Abstract
The progressive commercial deployment of multi-walled carbon nanotubes (MWCNTs) raises concerns about their terrestrial ecotoxicity. We exposed adult Cornu aspersum (150 snails; five replicates of three animals per time-point) to 50 mg L−1 MWCNT-dosed Lactuca sativa for 30 days and quantified five mechanistic biomarkers alongside survival. Hemocyte spread-cell area increased by 48% (from 243 ± 22 µm2 at day 0 to 360 ± 18 µm2 at day 14, p < 0.001). Lysosomal membrane stability (neutral red retention) fell twofold within 72 h and to 10 min by day 30 (controls ≈ 60 min), indicating early, persistent destabilization. Micronucleus frequency rose above the ecogenotoxic threshold of 5‰ after 7 days, peaking at 8.3 ± 0.7‰ on day 14 (p < 0.01). Hepatopancreas metallothionein concentrations doubled by day 3 (2.1 ± 0.3 vs. 1.0 ± 0.2 µg g−1 ww in controls) and remained >150% of control throughout exposure, consistent with metal impurity mobilization. Acetylcholinesterase activity in cephalic tissue declined by 50% after 7 days and by 73% after 30 days, revealing sustained neurotoxicity. Despite these pronounced sub-individual disturbances, cumulative mortality reached only 19% at day 30, suggesting substantial, but finite, physiological compensation. Collectively, the data demonstrate that a 50 mg L−1 dietary load of MWCNTs elicits rapid cytotoxic, genotoxic, and neurotoxic responses in C. aspersum that precede overt lethality, underscoring the utility of this gastropod and the chosen biomarker suite for monitoring nanotube contamination in agro-ecosystems and food-grade snail farming.
1. Introduction
In recent decades, the production and application of nanomaterials (NMs) have grown significantly. Carbon nanotubes (CNTs) represent a very interesting group of NMs for their large number of applications [1] due to their unique qualities, such as high mechanical strength, high thermal conductivity, high electrical conductivity, and good chemical stability in aggressive media, among others [2,3]. CNTs are widely used in large number of fields such as biomedicine [4], construction industry [5], electronic products [6], sensors [7], aerospace [8], antifouling products [9], agriculture [10], and have recently been employed in environmental pollution remediation [11,12].
The rising use of CNTs has led to an increase in their presence in the environment [13,14,15]. They can enter the environment directly through unintentional releases during the use and consumption of products containing CNTs, or as waste from wastewater treatment plants, waste incineration plants, and landfills [15,16]. Using these materials in agriculture could lead to persistent residues building up in the soil.
While the use of CNTs in agriculture could indeed represent a potential opportunity [10], an analysis of the risks this would pose to ecosystems and agroecosystems has yet to be carried out. Different authors have previously reported on the toxicity of these substances to various plant species [17,18,19,20,21]. Furthermore, these materials could bioaccumulate and enter the food chain [22,23].
Indeed, the high production rate of these CNTs also considerably increases the risk of environmental exposure, which may affect both vertebrate and invertebrate species [16,23] inhabiting terrestrial and aquatic ecosystems [15]. Several studies were conducted to assess the hazardousness of these NMs; the main aim was to understand the possible effects on the respiratory tract [24,25], due to their morphology, which is comparable to asbestos fibers [26], and, as such, makes them able to induce inflammatory patterns in the lung compartment causing an enhancement in the tumoral processes [27]. Their toxicity may differ on the basis of their diverse properties, such as length, surface area, aspect ratio, purity, degree of aggregation, concentration, and dose [28].
In the last decades, many studies have focused their efforts on terrestrial and aquatic organisms [14,23,29,30,31,32,33,34,35,36], with a main focus on the latter. This shift underlines the importance of understanding the effects of these NMs on different models of vertebrate and invertebrate organisms.
Studies conducted on different species of aquatic fauna have underlined that high levels of CNTs inhibit growth in zebrafish larvae, inhibit hatching, decrease survival rate, decrease heart rate, and decrease total length [30,31]. Whereas, in Artemia salina, there was a marked decrease in body growth, a decrease in hatching, and a decrease in severe mortality rates [32,33]. However, in comparison, the information available concerning the effects of CNTs on soil organisms is scarce; the most commonly used organism for these experiments was E. fetida, a terrestrial oligochaete [34,35]. Moreover, as reported by the Organisation for Economic Co-operation and Development (OECD) [37,38], multiwalled carbon nanotubes (MWCNTs) are less studied than single-walled carbon nanotubes (SWCNTs).
This results in a knowledge gap regarding the effects of carbon nanotubes on terrestrial gastropods (snails). They are an important group of molluscs used as bio-indicators of soil quality due to their ability to live in close contact with the soil and absorb pollutants through dermal contact with soil, ingestion of polluted soil/vegetation/water, and via inhalation [39,40,41]. These organisms are thoroughly studied and vastly employed for ecotoxicological studies [42,43,44,45,46], thus making them a robust experimental organism and an interesting ecotoxicological endpoint. Moreover, many species of snails are considered to be suitable for consumption by humans and are, as such, highly prized for culinary purposes [47]. Thus, these organisms play an important role in the transfer of pollutants to higher ecological levels within terrestrial trophic chains [48].
The aim of this study is to provide information on the transfer toxicity of MWCNTs in the trophic chain by the assessment of their effects on the terrestrial snail Cornu aspersum using several biomarkers such as AchE assay, morphological alteration of hemocytes, tissue levels of metallothioneins, micronuclei assay, and lysosomal membrane stability. A comparison was made between the biochemical and cellular responses to MWCNT exposure and individual-level adverse outcomes commonly used in ecotoxicology, such as impairment of mortality.
C. aspersum is a species endemic to the Mediterranean region, inhabiting coastal and maritime-influenced Mediterranean habitats. Therefore, the assessment of CNTs’ effect in this organism could also be of concern in food chains for potential effects on human health, due to the human consumption of this species.
2. Materials and Methods
2.1. Materials
Adult specimens of C. aspersum (mean wt 12.401 ± 0.2 g), obtained from a local farm, were reared in plastic cages (55 × 39 × 25 cm) under controlled conditions of temperature (20 ± 2 °C), photoperiod (18/6 light/dark regime) and humidity (85%) [49,50]. The floor of the cage was covered with blotting paper, dampened with tap water. Leaves of Lactuca sativa were administered ad libitum as food. Thin MWCNTs NANOCYL™ NC7000 were purchased from NANOCYL (Sambreville, Belgium). The MWCNTs have an average 9.5 nm external diameter by 1.5 μm mean length with a surface area of 250–300 m2/g. They were manufactured by a CCVD (catalytic carbon vapor deposition) process with a purity of 90% Carbon and 10% metal oxide, of which 9.6% was aluminium oxide with traces of iron and cobalt. This type of carbon nanotube was chosen because it has been extensively characterized by the OECD [51], but few toxicity tests have been performed (in particular on terrestrial environments) [37,51]. Diff-Quick® Kit was purchased from Dade Behring (Newark, NJ, USA), while the other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) at reagent grade.
2.2. Experimental Design
A total of 150 adult snails were selected for participation in the exposure experiment. These were subjected to a two-day period of starvation prior to the commencement of the experiment. Following this acclimatization period, the snails were divided into two groups and arranged for each condition in five replicate plastic boxes (14 × 10 × 7 cm), with each box containing three individuals. The first group (n = 75) was designated as the control group, while the second group (n = 75) was exposed to 50 mg/L MWCNTs according to other authors [52,53].
Due to the absence of a limit concentration defined by national or EU legislation to refer to for the purposes of the study carried out, it was decided to use a concentration of MWCNTs in line with other studies with different plant and invertebrate organisms [21,37,51,52,53,54,55]. The nanotubes were dispersed in water by sonication. Thereafter, to facilitate the absorption of the nanotubes by the lettuce, sections of Lactuca sativa were maintained in infusion for one hour in the aqueous solution containing nanotubes according to the method employed in other studies with different classes of contaminants [43,56,57]. Foliar consumption is one of the main natural routes of exposure to contaminants in terrestrial snails [56]. Sections were administered (every 2 days) to the treated adult group. The control group was fed L. sativa sections without MWCNTs. Animals were exposed for 30 days, and biomarker responses were monitored in control and treated animals over time (at 0, 3, 7, 14, and 30 days). Each experimental condition was performed in five replicates with three animals per box. Three animals per box were sampled at each time point. Each sample was subjected to hemolymph sampling using a sterilized hypodermic syringe (needle size 26G½: 0.45 mm × 13 mm) through a small hole made in the shell at the level of the hemocoele [58].
The hemolymph was immediately used for cytological staining of hemocytes and determination of cellular biomarkers (morphometric changes, lysosomal membrane stability, and micronuclei frequency). Each sample was then cold anaesthetized at 4 °C for 30 min, after which the cephalic portion and hepatopancreas were dissected, immersed in liquid nitrogen, and kept at −80 °C until it was used for acetylcholinesterase and metallothionein measurements. Finally, the number of deaths during the 30-day exposure period was considered as a biological endpoint at the organism level.
2.3. Morphometric Analysis
Morphometric alterations in hemocytes were determined by image analysis of Diff-Quick® (Dade Behring, Newark, NJ, USA) stained cells [57,59]. A volume of 40 μL of hemolymph diluted 1:1 in a snail ringer solution containing 97 mM NaCl, 2 mM KCl, 9 CaCl2, 9 mM HEPES, pH 7.5 with NaOH 1 M was placed on a poly-L-lysine coated slide, incubated in a humid chamber (16 °C) for 30 min and stained using the Diff-Quick® kit. The Diff-Quick stained hemocytes were observed with a light microscope (Axiostar Plus; Zeiss, Oberkochen, Germany), and the images obtained with a video camera (AxioCam ERc 5s, Zeiss, Oberkochen, Germany) were captured and digitized using ImageJ™ Version 1.54p software (public domain Java image processing program). Approximately 70 cells were analysed per sample.
2.4. Lysosomal Membrane Stability
Lysosomal membrane stability was assessed by the Neutral Red Retention Assay (NRRA) method [60] with minor modifications. Approximately 40 μL of hemolymph collected from individual snails (diluted 1:1 as above) was applied to a polylysine-coated slide and incubated in a humid chamber (16 °C) for 30 min; 40 μL of neutral red solution (995 μL saline and 5 μL neutral red solution obtained by dissolving 20 mg neutral red powder in 1 mL dimethyl sulfoxide) was added. The slides thus prepared were left in a humid chamber (16 °C) for 15 min and then observed under the microscope: every 15 min—for the first hour—and then every 30 min for the next 2 h. The time taken for 50% of the cell lysosomes to release the neutral red into the cytosol was determined.
2.5. Micronuclei Assay
For micronucleus detection, DAPI was used as a fluorescent DNA stain [61]. Briefly, 40 μL of hemolymph diluted 1:1 in the snail ringer solution described above was applied to slides, air dried, fixed in methanol, and stained for 3 min with DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) (1 μg/mL), a DNA-specific fluorescent probe. Two thousand cells with preserved cytoplasm were analyzed for each sample using an inverted photomicroscope (Zeiss AXIO Observer Z1, Zeiss, Oberkochen, Germany) equipped for fluorescence microscopy at 100× magnification. The results were expressed as MN‰ frequencies.
2.6. Metallothionein and Acetylcholinesterase Activity Measurement
Metallothionein content in C. aspersum hepatopancreas was determined by the spectrophotometric method [62], suitably modified for this species. Briefly, the single organ was homogenized in three volumes of 0.2 M sucrose, 20 mM Tris-HCl buffer, and pH 8.6, to which were added 0.006 mM leupeptine and 0.5 mM phenylmethylsulphonyl fluoride as antiproteolytic agents, and 0.01% β-mercaptoethanol as a reducing agent. The resulting homogenate was then subjected to ethanol/chloroform precipitation to obtain a partially purified metallothionein fraction. Finally, the MT concentration was quantified by evaluating the content of sulfhydryl residues according to the Ellman method, using DTNB and reduced glutathione (GSH) as standards. Data were expressed as μg MT/g wet weight.
Acetylcholinesterase (AChE) activity was measured on the cephalic portion according to the Ellman method [63]. The tissue was homogenized in Tris buffer (0.1 M, pH 7.5) at a 1:5 (w/v) ratio for 2 min. The homogenate was centrifuged at 9000× g for 20 min at 4 °C. The supernatant was removed and used for the determination of AChE activity. Enzyme activity was expressed as nmol of product developed per minute per milligram of protein. Protein concentration was determined by the Bradford assay using NanoDrop ND-1000 UV-Vis (Thermo Scientific, Waltham, MA, USA) [64].
2.7. Statistical Analysis
Statistical analysis was performed with software for analysis was GraphPad Prism™ 9 (GraphPad Software, San Diego, CA, USA). For each analysis, a Mann–Whitney test was performed. The Mann–Whitney test was used because the datasets were not normally distributed and violated the assumption of homoscedasticity.
3. Results
3.1. Cellular Biomarkers
Morphometric alterations in Cornu aspersum hemocytes following exposure to MWCNTs were assessed by analyzing spread cells stained with the Diff-Quick® kit. Cell area (μm2) was measured at multiple time points, from day 0 to day 30, using two-dimensional images processed with ImageJ® software (National Institutes of Health, USA). Compared to the controls (Figure 1A), the spread cells from exposed organisms (Figure 1B) exhibited an increase in cell area as early as day 3; however, statistically significant differences were observed at 7 days (p < 0.01), 14 days (p < 0.001), and 30 days (p < 0.001) (Figure 1C). The cell area progressively increased, reaching a maximum of approximately 360 μm2 at day 14, followed by a slight decrease that persisted until the end of the exposure period. Moreover, associated with the cell enlargement, a round cell shape was observed (Figure 1B). To further characterize the cellular effects of MWCNT exposure, lysosomal membrane stability and micronucleus formation assays were also performed.
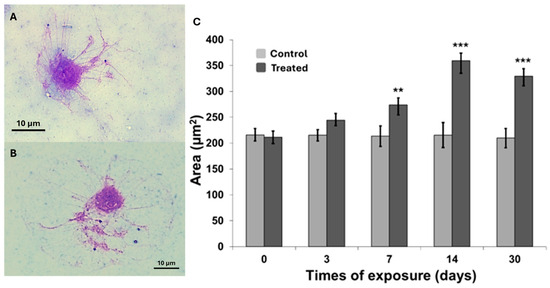
Figure 1.
Representative spread cells images from control (A) and treated (B) snails (100×, Bar 10 μm). (C) Area of two-dimensional digitized spread cells images in control and treated snails during the exposure experiments (see Materials and Methods). Data are reported as mean ± SEM (n = 70). The statistical significance of data was determined by Mann–Whitney. ** p < 0.01; *** p < 0.001.
Lysosomal membrane stability, assessed via neutral red retention assay, is presented in Figure 2. A statistically significant decrease in membrane stability was observed as early as day 3 of exposure (p < 0.05), with hemocytes from exposed organisms retaining approximately half the amount of dye compared to controls. Subsequent time points showed a progressive decline in dye retention time (Figure 2A,B), indicating increasing lysosomal membrane destabilization in treated cells. The most pronounced effect was recorded at day 30 (p < 0.001), where hemocytes retained the dye for only 10 min. Overall, a steady decrease in retention time was observed from day 3 through day 30. However, the relatively small difference between the values at days 14 and 30 suggests a potential onset of adaptive responses to the experimental conditions.
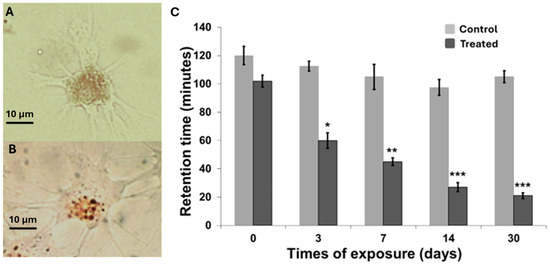
Figure 2.
Representative spread cells images stained with neutral red from control (A) and treated (B) snails after 14 days of exposure: (100×, Bar 10 μm). (C) Lysosomal membrane stability measured on spread cells by the NRRA method in snails exposed to carbon nanotubes. Data are expressed as mean ± SEM. The statistical significance of data was determined by Mann–Whitney. * p < 0.05, ** p < 0.01; *** p < 0.001.
Following the assessment of cell morphology alterations and lysosomal membrane stability, a genotoxicity biomarker was incorporated to further describe the effects of MWCNT exposure on C. aspersum hemocytes. Genotoxic damage was evaluated using the micronucleus (MNi) assay, with MNi frequency serving as an indicator of DNA damage induced by physical and/or chemical stressors [61]. The results, shown in Figure 3, display the frequency of MNi formation (Figure 3A) across the different exposure time points. A steep increase in MNi frequency (Figure 3B) was observed from day 0 to day 14, followed by a slight decrease at day 30. Notably, MNi frequency surpassed the 5‰ threshold from day 7 onwards, suggesting that genotoxic effects become clearly evident after one week of exposure.
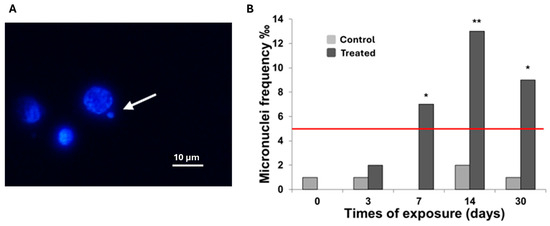
Figure 3.
(A) Representative images of micronucleus (arrow) (100×, Bar 10 μm). (B) Frequency of micronuclei was observed in snails spread cells. Data are expressed as frequency ‰. At least 1000 cells were identified for each condition examined. Statistical significance of the data (values greater than 5‰) was determined by Mann–Whitney. * p < 0.05, ** p < 0.01.
3.2. Metallothionein and Acetylcholinesterase Activity
Metallothionein (MT) levels were measured following MWCNT exposure to evaluate potential metal-binding protein responses in C. aspersum tissues. As shown in Figure 4, a significant increase in MT concentration was detected beginning at day 3 of exposure (p < 0.001), with levels continuing to rise up to day 14 (p < 0.001). Notably, MT concentrations were already more than double those of the control group at day 3 (p < 0.001). Prolonged exposure to MWCNTs stimulated a sustained increase in MT biosynthesis, resulting in an expanding divergence between treated and control organisms over time (p < 0.001). A slight decrease in MT levels was recorded by day 30, although concentrations remained elevated compared to controls.
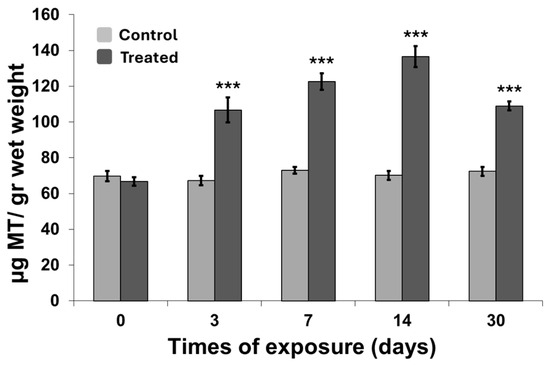
Figure 4.
Metallothionein concentration measured in organisms exposed to carbon nanotubes. Data are expressed as mean ± SEM (n = 15). The statistical significance of data was determined by Mann–Whitney. *** p < 0.001.
The acetylcholinesterase (AChE) activity of the cephalic region in C. aspersum following exposure is shown in Figure 5. In control organisms, AChE activity remained relatively stable over the 30-day period. In contrast, exposed individuals exhibited a continuous decline in AChE activity, evident from the early stages of exposure. A significant reduction was detected by day 7 (p < 0.01), with AChE activity in exposed organisms approximately 50% lower than that of controls. This downward trend persisted throughout the exposure period, culminating in markedly diminished activity by day 30 (p < 0.001), indicating sustained neurotoxic effects induced by MWCNT exposure.
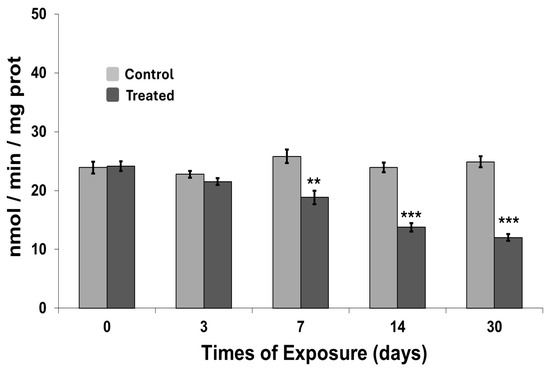
Figure 5.
Acetylcholinesterase activity measured in organisms exposed to carbon nanotubes. Data are expressed as mean ± SEM (n = 15). The statistical significance of data was determined by Mann–Whitney. ** p < 0.01; *** p < 0.001.
3.3. Mortality
Mortality rates in C. aspersum following exposure over the 30-day period are shown in Figure 6. During the initial phase (up to day 3), mortality remained within physiological limits, with no statistically significant differences between control and exposed groups. However, from day 7 onward, a significant increase in mortality was observed among exposed organisms. Although mortality remained ≤20% through day 14, it continued to rise with prolonged exposure, reaching 19% by day 30. These findings indicate a time-dependent increase in mortality associated with sustained MWCNT exposure.
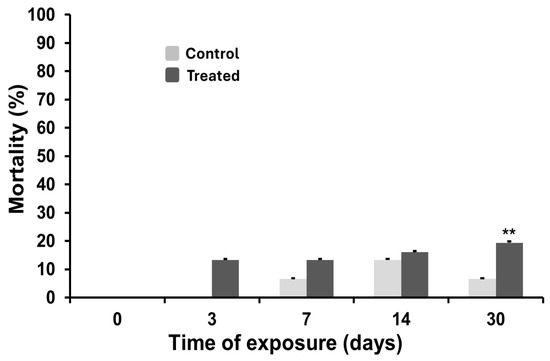
Figure 6.
Mortality rate in control and treated animals during the exposure to carbon nanotubes. The statistical significance of data was determined by Mann–Whitney. ** p < 0.01.
4. Discussion
The present study employed an integrated system of several biomarkers to assess the effects of carbon nanotubes on C. aspersum, an edible species of snail.
The focus on “cell dynamics” stems from the critical role played by the hemolymph in the overall physiology of the organism [65,66]. This fluid can serve as both a recipient and transporter of contaminants throughout the organism’s body [59,67]. Moreover, given the essential function of hemolymph’s cells in immune defence mechanisms, it is evident that morpho-functional alterations can directly influence the overall response to stress of the organism. Different studies have demonstrated that carbon nanotubes can induce alterations in immune system cells across a variety of organisms [35,36,67].
For instance, Girardello et al. [36] demonstrated that exposure to carbon nanotubes in leeches triggered inflammatory processes characterized by substantial monocyte-macrophage migration. Similarly, Calisi et al. [35] reported that carbon nanotubes directly affected earthworm granulocytes, inducing an increase in cell size and a loss of pseudopodia. Comparable results were obtained in the present study, where exposed hemocytes exhibited progressive enlargement, which was accompanied by cell rounding, suggesting a reduction in pseudopod formation (Figure 1). These findings support the hypothesis that carbon nanotubes may affect cytoskeletal organization, consistent with observations from other studies on earthworms exposed to different classes of contaminants [59]. In this study, lysosomal stability provided critical insights into the internalization kinetics of nanoparticles within lysosomes [60]. As reported by several authors, destabilization of the lysosomal membrane is recognized as a key mechanism of carbon nanotube toxicity [35,68,69,70]. It has been demonstrated that carbon nanotubes can induce oxidative stress, which may lead to lysosomal membrane destabilization through lipid peroxidation [67,71]. In the present study, a significant loss of lysosomal membrane integrity was observed as early as day 3 of exposure, with membrane stability decreasing by more than 50% compared to control organisms (Figure 2). These findings support the hypothesis that lysosomes are a primary cellular target for carbon nanotubes, enabling these nanoparticles to exert toxic effects following internalization [66,67,68,69,70].
Another adverse effect associated with carbon nanotubes, closely linked to oxidative stress, is DNA damage. In aquatic snails, the presence of carbon nanotubes has been shown to induce DNA damage because of oxidative stress [67]. In the present study, the micronucleus (MNi) test was employed to assess genotoxicity, revealing a substantial increase in MNi frequency in exposed organisms compared to controls, exceeding the 5‰ threshold (Figure 3). This is a clear indication of DNA damage and chromosomal alterations attributable to nanotube exposure, with effects becoming evident as early as seven days after the onset of treatment. Recently, Solorio-Rodriguez et al. [72] reported that MWCNTs show a higher genotoxic effect than SWCNTs in mice.
Tissue concentrations of metallothionein (MT) were also evaluated for two main reasons. First, although carbon nanotubes are composed predominantly of carbon atoms, they often contain impurities, including residual metal catalysts. In the present study, the nanotubes used exhibited a minimum impurity content of 10% (see Materials and Methods for details). Second, it was hypothesized that, upon internalization within lysosomes, carbon nanotubes could release metal particles into the intracellular environment, thereby inducing the upregulation of metal-chelating proteins such as metallothioneins [69].
An increase in metallothionein (MT) concentrations was observed as early as three days after exposure to MWCNTs. This rise peaked at day 14, followed by a slight decline (Figure 4), suggesting a potential adaptive response to the release of metal impurities from the nanotubes—an effect also reported in earthworms [35]. The elevated MT levels likely represent a protective cellular response to both the presence of metal impurities and metal-induced oxidative stress, as such contaminants can promote the generation of reactive oxygen species (ROS). Over time, ROS levels tend to decrease as antioxidant systems, including MTs, mitigate oxidative damage—a trend also observed in this study (Figure 5) [69,73]. The reduction in MT levels observed at day 30 may also result from early MT-mediated scavenging of free metal ions released from the nanotubes during the initial stages of exposure. Notably, previous studies have shown that not only heavy metals [74] but also iron—present among the impurities in the MWCNTs used in this study—can induce MT synthesis [58]. This finding supports the observed MT expression pattern and reinforces the role of MWCNT-derived metal contaminants in modulating metal-responsive proteins across diverse taxa.
CNTs have also been associated with neurotoxic effects, as reported by several authors [35,54,75]. Bernal Martinez et al. [54], for instance, have demonstrated that carbon nanotubes can attach to neuronal cell membranes in snails, potentially interfering with neural function. In the present study, a progressive inhibition of acetylcholinesterase (AChE) activity was observed over time in response to MWCNT exposure, supporting previous findings (Figure 5). Wang et al. [75] also reported that carbon nanotubes have a high affinity for AChE, promoting its adsorption and subsequent inhibition. Moreover, Albini et al. [76] demonstrate that MWCNTs themselves induce toxic effects in the rat brain, where accumulation leads to inflammation and amyloid deposition. Our findings are consistent with our own observations; indeed, amyloid deposition can result in long-term cellular toxicity, which may contribute to the development of neurodegenerative diseases and the subsequent inhibition of acetylcholinesterase activity [77].
Despite the marked alterations observed in immune function, DNA integrity, synaptic activity, and protective protein synthesis, the overall mortality rate remained relatively low. Mortality exceeded 20% only after 30 days of continuous exposure (Figure 6). This relatively low lethality, in the context of significant sublethal effects, suggests that C. aspersum could possess compensatory mechanisms capable of mitigating the toxic effects of MWCNTs over time. The mortality data, when considered alongside the observed cellular and molecular changes, indicate an ability of the organism to mount protective or adaptive responses under chronic exposure conditions.
Finally, in our study, the toxic effects of MWCNTs on snails were evaluated on nanotubes with a high percentage of impurities. Previous studies utilizing the same MWCNTs have reported a series of cascading effects arising from oxidative stress and inflammation [35,36,76]. Recent studies have demonstrated that varying degrees of purity in MWCNTs do not affect their toxicity [78,79], with all MWCNTs inducing oxidative stress [78]. The relationship between oxidative stress and the quantity of metallic impurities in MWCNTs is direct [79]. Furthermore, an increase in impurities resulted in an escalation of oxidative stress, which subsequently led to the development of inflammatory responses and subsequent damage to cells and their structures [78].
5. Conclusions
In conclusion, this study evaluated a group of cellular and biochemical responses in a bioindicator species to assess the ecotoxicological impact of multi-walled carbon nanotubes (MWCNTs) within agroecosystems and along trophic chains. The findings demonstrated that these biological responses are highly sensitive to MWCNT exposure. Compared to traditional homeostatic parameters or with individual-level adverse outcomes, the multi-biomarker approach revealed earlier onset and more pronounced effects. The use of C. aspersum as a bioindicator species in the context of a multi-biomarker approach encompassing multiple indicator species holds the potential to yield effective outcomes for detecting environmental contamination in agroecosystem health and product safety. Moreover, the integration of a multi-biomarker strategy may contribute meaningfully to the quality assessment of food-grade snails, supporting broader efforts in environmental monitoring and risk evaluation.
Author Contributions
Conceptualization, A.C.; methodology, A.C., D.G. and F.D.; software, D.G.; validation, A.C. and F.D.; formal analysis, D.G., A.C., A.F., T.S. and F.D.; investigation, A.C., D.R., C.L. and D.G.; data curation, A.C. and D.G.; writing—original draft preparation, D.G.; writing—review and editing, D.G., A.C., A.F., T.S. and F.D.; visualization, D.G.; supervision A.C. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union EXCELLENT SCIENCE—Marie Skłodowska-Curie Actions under grant agreement n° 101008099.
Institutional Review Board Statement
This study did not require ethical approval according to the legislation in force, that is, Directive 2010/63/EU of the European Parliament and of the Council.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available when required under the responsibility of the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of Carbon Nanotube Research and Development: Materials and Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Chen, X.; Ponraj, J.S.; Zhang, H. Hybrid carbon nanostructured fibers: Stepping stone for intelligent textile-based electronics. Nanoscale 2019, 11, 3046–3101. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. Chem. Med. Chem. 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Mahadevi, N.; Assein, D. Electronic applications of multi-walled carbon nanotubes in polymers: A short review. Mater. Today Proc. 2020, 33, 382–386. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Lan, H.; Nag, A.; Chen, Y.; Gao, J.; Deng, S. A review of multi-walled carbon nanotubes-based electrochemical sensors to detect heavy metals for food packaging applications. J. Alloys Compd. 2025, 1017, 179106. [Google Scholar] [CrossRef]
- Ramachandran, K.; Boopalan, V.; Bear, J.C.; Subramani, R. Multi-walled carbon nanotubes (MWCNTs)-reinforced ceramic nanocomposites for aerospace applications: A review. J. Mater. Sci. 2022, 57, 3923–3953. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Sun, T.; Liu, Q.; Pan, Y.; Qi, Z.; Ling, N.; Feng, Y.; Yu, M.; Ji, Y.; et al. Antifouling potential of multi-walled carbon nanotubes-modified chlorinated rubber-based composites on the colonization dynamics of pioneer biofilm-forming eukaryotic microbes. Int. Biodeterior. Biodegrad. 2020, 149, 104921. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Cao, J.; Zhao, Y.; Huang, J.; Zheng, Z.; Li, W.; Jiang, S.; Qiao, J.; Xing, B.; et al. Opportunities for graphene, single-walled and multi-walled carbonnanotube applications in agriculture: A review. Crop Design 2022, 1, 100006. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef] [PubMed]
- Ogunsola, S.S.; Oladipo, M.E.; Oladoye, P.O.; Kadhom, M. Carbon nanotubes for sustainable environmental remediation: A critical and comprehensive review. Nano-Struct. Nano-Objects 2024, 37, 101099. [Google Scholar] [CrossRef]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zepp, R.; Knightes, C.D. Environmental fate of multiwalled carbon nanotubes and graphene oxide across different aquatic ecosystems. NanoImpact 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Shu, X.; Zhang, W.; Kai, J.; Tang, M.; Gong, J.; Yang, J.; Lin, J.; Chai, Y.; et al. Effects of multi-walled carbon nanotubes in soil on earthworm growth and reproduction, enzymatic activities, and metabolomics. Ecotox Environ. Saf. 2022, 246, 114158. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and transport of engineered nanomaterials in the environment. J. Environ. Qual. 2010, 39, 1896–1908. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, C.; Wu, Y.; Dai, Z.; Tang, Q.; Cheng, C.; Xu, Y.; Hu, R.; Liu, C.; Chen, X.; et al. Insights into the mechanism of multi-walled carbon nanotubes phytotoxicity in Arabidopsis through transcriptome and m6A methylome analysis. Sci. Total Environ. 2021, 787, 147510. [Google Scholar] [CrossRef]
- Ikhtiari, R.; Begum, P.; Watari, F.; Fugetsu, B. Toxic effect of multiwalled carbon nanotubes on lettuce (Lactuca sativa). Nano Biomed. 2013, 5, 18–24. [Google Scholar]
- Hamdi, H.; De La Torre-Roche, R.; Hawthorne, J.; White, J.C. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.). Nanotoxicology 2014, 9, 172–180. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Zhang, W. Combined toxicity of multiwall carbon nanotubes and cadmium on rice (Oryza sativa L.) growth in soil. Front. Environ. Sci. 2024, 12, 1469172. [Google Scholar] [CrossRef]
- Zhuzhukin, K.V.; Evlakov, P.M.; Grodetskaya, T.A.; Gusev, A.A.; Zakharova, O.V.; Shuklinov, A.V.; Tomina, E.V. Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch. Forests 2023, 14, 163. [Google Scholar] [CrossRef]
- Larue, C.; Pinault, M.; Czarny, B.; Georgin, D.; Jaillard, D.; Bendiab, N.; Mayne-L’hermite, M.; Taran, F.; Dive, V.; Carrière, M. Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J. Hazard. Mat. 2012, 227–228, 155–163. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kühnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013, 7, 154. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Wang, L.; Rojanasakul, Y. The effects of carbon nanotubes on lung and dermal cellular behaviors. Nanomedicine 2014, 9, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Singh, K.P.; Gupta, S.; Dusinska, M.; Rahman, Q. Do Carbon Nanotubes and Asbestos Fibers Exhibit Common Toxicity Mechanisms? Nanomaterials 2022, 12, 1708. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [CrossRef]
- Girardi, F.A.; Bruch, G.E.; Peixoto, C.S.; Dal Bosco, L.; Sahoo, S.K.; Gonçalves, C.O.; Santos, A.P.; Furtado, C.A.; Fantini, C.; Barros, D.M. Toxicity of single-wall carbon nanotubes functionalized with polyethylene glycol in zebrafish (Danio rerio) embryos. J. Appl. Toxicol. 2017, 37, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhu, S.; Li, J.; Hui, X.; Wang, G.X. The developmental toxicity, bioaccumulation and distribution of oxidized single walled carbon nanotubes in Artemia salina. Toxicol. Res. 2018, 7, 897–906. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Tu, X.; Chen, W.C.; Zhu, B.; Wang, G.X. Developmental toxicity of oxidized multi-walled carbon nanotubes on Artemia salina cysts and larvae: Uptake, accumulation, excretion and toxic responses. Environ. Pollut. 2017, 229, 679–687. [Google Scholar] [CrossRef]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef]
- Calisi, A.; Grimaldi, A.; Leomanni, A.; Lionetto, M.G.; Dondero, F.; Schettino, T. Multibiomarker response in the earthworm Eisenia fetida as tool for assessing multi-walled carbon nanotube ecotoxicity. Ecotoxicology 2016, 25, 677–687. [Google Scholar] [CrossRef]
- Girardello, R.; Tasselli, S.; Baranzini, N.; Valvassori, R.; de Eguileor, M.; Grimaldi, A. Effects of Carbon Nanotube Environmental Dispersion on an Aquatic Invertebrate, Hirudo medicinalis. PLoS ONE 2015, 10, e0144361. [Google Scholar] [CrossRef] [PubMed]
- OECD. Multiwalled Carbon Nanotubes (MWCNT): Summary of the Dossier; OECD Series on the Safety of Manufactured Nanomaterials and other Advanced Materials; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Single Walled Carbon Nanotubes (SWCNTs): Summary of the Dossier; OECD Series on the Safety of Manufactured Nanomaterials and other Advanced Materials; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Helix aspersa as sentinel of development damage for biomonitoring purpose: A validation study. Mol. Reprod. Dev. 2019, 86, 1283–1291. [Google Scholar] [CrossRef]
- El-Gendy, K.S.; Gad, A.F.; Radwan, M.A. Physiological and behavioral responses of land molluscs as biomarkers for pollution impact assessment: A review. Environ. Res. 2021, 193, 110558. [Google Scholar] [CrossRef]
- Gomot-de, V.A.; Pihan, F. Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails: Metal bioavailability and bioaccumulation. Environ. Toxicol. Chem. 2002, 21, 820–827. [Google Scholar] [CrossRef]
- Dhiman, V.; Pant, D. Environmental biomonitoring by snails. Biomarkers 2021, 26, 221–239. [Google Scholar] [CrossRef]
- Leomanni, A.; Schettino, T.; Calisi, A.; Gorbi, S.; Mezzelani, M.; Regoli, F.; Lionetto, M.G. Antioxidant and oxidative stress related responses in the mediterranean land snail Cantareus apertus exposed to the carbammate pesticide carbaryl. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 168, 20–27. [Google Scholar] [CrossRef]
- Vranković, J.; Janković-Tomanić, M.; Vukov, T. Comparative assessment of biomarker response to tissue metal concentrations in urban populations of the land snail Helix pomatia (Pulmonata: Helicidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 245, 110448. [Google Scholar] [CrossRef]
- de Vaufleury, A. Landsnail for Ecotoxicological Assessment of Chemicals and Soil Contamination–Ecotoxicological Assessment of Chemicals and Contaminated Soils Using the Terrestrial Snail, Helix aspersa, at Various Stage of Its Life Cycle: A Review. In Environmental Indicators; Armon, R., Hänninen, O., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 365–391. [Google Scholar] [CrossRef]
- Coeurdassier, M.; Gomot-De Vaufleury, A.; Saint-Denis, M.; Ribera, D.; Narbonne, J.F.; Badot, P.M. Effects of dimethoate on snail B-esterase and growth as a function of dose, time and exposure route in a laboratory bioassay. Biomarkers 2002, 7, 138–150. [Google Scholar] [CrossRef]
- Rygało-Galewska, A.; Zglińska, K.; Niemiec, T. Edible Snail Production in Europe. Animals 2022, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Staikou, A.; Lazaridou, M. Effect of crowding on growth and mortality in the edible snail Helix lucorum (Gastropoda: Pulmonata) in Greece. Isr. J. Zool. 2013, 36, 1–9. [Google Scholar] [CrossRef]
- De Vaufleury, A.; Gimbert, F. Obtention du cycle de vie complete d’Helix aperta Born de sites tunisiens en conditions contrôlées. Influence de la photopériode. C.R. Biol. 2009, 332, 795–805. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS 15952; Soil Quality—Effects of Pollutants on Juvenile Land Snails (Helicidae)—Determination of the Effects on Growth by Soil Contamination. International Organization for Standardization: Geneva, Switzerland, 2006.
- OECD. PART 1—Series on the Safety of Manufactured Nanomaterials No. 49. In Dossier on Multiwalled Carbon Nanotubes (MWCNT); OECD Publishing: Paris, France, 2015. [Google Scholar]
- Scott-Fordsman, J.J.; Krogh, P.H.; Schaefer, M.; Johansen, A. The toxicity testing of double-walled nanotubes-contaminated food to Eisenia veneta earthworms. Ecotoxicol. Environ. Saf. 2008, 71, 616–619. [Google Scholar] [CrossRef]
- Petersen, E.J.; Pinto, R.A.; Mai, D.J.; Landrum, P.F.; Weber, W.J., Jr. Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna. Environ. Sci. Technol. 2011, 45, 1133–1138. [Google Scholar] [CrossRef]
- Bernal-Martinez, J.; Godínez-Fernández, R.; Aguilar-Elgue-zabal, A. Suitability of the Composite Made of Multi Wall Carbon Nanotubes-Polyvinylpyrrolidone for Culturing Invertebrate Helix aspersa Neurons. J. Mater. Sci. Chem. Eng. 2017, 5, 41–50. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hunziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) is regulated by single one cell type: Differently responsive metal pools in rhogocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Leomanni, A.; Schettino, T.; Calisi, A.; Lionetto, M.G. Mercury induced haemocyte alterations in the terrestrial snail Cantareus apertus as novel biomarker. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 183, 20–27. [Google Scholar] [CrossRef]
- Regoli, F.; Gorbi, S.; Fattorini, D.; Tedesco, S.; Notti, A.; Machella, N.; Bocchetti, R.; Benedetti, M.; Piva, F. Use of the land snail Helix aspersa as a sentinel organism for monitoring ecotoxicology effects of urban pollution: An integrated approach. Environ. Health Perspect. 2006, 114, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Calisi, A.; Cappello, T.; Angelelli, M.; Maisano, M.; Rotondo, D.; Gualandris, D.; Semeraro, T.; Dondero, F. Non-destructive biomarkers in non-target species earthworm Lumbricus terrestris for assessment of different agrochemicals. Environments 2024, 11, 276. [Google Scholar] [CrossRef]
- Lowe, D.M.; Pipe, R.K. Contaminant induced lysosomal membrane damage in marine mussel digestive cells: An in vivo study. Aquat. Toxicol. 1994, 30, 357–365. [Google Scholar] [CrossRef]
- Calisi, A.; Giordano, M.E.; Dondero, F.; Maisano, M.; Fasulo, S.; Lionetto, M.G. Morphological and functional alterations in hemocytes of Mytilus galloprovincialis exposed in high-impact anthropogenic sites. Mar. Environ. Res. 2023, 188, 105988. [Google Scholar] [CrossRef]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterrarenan and Antarctic molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Ellman, G.L.; Cortney, K.D.; Andrea, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Desjardins, P.; Hansen, J.B.; Allen, M. Microvolume protein concentration determination using the NanoDrop 2000c spectrophotometer. J. Vis. Exp. 2009, 33, 1610. [Google Scholar]
- Louzon, M.; de Vaufleury, A.; Capelli, N. Ecogenotoxicity assessment with land snails: A mini-review. Mutat. Res.–Rev. Mutat. Res. 2023, 792, 108472. [Google Scholar] [CrossRef]
- Baroudi, F.; Al Alam, J.; Fajloun, Z.; Millet, M. Snail as sentinel organism for monitoring the environmental pollution; a review. Ecol. Indic. 2020, 113, 106240. [Google Scholar] [CrossRef]
- Ali, D.; Ahmed, M.; Alarifi, S.; Ali, H. Ecotoxicity of single-wall carbon nanotubes to freshwater snail Lymnaea luteola L.: Impacts on oxidative stress and genotoxicity. Environ. Toxicol. 2015, 30, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Li, Y.; Yin, J.-J.; Liu, Y.; Wang, L.; Zhao, Y.; Chen, C. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4, e32. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef]
- Møller, P.; Vest Christophersen, D.; Jensen, D.M.; Kermanizadeh, A.; Roursgaard, M.; Jacobsen, N.R.; Hemmingsen, J.G.; Danielsen, P.H.; Cao, Y.; Jantzen, K.; et al. Role of oxidative stress in carbon nanotube-generated health effects. Arch. Toxicol. 2014, 88, 1939–1964. [Google Scholar] [CrossRef]
- Solorio-Rodriguez, S.A.; Williams, A.; Poulsen, S.S.; Knudsen, K.B.; Jensen, K.A.; Clausen, P.A.; Danielsen, P.H.; Wallin, H.; Vogel, U.; Halappanavar, S. Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs. Nanomaterials 2023, 13, 1059. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Gualandris, D.; Rotondo, D.; Lorusso, C.; La Terza, A.; Calisi, A.; Dondero, F. The Metallothionein System in Tetrahymena thermophila Is Iron-Inducible. Toxics 2024, 12, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere 2009, 77, 67–73. [Google Scholar] [CrossRef]
- Noonan, D.M.; Pagani, A.; Pulze, L.; Bruno, A.; Principi, E.; Congiu, T.; Grimaldi, A.; Bassani, B.; De Flora, S.; Gini, E.; et al. Environmental impact of multi-wall carbon nanotubes in a novel model of exposure: Systemic distribution, macrophage accumulation, and amyloid deposition. Int. J. Nanomed. 2015, 10, 6133–6145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, S.H.; Park, J.C.; Byun, M.S.; Yi, D.; Lee, J.H.; Lee, D.Y.; Jung, I.M. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol. Aging 2019, 73, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Vitkina, T.I.; Yankova, V.I.; Gvozdenko, T.A.; Kuznetsov, V.L.; Krasnikov, D.V.; Nazarenko, A.V. The impact of multi-walled carbon nanotubes with different amount of metallic impurities on immunometabolic parameters in healthy volunteers. Food Chem. Toxicol. 2016, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F.; Lim, C.T.; Leong, D.T.; Neto, A.H.C.; Garaj, S.; Rosa, V. Cytotoxicity survey of commercial graphene materials from worldwide. NPJ 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).