Environmental Behavior of Novel “Smart” Anti-Corrosion Nanomaterials in a Global Change Scenario
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of Dispersions and Experimental Design
2.2. Environmental BEHAVIOR
2.2.1. Nanomaterials Hydrodynamic Size
2.2.2. Release of Corrosion Inhibitors
2.2.3. Release of Metallic Elements
2.3. Data Analysis
3. Results
3.1. ZnAl LDH-BTA
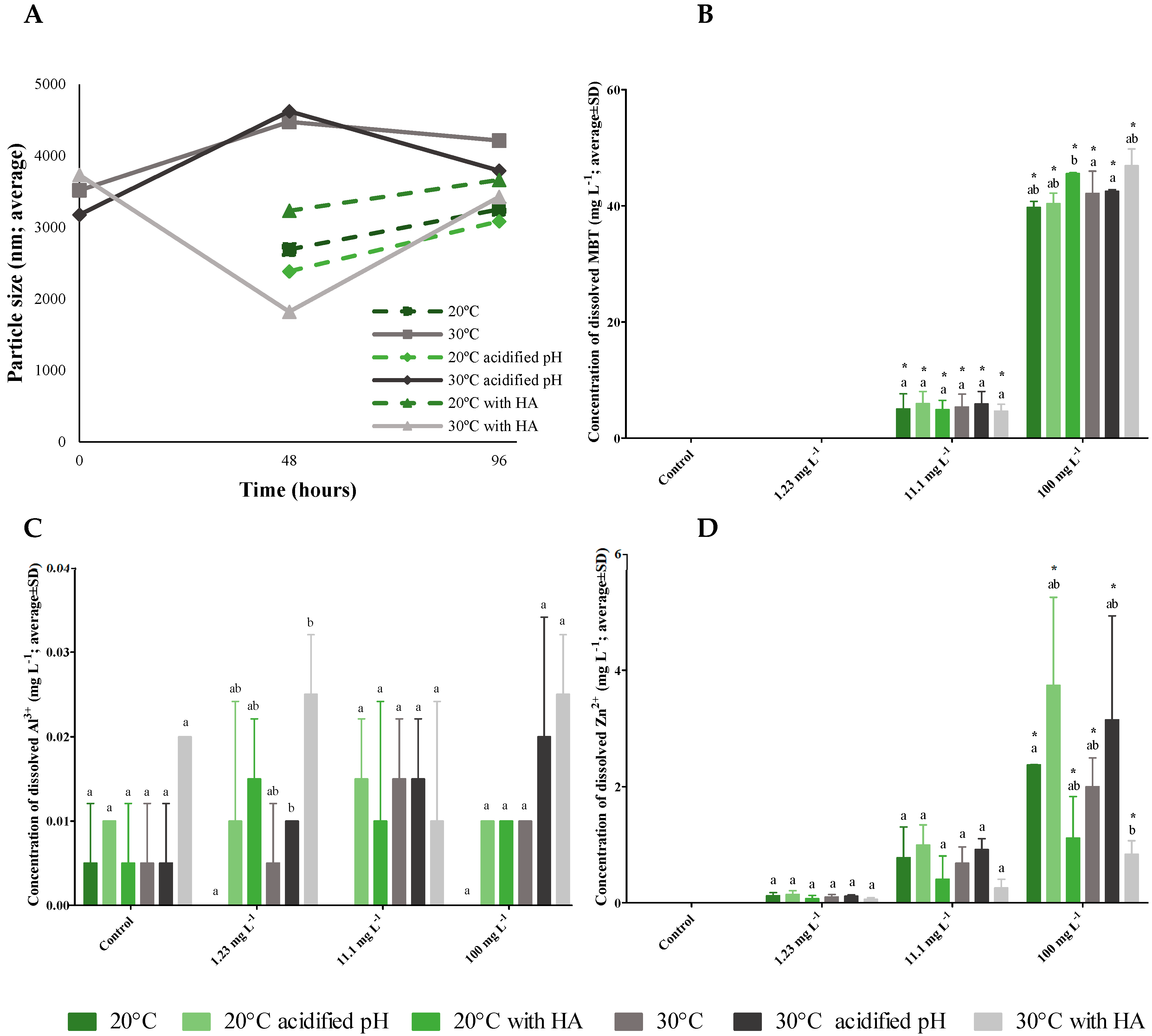
3.2. ZnAl LDH-MBT
3.3. ZnAl LDH-Gluconate
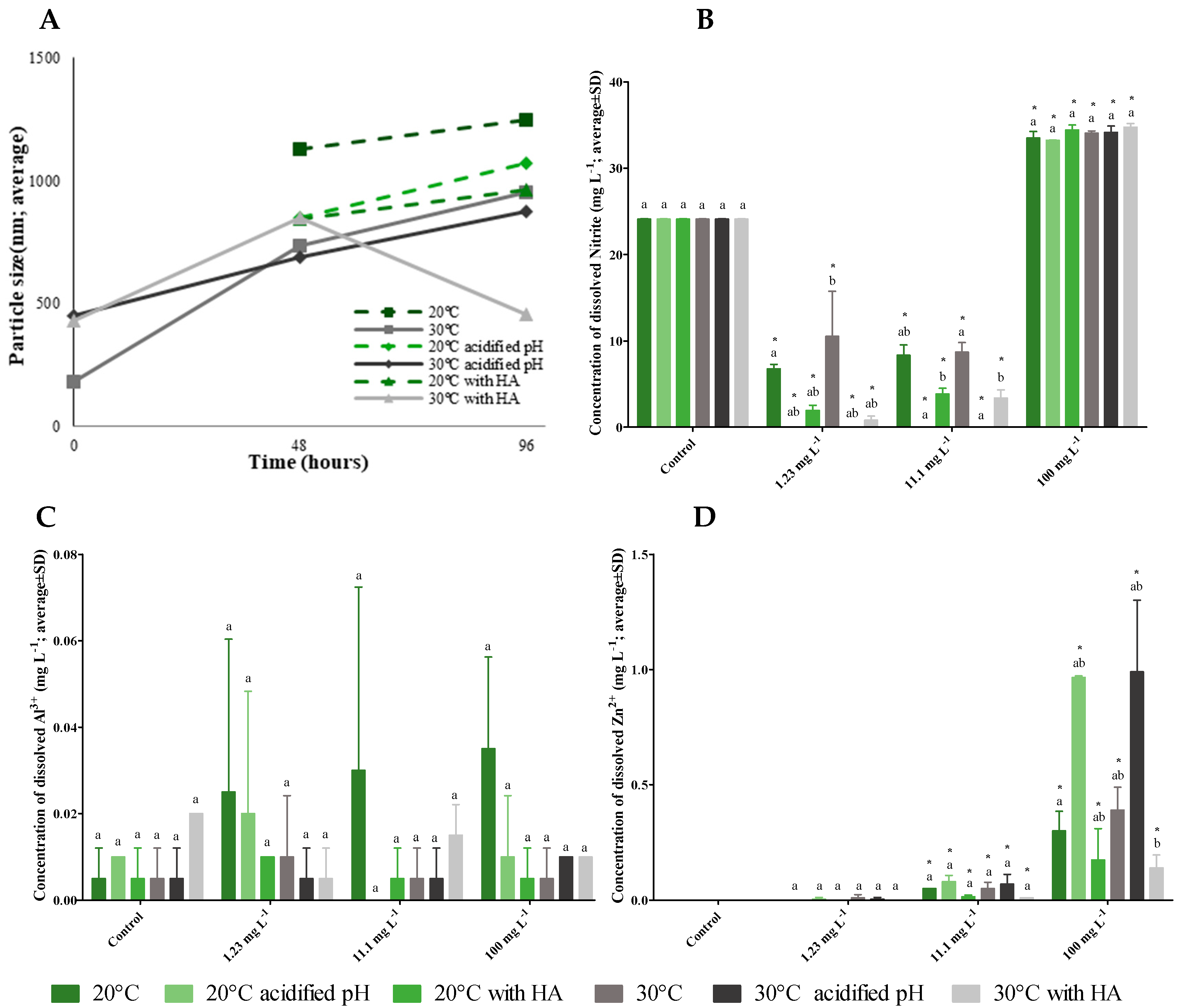
3.4. ZnAl-LDH-NO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIs | Corrosion Inhibitors |
| LDH | Layered Double Hydroxides |
| ASW | Artificial Salt Water |
| MBT | 2-mercaptobenzothiazole |
| BTA | Benzotriazole |
| SG | Sodium Gluconate |
| NO2− | Nitrite |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-ray Diffraction |
| TGA | Thermogravimetric Analysis |
| OCP | Open Circuit Potential |
| EIS | Electrochemical Impedance Spectroscopy |
| I.E. | Inhibition Efficiency |
| IPCC | Intergovernmental Panel on Climate Change |
| HA | Humic Acid |
| NOM | Natural Organic Matter |
| DLS | Dynamic Light Scattering |
| HPLC | High-Performance Liquid Chromatography |
| MEC | Measured Environmental Concentrations |
References
- Figueira, R.B. Hybrid sol–gel coatings for corrosion mitigation: A critical review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef]
- Loukil, N.; Feki, M. Zn–Mn electrodeposition: A literature review. J. Electrochem. Soc. 2020, 167, 022503. [Google Scholar] [CrossRef]
- Palou, R.M.; Olivares-Xomelt, O.; Likhanova, N.V. Environmentally friendly corrosion inhibitors. Dev. Corros. Prot. 2014, 19, 431–465. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Vaghefinazari, B.; Mohedano, M.; Visser, P.; Posner, R.; Blawert, C.; Zheludkevich, M.; Lamaka, S.; Matykina, E.; Arrabal, R. Chromate-free corrosion protection strategies for magnesium alloys—A review: Part II—PEO and anodizing. Materials 2022, 15, 8515. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Appl. Mater. Interfac. 2010, 2, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Oliveira, T.; Santos, C.; Kuznetsova, A.; Ferreira, V.; Avelelas, F.; Caetano, A.; Tedim, J.; Ferreira, M.; Freitas, R.; et al. Effects of a novel anti-corrosion engineered nanomaterial on the bivalve Ruditapes philippinarum. Environ. Sci. Nano 2017, 4, 1064–1076. [Google Scholar] [CrossRef]
- Dias, I.A.; Perina, F.; Figueiredo, J.; Silva, A.R.R.; Cardoso, D.N.; Martins, R. Sub-lethal effects of innovative anti-corrosion nanoadditives on the marine bivalve Ruditapes philippinarum. Environ. Pollut. 2025, 368, 125662. [Google Scholar] [CrossRef]
- Figueiredo, J.; Perina, F.; Carneiro, D.; Iqbal, M.A.; Oliveira, T.; Rocha, C.; Tedim, J.; Martins, R. Environmental behavior, hazard and anti-corrosion performance of benzotriazole-based nanomaterials for sustainable maritime applications. Environ. Sci. Nano 2025, 12, 3565–3580. [Google Scholar] [CrossRef]
- Liao, C.; Kim, U.J.; Kannan, K. A review of environmental occurrence, fate, exposure, and toxicity of benzothiazoles. Environ. Sci. Technol. 2018, 52, 5007–5026. [Google Scholar] [CrossRef]
- Refaey, S.A.M. Inhibition of chloride pitting corrosion of mild steel by sodium gluconate. Appl. Surf. Sci. 2000, 157, 199–206. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Verma, C.; Chauhan, D.S.; Aslam, R.; Banerjee, P.; Aslam, J.; Quadri, T.W.; Zehra, S.; Verma, D.K.; Quraishi, M.A.; Dubey, S.; et al. Principles and theories of green chemistry for corrosion science and engineering: Design and application. Green Chem. 2024, 26, 4270. [Google Scholar] [CrossRef]
- Verma, C.; Kumar, A.M.; Mazumder, M.A.J.; Quraishi, M.A. Chitosan-Based Green and Sustainable Corrosion Inhibitors for Carbon Steel. Chitin-Chitosan-Myriad Funct. Sci. Technol. 2018, 143. [Google Scholar] [CrossRef]
- Shen, M.; Furman, A.; Kharshan, R. Investigation of Bio-Based Aromatic Acids as Corrosion Inhibitor. In Proceedings of the CORROSION 2015, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Gomes, C.; Mir, Z.; Sampaio, R.; Bastos, A.; Tedim, J.; Maia, F.; Rocha, C.; Ferreira, M. Use of ZnAl-Layered Double Hydroxide (LDH) to Extend the Service Life of Reinforced Concrete. Materials 2020, 13, 1769. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Qiu, S.; Yang, D.; Liu, S.; Zhao, H.; Wang, L.; Xue, Q. Active Anti-corrosion of Epoxy Coating by Nitrite Ions Intercalated MgAl LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar] [CrossRef]
- Zuo, J.; Wu, B.; Dong, B.; Xing, F.; Ma, J.; Wei, G. Effects of nitrite ion intercalated CaAl and MgAl layered double hydroxides on the properties of concrete mortar. Cem. Concr. Compos. 2024, 145, 105306. [Google Scholar] [CrossRef]
- Poznyak, S.K.; Tedim, J.; Rodrigues, L.M.; Salak, A.N.; Zheludkevich, M.L.; Dick, L.F.P.; Ferreira, M.G.S. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anti-corrosion applications. ACS Appl. Mater. Interfac. 2009, 1, 2353–2362. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Rodriguez, J.; Bollen, E.; Nguyen, T.D.; Portier, A.; Paint, Y.; Olivier, M.G. Incorporation of layered double hydroxides modified with benzotriazole into an epoxy resin for the corrosion protection of Zn-Mg coated steel. Prog. Org. Coat. 2020, 149, 105894. [Google Scholar] [CrossRef]
- Pellanda, A.C.; Neto, A.G.C.; Jorge, A.R.C.; Berton, M.A.C.; Florian, J.B.; Thomas, S.; Vijayan, P. Performance evaluation of layered double hydroxides containing benzotriazole and nitrogen oxides as autonomic protection particles against corrosion. Int. J. Polym. Sci. 2021, 1, 6630194. [Google Scholar] [CrossRef]
- Amanian, S.; Naderi, R.; Mahdavian, M. Benzotriazole modified Zn-Al layered double hydroxide conversion coating on galvanized steel for improved corrosion resistance. J. Taiwan Inst. Chem. Eng. 2023, 150, 105072. [Google Scholar] [CrossRef]
- Leal, D.A.; Sousa, I.; Bastos, A.C.; Tedim, J.; Wypych, F.; Marino, C.E.B. Combination of layered-based materials as an innovative strategy for improving active corrosion protection of carbon steel. Surf. Coat. Technol. 2023, 473, 129972. [Google Scholar] [CrossRef]
- Deip, A.R.; Leal, D.A.; Sakae, G.H.; Maia, F.; Berton, M.A.C.; Ferreira, M.G.S.; Marino, C.E.B. Performance of commercial LDH traps for chloride ion in a commercial corrosion protection primer for petrochemical industry. Corros. Eng. Sci. Technol. 2020, 55, 66–74. [Google Scholar] [CrossRef]
- Seniski, A.; Monteiro, R.F.; Carrera, G.T.; Bragança, M.D.O.G.P.; Portella, K.F. The inhibitory and comparative effects of Zn-Al layered double hydroxide microcontainers intercalated with benzotriazole and nitrite for corrosion protection coatings on AISI 1010 carbon steel. Matéria 2020, 25, e-12664. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; Tan, Q.; Jiang, L. Chloride absorption by nitrate, nitrite and aminobenzoate intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 5908–5916. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.P.; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.; et al. (Eds.) IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Dedman, C.J. Nano-ecotoxicology in a changing ocean. SN Appl. Sci. 2022, 4, 264. [Google Scholar] [CrossRef]
- Walters, C.; Pool, E.; Somerset, V. Aggregation and dissolution of silver nanoparticles in a laboratory-based freshwater microcosm under simulated environmental conditions. Toxicol. Environ. Chem. 2014, 95, 1690–1701. [Google Scholar] [CrossRef]
- Li, L.; Fernández-Cruz, M.L.; Connolly, M.; Schuster, M.; Navas, J.M. Dissolution and aggregation of Cu nanoparticles in culture media: Effects of incubation temperature and particles size. J. Nanopart. Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Huang, X.; Lin, D.; Ning, K.; Sui, Y.; Hu, M.; Lu, W.; Wang, Y. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquat. Toxicol. 2016, 180, 1–10. [Google Scholar] [CrossRef]
- De Marchi, L.; Pretti, C.; Chiellini, F.; Morelli, A.; Neto, V.; Soares, A.; Figueira, E.; Freitas, R. The influence of simulated global ocean acidification on the toxic effects of carbon nanoparticles on polychaetes. Sci. Total Environ. 2019, 666, 1178–1187. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Biglari, H.; Mahvi, A.H. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. J. Chem. 2012, 9, 2453–2461. [Google Scholar] [CrossRef]
- Martins, R.; Figueiredo, J.; Sushkova, A.; Wilhelm, M.; Tedim, J.; Loureiro, S. “Smart” nanosensors for early detection of corrosion: Environmental behavior and effects on marine organisms. Environ. Pollut. 2022, 302, 118973. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.C.; Campos, D.; Kaczerewska, O.; Figueiredo, J.; Silva, S.; Sousa, I.; Maia, F.; Tedim, J.; Abessa, D.; Posão-Ferreira, P.; et al. Can the toxicity of polyethylene microplastics and engineered nanoclays on flatfish (Solea senegalensis) be influenced by the presence of each other? Sci. Total Environ. 2022, 804, 150188. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Eberl, R. Marine ecotoxicity and hazard of smart antifouling nanomaterials. In Advances in Nanotechnology for Marine Antifouling; Elsevier: Amsterdam, The Netherlands, 2023; pp. 363–378. [Google Scholar] [CrossRef]
- Yasaei, M.; Khakbiz, M.; Zamanian, A.; Ghasemi, E. Synthesis and characterization of Zn/Al-LDH SiO2 nanohybrid: Intercalation and release behavior of vitamin C. Mater. Sci. Eng. C 2019, 103, 109816. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, R.; Jing, Z.; Wan, X.; Tong, J.; Huang, W.; Liu, J. Synthesis of Zn/Al layered double hydroxides magnetic-nanoparticle for removal of humic acid. Desalinat. Water Treat. 2024, 317, 100097. [Google Scholar] [CrossRef]
- Wang, D.; Wang, P.; Wang, C.; Ao, Y. Effects of interactions between humic acid and heavy metal ions on the aggregation of TiO2 nanoparticles in water environment. Environ. Pollut. 2019, 248, 834–844. [Google Scholar] [CrossRef]
- Campos, F.; Silva, P.V.; Soares, A.M.; Martins, R.; Loureiro, S. Harmonizing nanomaterial exposure methodologies in ecotoxicology: The effects of two innovative nanoclays in the freshwater microalgae Raphidocelis subcapitata. Nanotoxicology 2023, 17, 401–419. [Google Scholar] [CrossRef]
- Carneiro, D.; Damasceno, E.P.; Ferreira, V.; Charlie-Silva, I.; Tedim, J.; Maia, F.; Loureiro, S.; Martins, R.; Pavlaki, M.D. Zn-Al layered double hydroxides induce embryo malformations and impair locomotion behavior in Danio rerio. NanoImpact 2023, 30, 100457. [Google Scholar] [CrossRef]
- Bordin, E.R.; Ramsdorf, W.A.; Domingos, L.M.L.; de Souza Miranda, L.P.; Mattoso Filho, N.P.; Cestari, M.M. Ecotoxicological effects of zinc oxide nanoparticles (ZnO-NPs) on aquatic organisms: Current research and emerging trends. J. Environ. Manag. 2024, 349, 119396. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Huang, L.; Fortin, C.; Campbell, P.G. Aluminum effects on marine phytoplankton: Implications for a revised iron hypothesis (iron–aluminum hypothesis). Biogeochemistry 2018, 139, 123–137. [Google Scholar] [CrossRef]
- Neff, J. Bioaccumulation in marine organisms. In Chapter 10—Zinc in the Ocean; Elsevier: Amsterdam, The Netherlands, 2002; pp. 175–189. [Google Scholar] [CrossRef]
- Chesne, R.B.; Kim, C.S. Zn (II) and Cu (II) adsorption and retention onto iron oxyhydroxide nanoparticles: Effects of particle aggregation and salinity. Geochem. Trans. 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Qiu, Z.; Lu, S.; Shi, X. Functionalized layered double hydroxide applied to heavy metal ions absorption: A review. Nanotechnol. Rev. 2020, 9, 800–819. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Goswami, O.; Rouff, A.A.; Elzinga, E.J. Effects of humic substances on Fe (II) sorption onto aluminum oxide and clay. Geochem. Trans. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural memory effect of Mg–Al and Zn–Al layered double hydroxides in the presence of different natural humic acids: Process and mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef]
- Yang, R.; Van den Berg, C.M. Metal complexation by humic substances in seawater. Environ. Sci. Technol. 2009, 43, 7192–7197. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wu, J.; Wu, P.; Wang, J.; Niu, W.; Yang, S.; Chen, M.; Rehman, S.; Zhu, N. Enhancing peroxymonosulfate activation of Fe-Al layered double hydroxide by dissolved organic matter: Performance and mechanism. Water Res. 2020, 185, 116246. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Hou, J.; Xu, C.; Wang, Y.; Li, J.; Xiao, F.; Wang, D. Enhanced removal of natural organic matters by calcined Mg/Al layered double hydroxide nanocrystalline particles: Adsorption, reusability and mechanism studies. Appl. Surf. Sci. 2018, 442, 45–53. [Google Scholar] [CrossRef]
- McMahon, S.J.; Munday, P.L.; Wong, M.Y.; Donelson, J.M. Elevated CO2 and food ration affect growth but not the size-based hierarchy of a reef fish. Sci. Rep. 2019, 9, 19706. [Google Scholar] [CrossRef]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behavior of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef]
- Li, D.; Cui, X.; Wen, X.; Jin, G.; Zheng, W.; Li, J.; Shi, L. Study on growth behavior and properties of LDH nanosheet arrays enhanced Ni-based coatings on Mg-Li alloy. Surf. Coat. Technol. 2023, 474, 130088. [Google Scholar] [CrossRef]
- Furxhi, I.; Costa, A.; Vázquez-Campos, S.; Fito-López, C.; Hristozov, D.; Ramos, J.A.T.; Resch, S.; Cioffi, M.; Friedrichs, S.; Rocca, C.; et al. Status, implications and challenges of European safe and sustainable by design paradigms applicable to nanomaterials and advanced materials. RSC Sustain. 2023, 1, 234–250. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, M.; Figueiredo, J.; Perina, F.C.; Abessa, D.M.S.; Martins, R. Environmental Behavior of Novel “Smart” Anti-Corrosion Nanomaterials in a Global Change Scenario. Environments 2025, 12, 264. https://doi.org/10.3390/environments12080264
Bruni M, Figueiredo J, Perina FC, Abessa DMS, Martins R. Environmental Behavior of Novel “Smart” Anti-Corrosion Nanomaterials in a Global Change Scenario. Environments. 2025; 12(8):264. https://doi.org/10.3390/environments12080264
Chicago/Turabian StyleBruni, Mariana, Joana Figueiredo, Fernando C. Perina, Denis M. S. Abessa, and Roberto Martins. 2025. "Environmental Behavior of Novel “Smart” Anti-Corrosion Nanomaterials in a Global Change Scenario" Environments 12, no. 8: 264. https://doi.org/10.3390/environments12080264
APA StyleBruni, M., Figueiredo, J., Perina, F. C., Abessa, D. M. S., & Martins, R. (2025). Environmental Behavior of Novel “Smart” Anti-Corrosion Nanomaterials in a Global Change Scenario. Environments, 12(8), 264. https://doi.org/10.3390/environments12080264







