRef]
- Grimsditch, G.; Mwaura, J.M.; Kilonzo, J.; Amiyo, N. The Effects of Habitat on Coral Bleaching Responses in Kenya. AMBIO 2010, 39, 295–304. [Google Scholar] [CrossRef]
- Montano, S.; Seveso, D.; Galli, P.; Obura, D.O. Assessing Coral Bleaching and Recovery with a Colour Reference Card in Watamu Marine Park, Kenya. Hydrobiologia 2010, 655, 99–108. [Google Scholar] [CrossRef]
- Mbugua, J.; Aboud, S.; Obura, D. Western Indian Ocean-Regional Coral Bleaching Report 2024. Zenodo: 2024. Available online: https://icriforum.org/wp-content/uploads/2024/07/WIO-bleaching-2024_summary-report.pdf (accessed on 15 May 2025).
- Lambo, A.L.; Ormond, R.F.G. Continued Post-Bleaching Decline and Changed Benthic Community of a Kenyan Coral Reef. Mar. Pollut. Bull. 2006, 52, 1617–1624. [Google Scholar] [CrossRef]
- Head, C.E.I.; Bayley, D.T.I.; Rowlands, G.; Roche, R.C.; Tickler, D.M.; Rogers, A.D.; Koldewey, H.; Turner, J.R.; Andradi-Brown, D.A. Coral Bleaching Impacts from Back-to-Back 2015–2016 Thermal Anomalies in the Remote Central Indian Ocean. Coral Reefs 2019, 38, 605–618. [Google Scholar] [CrossRef]
- Remmers, T.; Grech, A.; Roelfsema, C.; Gordon, S.; Lechene, M.; Ferrari, R. Close-Range Underwater Photogrammetry for Coral Reef Ecology: A Systematic Literature Review. Coral Reefs 2024, 43, 35–52. [Google Scholar] [CrossRef]
- Gleason, D.F.; Wellington, G.M. Ultraviolet Radiation and Coral Bleaching. Nature 1993, 365, 836–838. [Google Scholar] [CrossRef]
- Knoester, E.G.; Rienstra, J.J.; Schürmann, Q.J.F.; Wolma, A.E.; Murk, A.J.; Osinga, R. Community-Managed Coral Reef Restoration in Southern Kenya Initiates Reef Recovery Using Various Artificial Reef Designs. Front. Mar. Sci. 2023, 10, 1152106. [Google Scholar] [CrossRef]
- ASCLME. National Marine Ecosystem Diagnostic Analysis. Kenya. Contribution to the Agulhas and Somali Current Large Marine Ecosystems Project (Supported by UNDP with GEF Grant Financing); 2012. Available online: https://nairobiconvention.org/clearinghouse/sites/default/files/National%20Marine%20Ecosystem%20Diagnostic%20Analysis%20%28MEDA%29%20-%20Kenya.pdf (accessed on 2 July 2025).
- Hamylton, S.M.; Zhou, Z.; Wang, L. What Can Artificial Intelligence Offer Coral Reef Managers? Front. Mar. Sci. 2020, 7, 603829. [Google Scholar] [CrossRef]
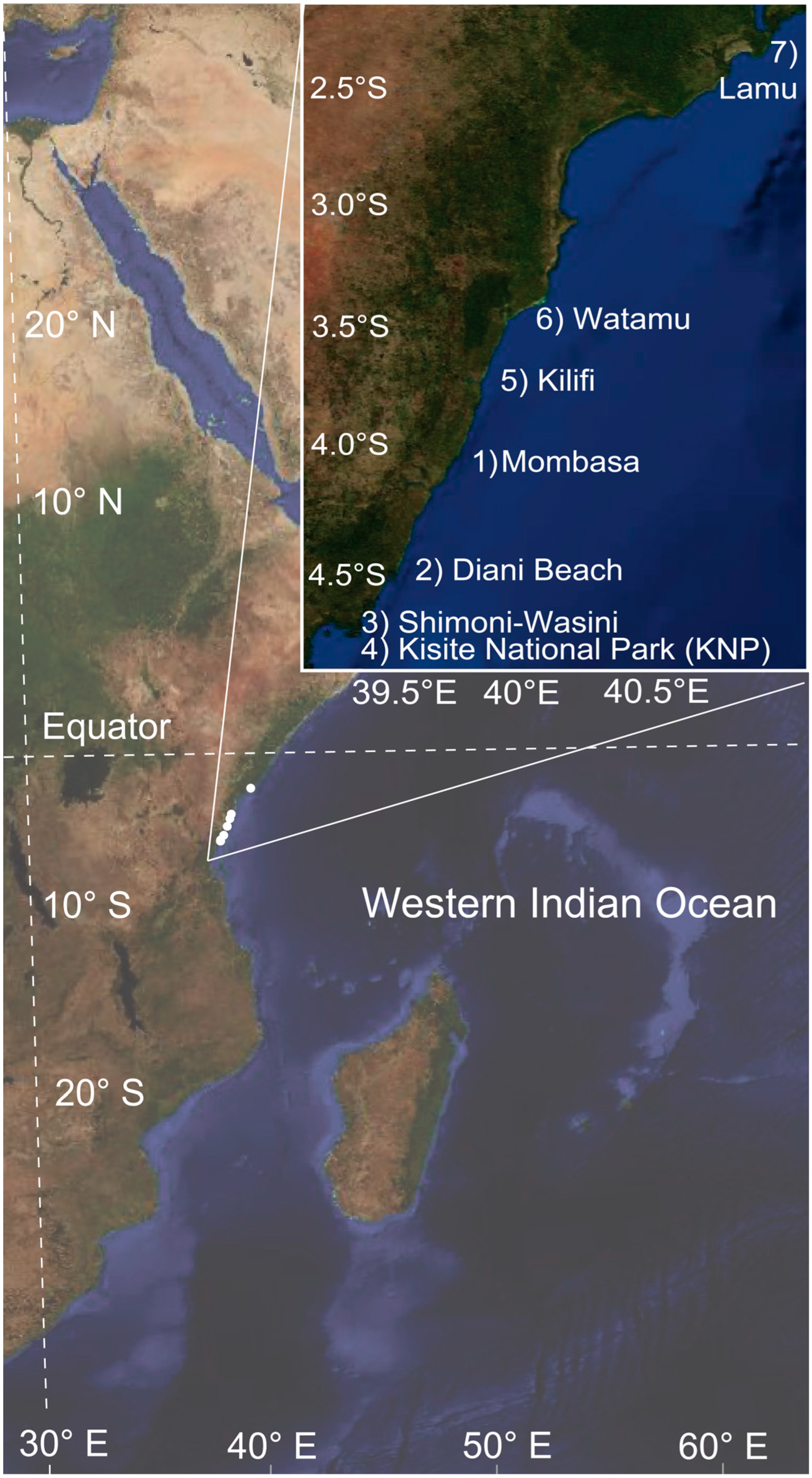
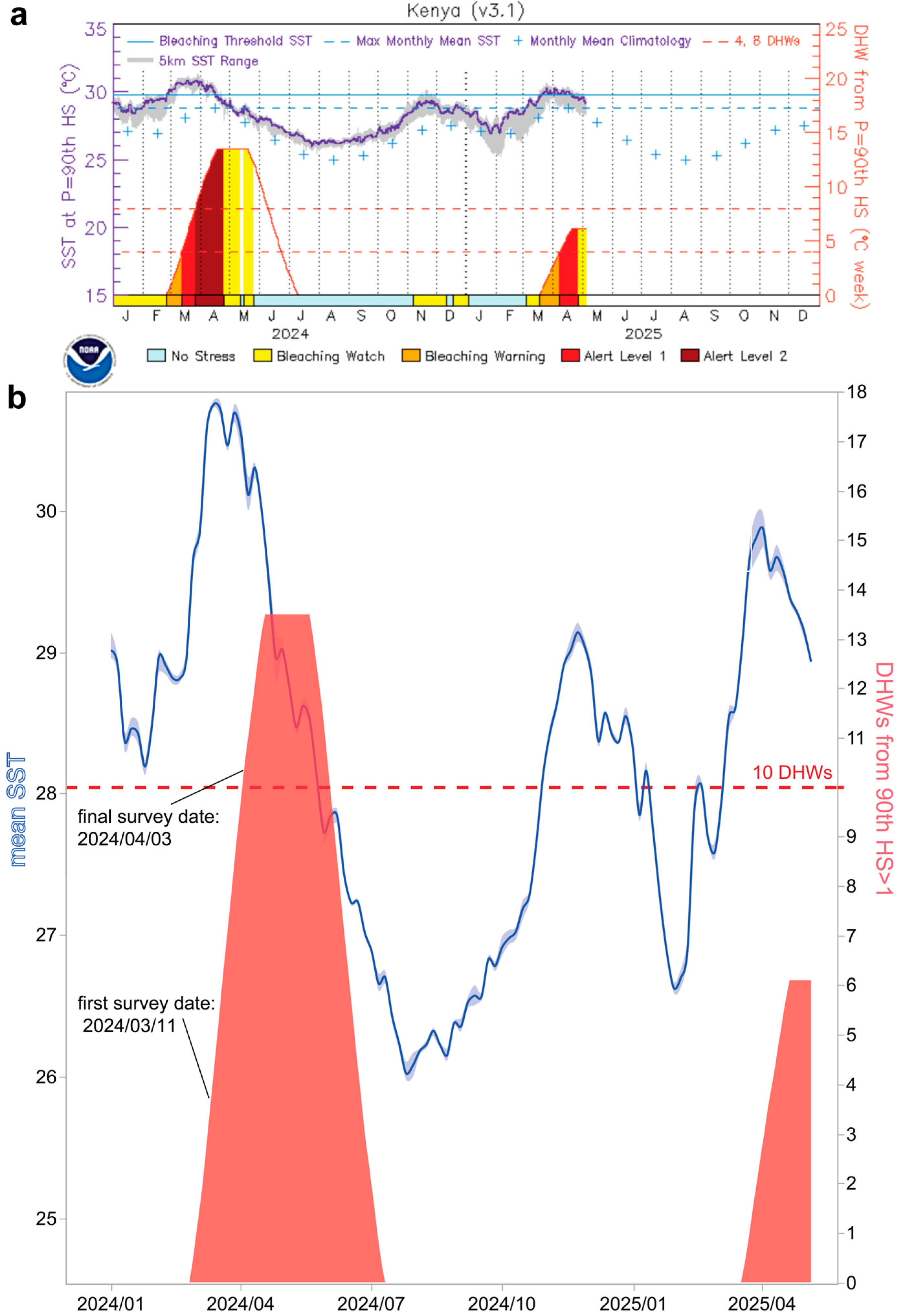
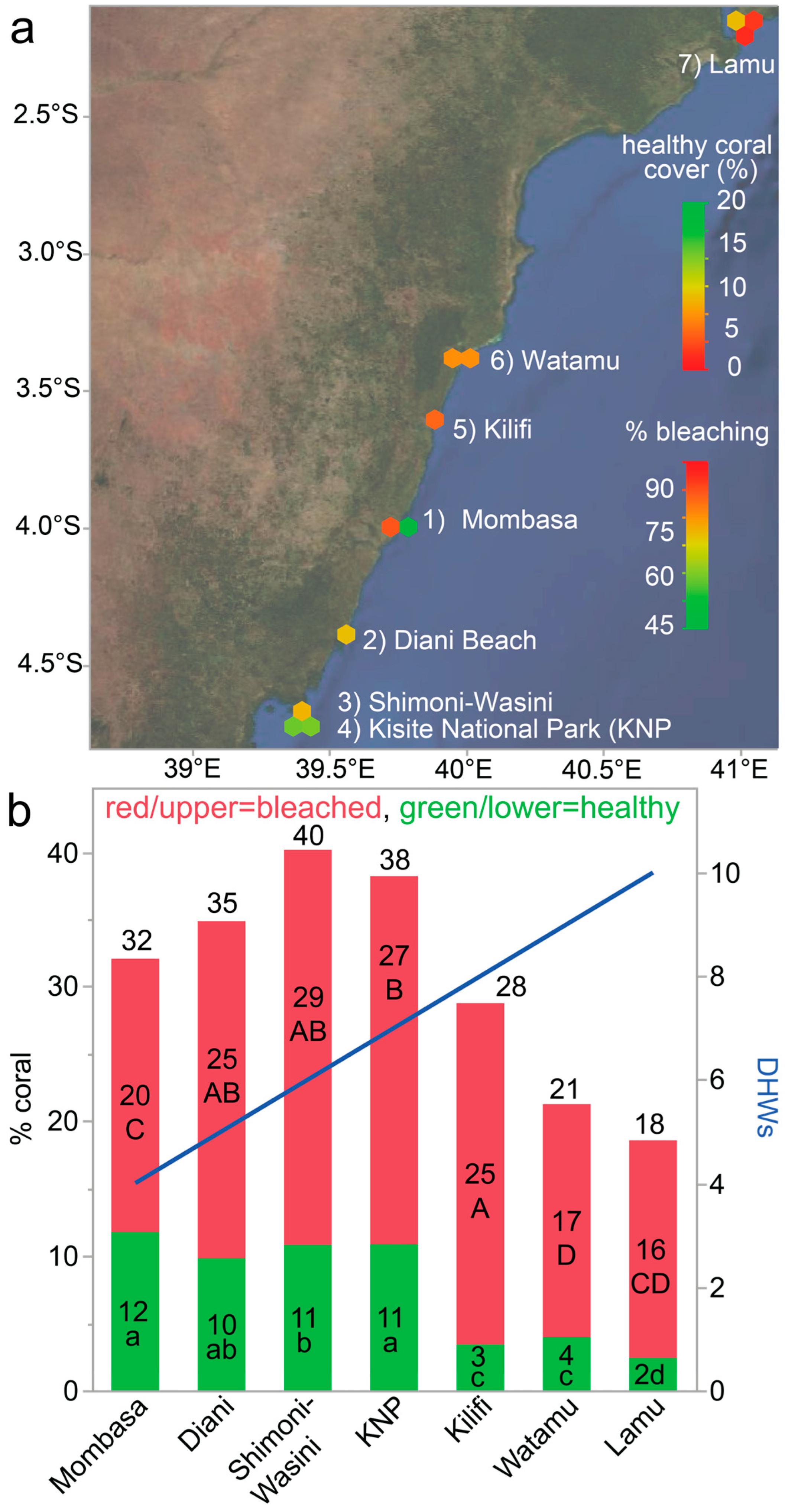

| Region | #TS/All Sites | Mean Image Depth (m) (max.) | Dates (2024) | Temp.- °C (DHWs) | Latitudinal Range (°S) | Longitudinal Range (°E) | Key Local Agency |
|---|---|---|---|---|---|---|---|
| Mombasa | 3/3 | 9.7 ± 5.9 (17) | March 11–14 | 32.5 (4) | −3.975 to −3.998 | 39.745 to 39.767 | KMFRI |
| Diani Beach | 2/2 | 2.0 ± 3.0 (8) | March 16 | 32.0 (5) | −4.377 to −4.434 | 39.548 to 39.571 | Whale Shark Adventures |
| Shimoni-Wasini | 0/2 | 5.5 ± 2.0 (15) | March 20 | 32.0 (6) | −4.65944 | 39.38222 | REEFoLution |
| KNP | 2/6 | 5.3 ± 2.6 (13) | March 21 | 31.6 (7) | −4.685 to −4.716 | 39.366 to 39.414 | REEFoLution |
| Kilifi | 2/2 | 10.8 ± 1.0 (21) | March 26 | 31.3 (8) | −3.606 to −3.626 | 39.892 to 39.897 | 3 Degrees South |
| Watamu | 2/4 | 7.1 ± 6.5 (26) & | March 27–29 | 32.3 (9) | −3.374 to −3.403 | 39.988 to 40.011 | A Rocha |
| Lamu | 0/3 | 2.8 ± 1.5 (8) | April 3 | 32 + (10) | −2.166 to −2.214 | 41.004 to 41.039 | Northern Rangelands Trust * |
| Metric | Value | Notes/Details |
|---|---|---|
| #Images taken for informal “tourist diver” analysis | 2322 | |
| #Images used in tourist diver CoralNet analysis | 2300 | Twenty-two images failed quality control. |
| #Images used for initial CoralNet AI training | 360 | Scored 10,800 features manually. |
| CoralNet-calculated classification accuracy | 81% | See “Classifier Overview” (https://coralnet.ucsd.edu/source/4963/ (accessed on 2 June 2025)) for details. |
| #Images for post-hoc CoralNet accuracy verification | 400 | Scored 12,000 features manually. |
| Manually calculated classification accuracy (%) | 84 ± 12% | |
| #Images for assessment of FPR and FNR | 140 | Scored 4200 features manually. |
| Total #images scored manually for CoralNet training | 900 | |
| Total #images classified automatically by CoralNet | 1400 | |
| Total #features scored by CoralNet across 1400 images | 42,000 | |
| Total #features scored by human and CoralNet | 69,000 | |
| #Coral colonies scored for assessment of FPR and FNR | 1197 | |
| Overall accuracy of coral bleaching analysis | 88.1% | |
| FPR (%) | 24.2% | |
| FNR (%) | 2.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayfield, A.B. A Citizen Science Approach for Documenting Mass Coral Bleaching in the Western Indian Ocean. Environments 2025, 12, 276. https://doi.org/10.3390/environments12080276
Mayfield AB. A Citizen Science Approach for Documenting Mass Coral Bleaching in the Western Indian Ocean. Environments. 2025; 12(8):276. https://doi.org/10.3390/environments12080276
Chicago/Turabian StyleMayfield, Anderson B. 2025. "A Citizen Science Approach for Documenting Mass Coral Bleaching in the Western Indian Ocean" Environments 12, no. 8: 276. https://doi.org/10.3390/environments12080276
APA StyleMayfield, A. B. (2025). A Citizen Science Approach for Documenting Mass Coral Bleaching in the Western Indian Ocean. Environments, 12(8), 276. https://doi.org/10.3390/environments12080276








