Effect of Multi-Walled Carbon Nanotubes in the Snail Cornu aspersum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Morphometric Analysis
2.4. Lysosomal Membrane Stability
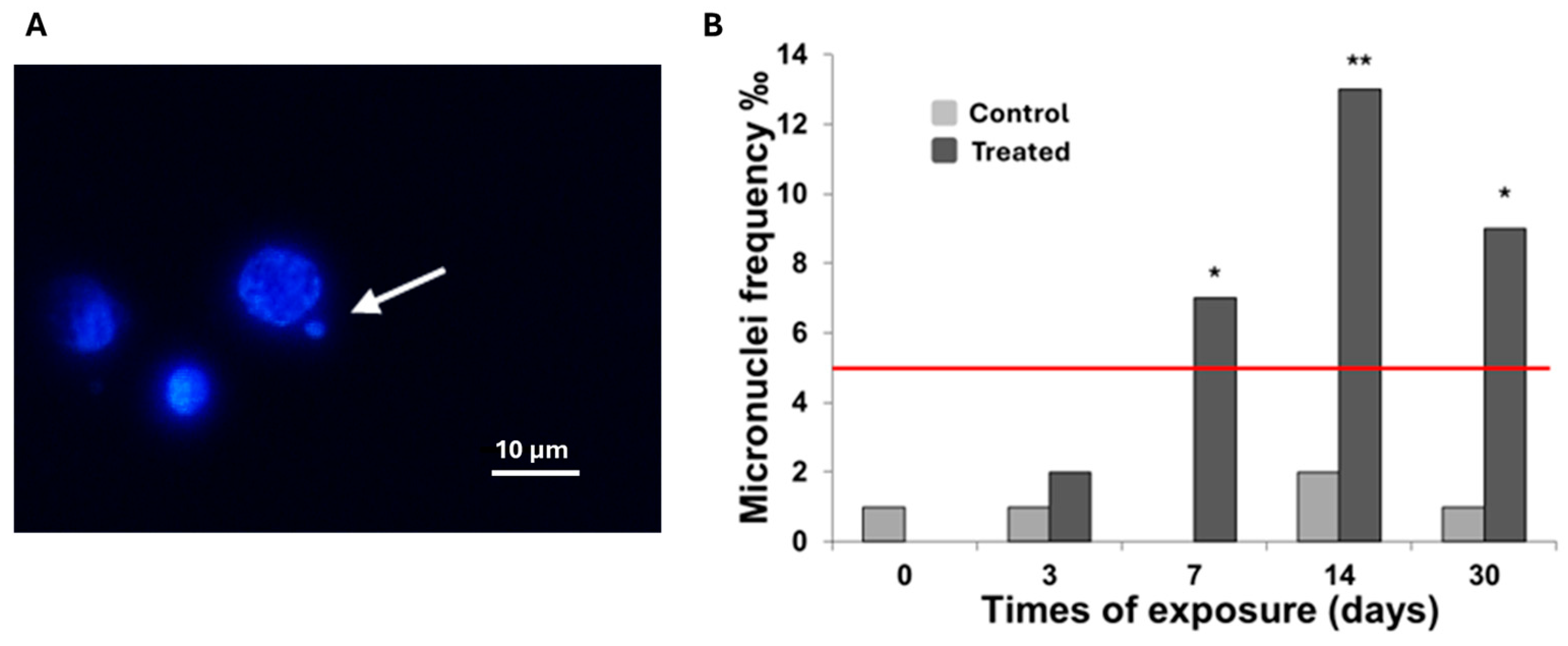
2.5. Micronuclei Assay
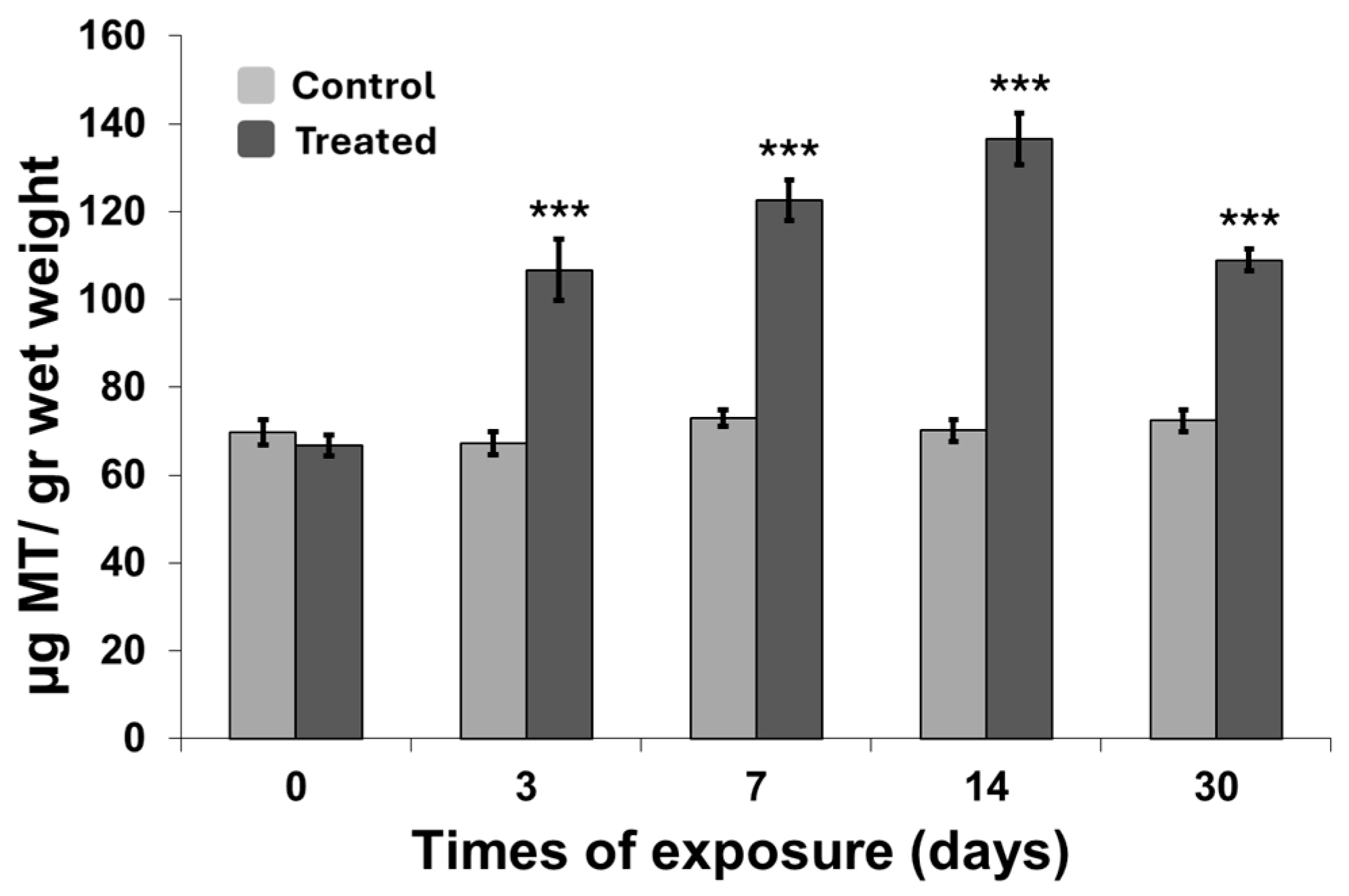
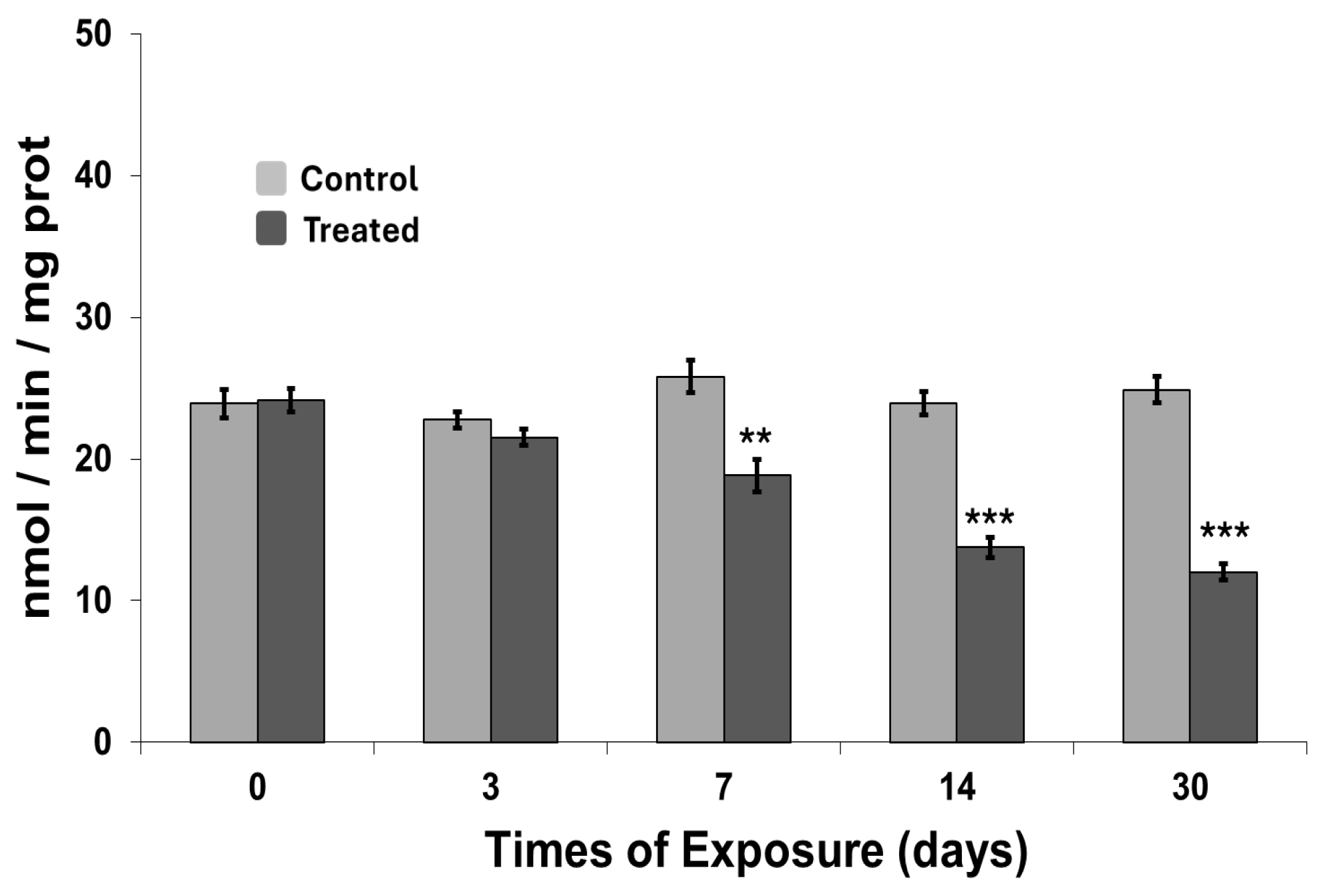
2.6. Metallothionein and Acetylcholinesterase Activity Measurement
2.7. Statistical Analysis
3. Results
3.1. Cellular Biomarkers
3.2. Metallothionein and Acetylcholinesterase Activity
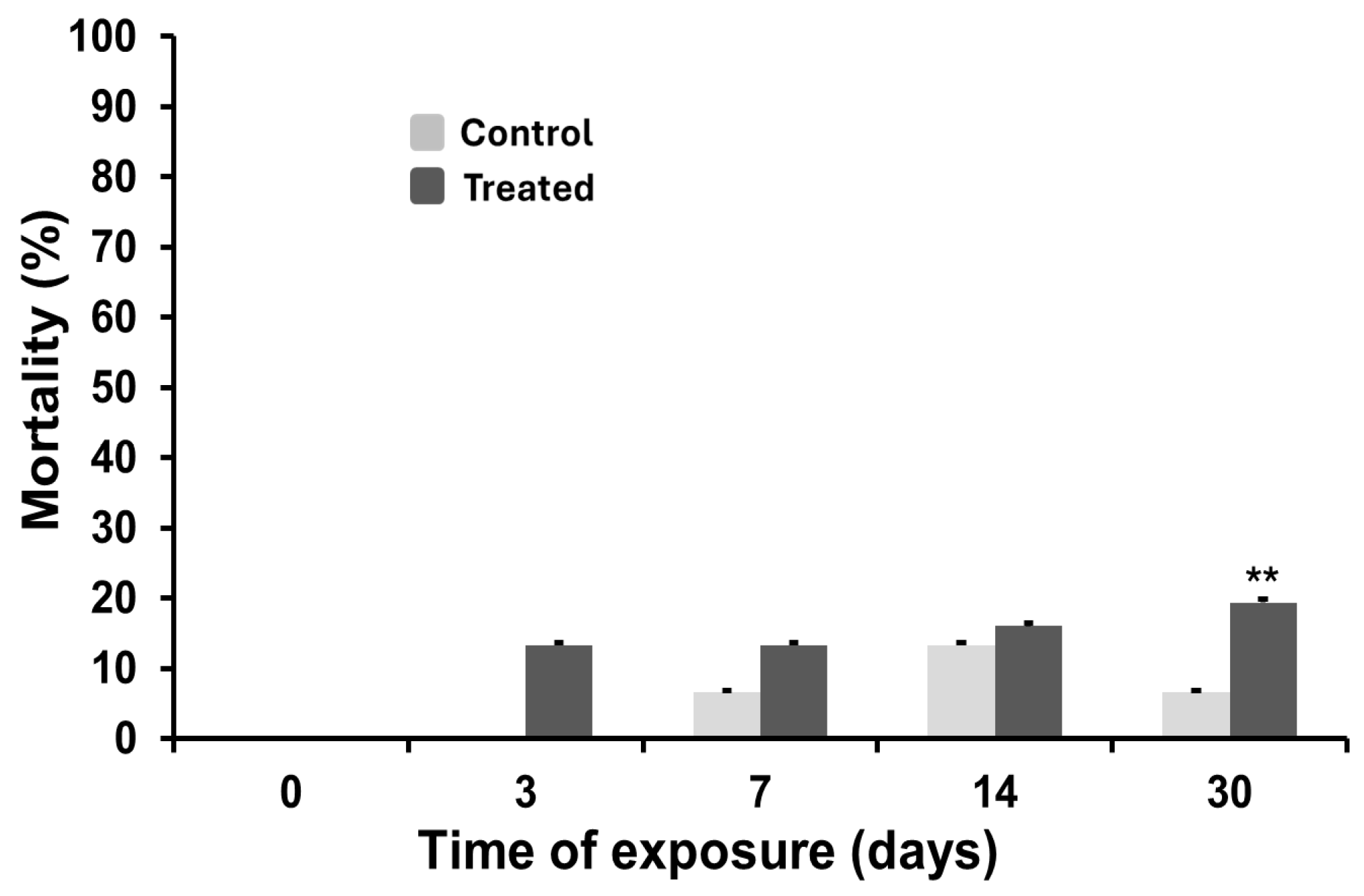
3.3. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of Carbon Nanotube Research and Development: Materials and Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Chen, X.; Ponraj, J.S.; Zhang, H. Hybrid carbon nanostructured fibers: Stepping stone for intelligent textile-based electronics. Nanoscale 2019, 11, 3046–3101. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. Chem. Med. Chem. 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Mahadevi, N.; Assein, D. Electronic applications of multi-walled carbon nanotubes in polymers: A short review. Mater. Today Proc. 2020, 33, 382–386. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Lan, H.; Nag, A.; Chen, Y.; Gao, J.; Deng, S. A review of multi-walled carbon nanotubes-based electrochemical sensors to detect heavy metals for food packaging applications. J. Alloys Compd. 2025, 1017, 179106. [Google Scholar] [CrossRef]
- Ramachandran, K.; Boopalan, V.; Bear, J.C.; Subramani, R. Multi-walled carbon nanotubes (MWCNTs)-reinforced ceramic nanocomposites for aerospace applications: A review. J. Mater. Sci. 2022, 57, 3923–3953. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Sun, T.; Liu, Q.; Pan, Y.; Qi, Z.; Ling, N.; Feng, Y.; Yu, M.; Ji, Y.; et al. Antifouling potential of multi-walled carbon nanotubes-modified chlorinated rubber-based composites on the colonization dynamics of pioneer biofilm-forming eukaryotic microbes. Int. Biodeterior. Biodegrad. 2020, 149, 104921. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Cao, J.; Zhao, Y.; Huang, J.; Zheng, Z.; Li, W.; Jiang, S.; Qiao, J.; Xing, B.; et al. Opportunities for graphene, single-walled and multi-walled carbonnanotube applications in agriculture: A review. Crop Design 2022, 1, 100006. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef] [PubMed]
- Ogunsola, S.S.; Oladipo, M.E.; Oladoye, P.O.; Kadhom, M. Carbon nanotubes for sustainable environmental remediation: A critical and comprehensive review. Nano-Struct. Nano-Objects 2024, 37, 101099. [Google Scholar] [CrossRef]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zepp, R.; Knightes, C.D. Environmental fate of multiwalled carbon nanotubes and graphene oxide across different aquatic ecosystems. NanoImpact 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Shu, X.; Zhang, W.; Kai, J.; Tang, M.; Gong, J.; Yang, J.; Lin, J.; Chai, Y.; et al. Effects of multi-walled carbon nanotubes in soil on earthworm growth and reproduction, enzymatic activities, and metabolomics. Ecotox Environ. Saf. 2022, 246, 114158. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and transport of engineered nanomaterials in the environment. J. Environ. Qual. 2010, 39, 1896–1908. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, C.; Wu, Y.; Dai, Z.; Tang, Q.; Cheng, C.; Xu, Y.; Hu, R.; Liu, C.; Chen, X.; et al. Insights into the mechanism of multi-walled carbon nanotubes phytotoxicity in Arabidopsis through transcriptome and m6A methylome analysis. Sci. Total Environ. 2021, 787, 147510. [Google Scholar] [CrossRef]
- Ikhtiari, R.; Begum, P.; Watari, F.; Fugetsu, B. Toxic effect of multiwalled carbon nanotubes on lettuce (Lactuca sativa). Nano Biomed. 2013, 5, 18–24. [Google Scholar]
- Hamdi, H.; De La Torre-Roche, R.; Hawthorne, J.; White, J.C. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.). Nanotoxicology 2014, 9, 172–180. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Zhang, W. Combined toxicity of multiwall carbon nanotubes and cadmium on rice (Oryza sativa L.) growth in soil. Front. Environ. Sci. 2024, 12, 1469172. [Google Scholar] [CrossRef]
- Zhuzhukin, K.V.; Evlakov, P.M.; Grodetskaya, T.A.; Gusev, A.A.; Zakharova, O.V.; Shuklinov, A.V.; Tomina, E.V. Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch. Forests 2023, 14, 163. [Google Scholar] [CrossRef]
- Larue, C.; Pinault, M.; Czarny, B.; Georgin, D.; Jaillard, D.; Bendiab, N.; Mayne-L’hermite, M.; Taran, F.; Dive, V.; Carrière, M. Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J. Hazard. Mat. 2012, 227–228, 155–163. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kühnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013, 7, 154. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Wang, L.; Rojanasakul, Y. The effects of carbon nanotubes on lung and dermal cellular behaviors. Nanomedicine 2014, 9, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Singh, K.P.; Gupta, S.; Dusinska, M.; Rahman, Q. Do Carbon Nanotubes and Asbestos Fibers Exhibit Common Toxicity Mechanisms? Nanomaterials 2022, 12, 1708. [Google Scholar] [CrossRef]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [CrossRef]
- Girardi, F.A.; Bruch, G.E.; Peixoto, C.S.; Dal Bosco, L.; Sahoo, S.K.; Gonçalves, C.O.; Santos, A.P.; Furtado, C.A.; Fantini, C.; Barros, D.M. Toxicity of single-wall carbon nanotubes functionalized with polyethylene glycol in zebrafish (Danio rerio) embryos. J. Appl. Toxicol. 2017, 37, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhu, S.; Li, J.; Hui, X.; Wang, G.X. The developmental toxicity, bioaccumulation and distribution of oxidized single walled carbon nanotubes in Artemia salina. Toxicol. Res. 2018, 7, 897–906. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Tu, X.; Chen, W.C.; Zhu, B.; Wang, G.X. Developmental toxicity of oxidized multi-walled carbon nanotubes on Artemia salina cysts and larvae: Uptake, accumulation, excretion and toxic responses. Environ. Pollut. 2017, 229, 679–687. [Google Scholar] [CrossRef]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef]
- Calisi, A.; Grimaldi, A.; Leomanni, A.; Lionetto, M.G.; Dondero, F.; Schettino, T. Multibiomarker response in the earthworm Eisenia fetida as tool for assessing multi-walled carbon nanotube ecotoxicity. Ecotoxicology 2016, 25, 677–687. [Google Scholar] [CrossRef]
- Girardello, R.; Tasselli, S.; Baranzini, N.; Valvassori, R.; de Eguileor, M.; Grimaldi, A. Effects of Carbon Nanotube Environmental Dispersion on an Aquatic Invertebrate, Hirudo medicinalis. PLoS ONE 2015, 10, e0144361. [Google Scholar] [CrossRef] [PubMed]
- OECD. Multiwalled Carbon Nanotubes (MWCNT): Summary of the Dossier; OECD Series on the Safety of Manufactured Nanomaterials and other Advanced Materials; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Single Walled Carbon Nanotubes (SWCNTs): Summary of the Dossier; OECD Series on the Safety of Manufactured Nanomaterials and other Advanced Materials; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Helix aspersa as sentinel of development damage for biomonitoring purpose: A validation study. Mol. Reprod. Dev. 2019, 86, 1283–1291. [Google Scholar] [CrossRef]
- El-Gendy, K.S.; Gad, A.F.; Radwan, M.A. Physiological and behavioral responses of land molluscs as biomarkers for pollution impact assessment: A review. Environ. Res. 2021, 193, 110558. [Google Scholar] [CrossRef]
- Gomot-de, V.A.; Pihan, F. Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails: Metal bioavailability and bioaccumulation. Environ. Toxicol. Chem. 2002, 21, 820–827. [Google Scholar] [CrossRef]
- Dhiman, V.; Pant, D. Environmental biomonitoring by snails. Biomarkers 2021, 26, 221–239. [Google Scholar] [CrossRef]
- Leomanni, A.; Schettino, T.; Calisi, A.; Gorbi, S.; Mezzelani, M.; Regoli, F.; Lionetto, M.G. Antioxidant and oxidative stress related responses in the mediterranean land snail Cantareus apertus exposed to the carbammate pesticide carbaryl. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 168, 20–27. [Google Scholar] [CrossRef]
- Vranković, J.; Janković-Tomanić, M.; Vukov, T. Comparative assessment of biomarker response to tissue metal concentrations in urban populations of the land snail Helix pomatia (Pulmonata: Helicidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 245, 110448. [Google Scholar] [CrossRef]
- de Vaufleury, A. Landsnail for Ecotoxicological Assessment of Chemicals and Soil Contamination–Ecotoxicological Assessment of Chemicals and Contaminated Soils Using the Terrestrial Snail, Helix aspersa, at Various Stage of Its Life Cycle: A Review. In Environmental Indicators; Armon, R., Hänninen, O., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 365–391. [Google Scholar] [CrossRef]
- Coeurdassier, M.; Gomot-De Vaufleury, A.; Saint-Denis, M.; Ribera, D.; Narbonne, J.F.; Badot, P.M. Effects of dimethoate on snail B-esterase and growth as a function of dose, time and exposure route in a laboratory bioassay. Biomarkers 2002, 7, 138–150. [Google Scholar] [CrossRef]
- Rygało-Galewska, A.; Zglińska, K.; Niemiec, T. Edible Snail Production in Europe. Animals 2022, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Staikou, A.; Lazaridou, M. Effect of crowding on growth and mortality in the edible snail Helix lucorum (Gastropoda: Pulmonata) in Greece. Isr. J. Zool. 2013, 36, 1–9. [Google Scholar] [CrossRef]
- De Vaufleury, A.; Gimbert, F. Obtention du cycle de vie complete d’Helix aperta Born de sites tunisiens en conditions contrôlées. Influence de la photopériode. C.R. Biol. 2009, 332, 795–805. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS 15952; Soil Quality—Effects of Pollutants on Juvenile Land Snails (Helicidae)—Determination of the Effects on Growth by Soil Contamination. International Organization for Standardization: Geneva, Switzerland, 2006.
- OECD. PART 1—Series on the Safety of Manufactured Nanomaterials No. 49. In Dossier on Multiwalled Carbon Nanotubes (MWCNT); OECD Publishing: Paris, France, 2015. [Google Scholar]
- Scott-Fordsman, J.J.; Krogh, P.H.; Schaefer, M.; Johansen, A. The toxicity testing of double-walled nanotubes-contaminated food to Eisenia veneta earthworms. Ecotoxicol. Environ. Saf. 2008, 71, 616–619. [Google Scholar] [CrossRef]
- Petersen, E.J.; Pinto, R.A.; Mai, D.J.; Landrum, P.F.; Weber, W.J., Jr. Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna. Environ. Sci. Technol. 2011, 45, 1133–1138. [Google Scholar] [CrossRef]
- Bernal-Martinez, J.; Godínez-Fernández, R.; Aguilar-Elgue-zabal, A. Suitability of the Composite Made of Multi Wall Carbon Nanotubes-Polyvinylpyrrolidone for Culturing Invertebrate Helix aspersa Neurons. J. Mater. Sci. Chem. Eng. 2017, 5, 41–50. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Hödl, E.; Prem, C.; Hunziker, P.; Manzl, C. Copper in Helix pomatia (Gastropoda) is regulated by single one cell type: Differently responsive metal pools in rhogocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Leomanni, A.; Schettino, T.; Calisi, A.; Lionetto, M.G. Mercury induced haemocyte alterations in the terrestrial snail Cantareus apertus as novel biomarker. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 183, 20–27. [Google Scholar] [CrossRef]
- Regoli, F.; Gorbi, S.; Fattorini, D.; Tedesco, S.; Notti, A.; Machella, N.; Bocchetti, R.; Benedetti, M.; Piva, F. Use of the land snail Helix aspersa as a sentinel organism for monitoring ecotoxicology effects of urban pollution: An integrated approach. Environ. Health Perspect. 2006, 114, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Calisi, A.; Cappello, T.; Angelelli, M.; Maisano, M.; Rotondo, D.; Gualandris, D.; Semeraro, T.; Dondero, F. Non-destructive biomarkers in non-target species earthworm Lumbricus terrestris for assessment of different agrochemicals. Environments 2024, 11, 276. [Google Scholar] [CrossRef]
- Lowe, D.M.; Pipe, R.K. Contaminant induced lysosomal membrane damage in marine mussel digestive cells: An in vivo study. Aquat. Toxicol. 1994, 30, 357–365. [Google Scholar] [CrossRef]
- Calisi, A.; Giordano, M.E.; Dondero, F.; Maisano, M.; Fasulo, S.; Lionetto, M.G. Morphological and functional alterations in hemocytes of Mytilus galloprovincialis exposed in high-impact anthropogenic sites. Mar. Environ. Res. 2023, 188, 105988. [Google Scholar] [CrossRef]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterrarenan and Antarctic molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Ellman, G.L.; Cortney, K.D.; Andrea, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Desjardins, P.; Hansen, J.B.; Allen, M. Microvolume protein concentration determination using the NanoDrop 2000c spectrophotometer. J. Vis. Exp. 2009, 33, 1610. [Google Scholar]
- Louzon, M.; de Vaufleury, A.; Capelli, N. Ecogenotoxicity assessment with land snails: A mini-review. Mutat. Res.–Rev. Mutat. Res. 2023, 792, 108472. [Google Scholar] [CrossRef]
- Baroudi, F.; Al Alam, J.; Fajloun, Z.; Millet, M. Snail as sentinel organism for monitoring the environmental pollution; a review. Ecol. Indic. 2020, 113, 106240. [Google Scholar] [CrossRef]
- Ali, D.; Ahmed, M.; Alarifi, S.; Ali, H. Ecotoxicity of single-wall carbon nanotubes to freshwater snail Lymnaea luteola L.: Impacts on oxidative stress and genotoxicity. Environ. Toxicol. 2015, 30, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Li, Y.; Yin, J.-J.; Liu, Y.; Wang, L.; Zhao, Y.; Chen, C. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4, e32. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef]
- Møller, P.; Vest Christophersen, D.; Jensen, D.M.; Kermanizadeh, A.; Roursgaard, M.; Jacobsen, N.R.; Hemmingsen, J.G.; Danielsen, P.H.; Cao, Y.; Jantzen, K.; et al. Role of oxidative stress in carbon nanotube-generated health effects. Arch. Toxicol. 2014, 88, 1939–1964. [Google Scholar] [CrossRef]
- Solorio-Rodriguez, S.A.; Williams, A.; Poulsen, S.S.; Knudsen, K.B.; Jensen, K.A.; Clausen, P.A.; Danielsen, P.H.; Wallin, H.; Vogel, U.; Halappanavar, S. Single-Walled vs. Multi-Walled Carbon Nanotubes: Influence of Physico-Chemical Properties on Toxicogenomics Responses in Mouse Lungs. Nanomaterials 2023, 13, 1059. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Gualandris, D.; Rotondo, D.; Lorusso, C.; La Terza, A.; Calisi, A.; Dondero, F. The Metallothionein System in Tetrahymena thermophila Is Iron-Inducible. Toxics 2024, 12, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere 2009, 77, 67–73. [Google Scholar] [CrossRef]
- Noonan, D.M.; Pagani, A.; Pulze, L.; Bruno, A.; Principi, E.; Congiu, T.; Grimaldi, A.; Bassani, B.; De Flora, S.; Gini, E.; et al. Environmental impact of multi-wall carbon nanotubes in a novel model of exposure: Systemic distribution, macrophage accumulation, and amyloid deposition. Int. J. Nanomed. 2015, 10, 6133–6145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, S.H.; Park, J.C.; Byun, M.S.; Yi, D.; Lee, J.H.; Lee, D.Y.; Jung, I.M. Blood acetylcholinesterase level is a potential biomarker for the early detection of cerebral amyloid deposition in cognitively normal individuals. Neurobiol. Aging 2019, 73, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Vitkina, T.I.; Yankova, V.I.; Gvozdenko, T.A.; Kuznetsov, V.L.; Krasnikov, D.V.; Nazarenko, A.V. The impact of multi-walled carbon nanotubes with different amount of metallic impurities on immunometabolic parameters in healthy volunteers. Food Chem. Toxicol. 2016, 87, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F.; Lim, C.T.; Leong, D.T.; Neto, A.H.C.; Garaj, S.; Rosa, V. Cytotoxicity survey of commercial graphene materials from worldwide. NPJ 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualandris, D.; Dondero, F.; Franzin, A.; Rotondo, D.; Lorusso, C.; Semeraro, T.; Calisi, A. Effect of Multi-Walled Carbon Nanotubes in the Snail Cornu aspersum. Environments 2025, 12, 213. https://doi.org/10.3390/environments12070213
Gualandris D, Dondero F, Franzin A, Rotondo D, Lorusso C, Semeraro T, Calisi A. Effect of Multi-Walled Carbon Nanotubes in the Snail Cornu aspersum. Environments. 2025; 12(7):213. https://doi.org/10.3390/environments12070213
Chicago/Turabian StyleGualandris, Davide, Francesco Dondero, Alberico Franzin, Davide Rotondo, Candida Lorusso, Teodoro Semeraro, and Antonio Calisi. 2025. "Effect of Multi-Walled Carbon Nanotubes in the Snail Cornu aspersum" Environments 12, no. 7: 213. https://doi.org/10.3390/environments12070213
APA StyleGualandris, D., Dondero, F., Franzin, A., Rotondo, D., Lorusso, C., Semeraro, T., & Calisi, A. (2025). Effect of Multi-Walled Carbon Nanotubes in the Snail Cornu aspersum. Environments, 12(7), 213. https://doi.org/10.3390/environments12070213













