Composted Sludge and Trichoderma harzianum T-22 as a Dual Strategy to Enhance Wheat Growth and Soil Microbial Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design for Trichoderma Inoculation in Wheat (Triticum turgidum L. Var. Durum)
2.2. Sampling and Measurement
2.2.1. Crop
2.2.2. Characterization of Substrates
Determination of Physicochemical Parameters
2.2.3. Determination of Mycoflora of Substrates Before Sowing
2.2.4. Statistical Procedure
3. Results and Discussion
3.1. Results in Substrates
3.2. Results in Plant Material
3.2.1. Physical Parameters
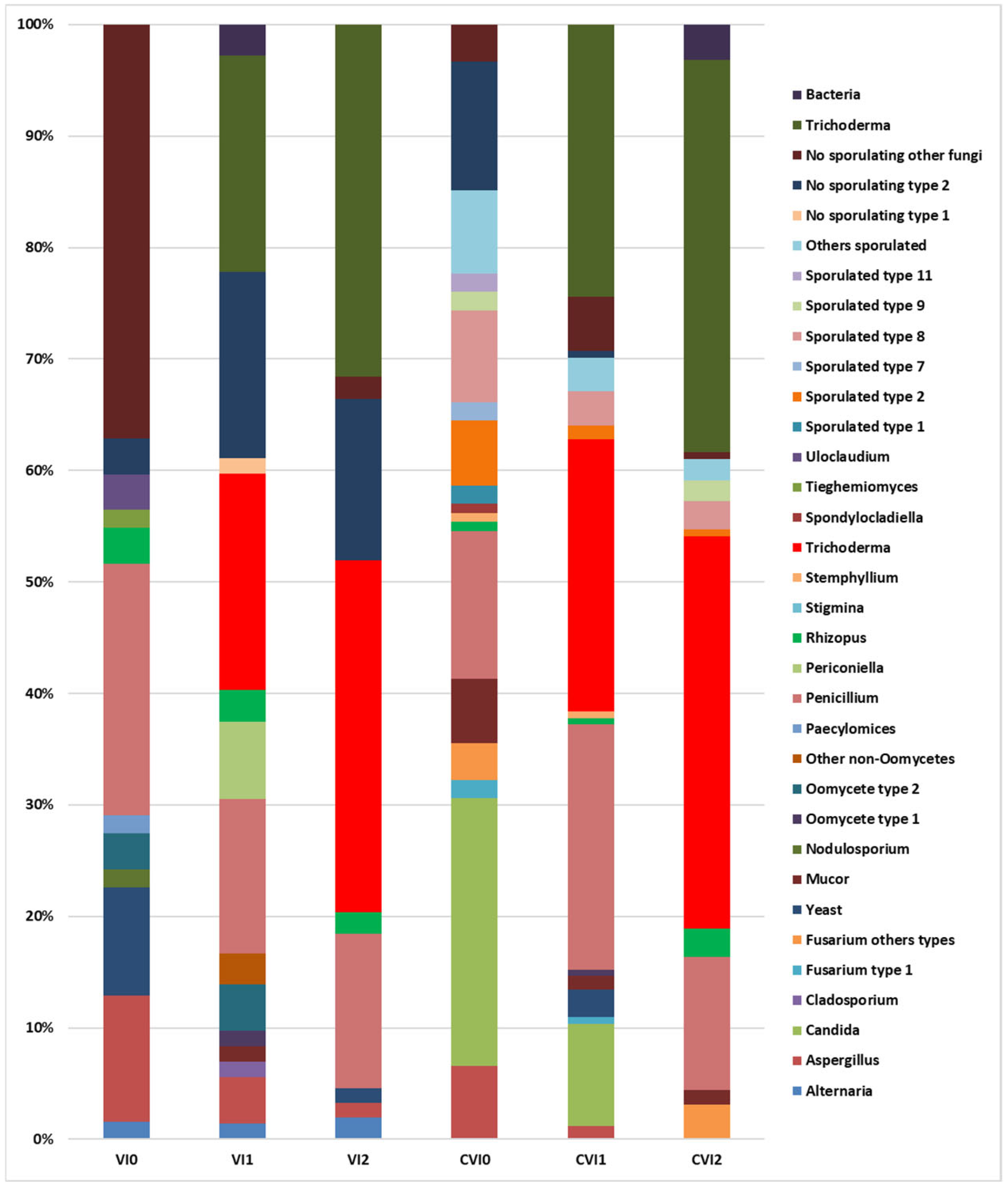
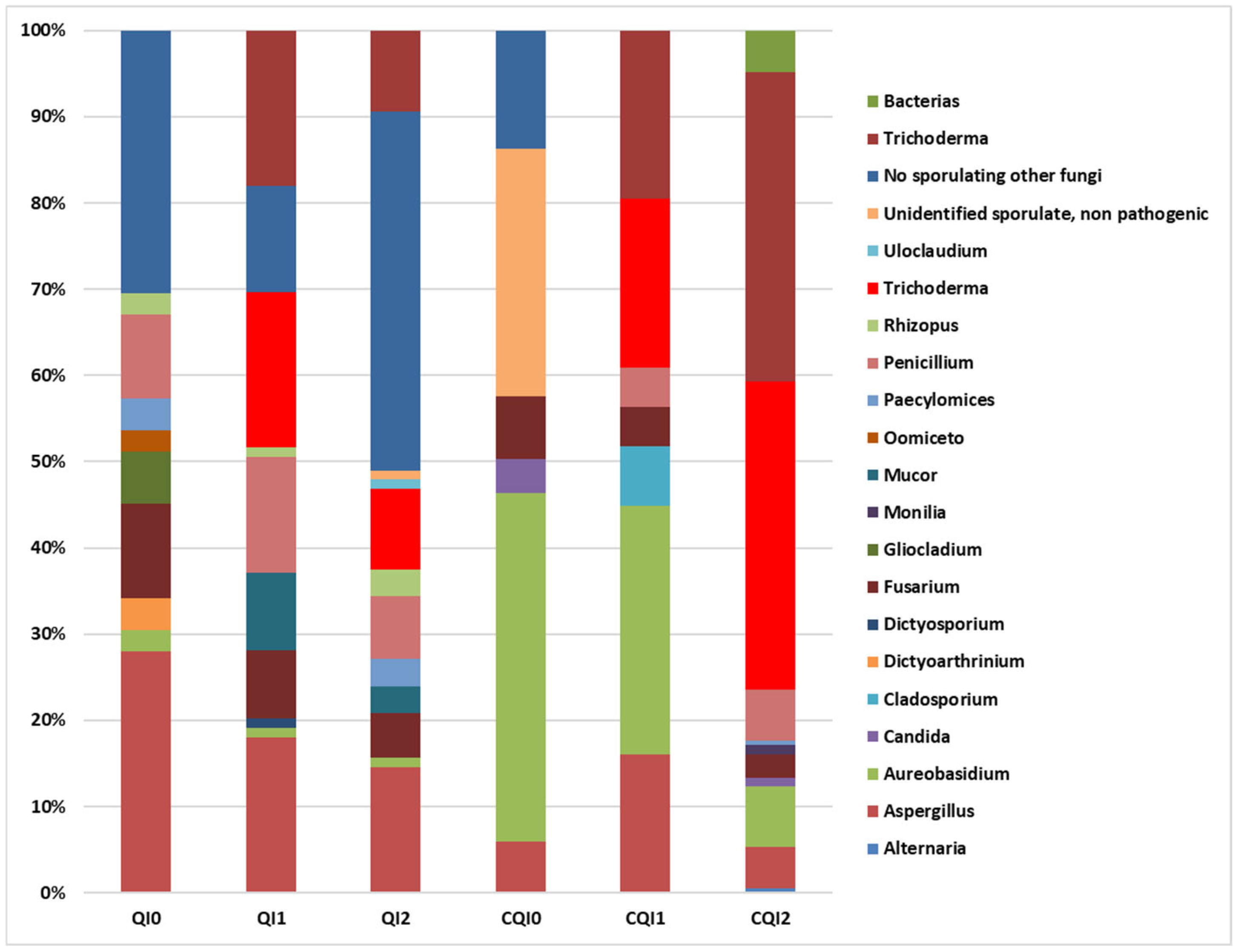
3.2.2. Mycoflora
- VI0:
- Neck: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one Penicillium sp.
- Roots: five isolated fungi: one non-sporulated fungus (suspected of Fusarium sp.), two non-sporulated fungi distinct from each other, and two isolates identified as possible Dendryphiopsis.
- VI1:
- Neck: three isolated fungi: one non-sporulated fungus (suspected of Fusarium sp.), one Mucor sp., and one Helminthosporium sp.
- Roots: eight isolated fungi: one non-sporular (suspected of Fusarium sp.), one non-sporular, one Fusarium sp., one Penicillium sp., three isolates identified as possible Dendryphiopsis, and one unidentified sporulated isolate.
- VI2:
- Neck: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one Helminthosporum sp.
- Roots: six isolated fungi: three non-sporulated fungi (suspected of Fusarium sp.), two non-sporulated fungi, and one possible Dendryphiopsis.
- CVI0:
- Neck: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.), and one Helminthosporium sp.
- Roots: three isolated fungi: three non-sporulated fungi (suspected of Fusarium sp.).
- CVI1:
- Neck: two isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.).
- Roots: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one possible Dendryphiopsis.
- CVI2:
- Neck: three isolated fungi: two non-sporular (suspected of Fusarium sp.) and one non-sporular.
- Roots: four isolated fungi: one non-sporular (suspected of Fusarium sp.), one non-sporular, two Fusarium sp.
- QI0:
- Neck: two isolated fungi: two Fusarium sp. (different from each other).
- Roots: six isolated fungi: one non-sporulated fungus (suspected of Fusarium sp.), two different non-sporulated fungi, and three equal non-sporulated fungi.
- QI1:
- Neck: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one Helminthosporum sp.
- Roots: five isolated fungi: four non-sporular (suspected of Fusarium sp.) and one Fusarium sp.
- QI2:
- Neck: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one Rhizopus sp.
- Roots: five isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.), one Fusarium sp., one Penicillium sp., and one non-sporulated fungi.
- CQI0:
- Neck: three isolated fungi: three non-sporulated fungi (suspected of Fusarium sp.).
- Roots: four isolated fungi: four non-sporulated fungi (suspected of Fusarium sp.).
- CQI1:
- Neck: two isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.).
- Roots: three isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.) and one Penicillium sp.
- CQI2:
- Neck: two isolated fungi: two non-sporulated fungi (suspected of Fusarium sp.).
- Roots: four isolated fungi: two non-sporular (suspected of Fusarium sp.), one Fusarium sp., one non-sporular.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moebius-Clune, B.L.; van Es, H.M.; Schindelbeck, R.R.; Kurtz, K.S.; Moebius-Clune, D.J.; Thies, R.H.; Decker, O.C.; Wolfe, D.W. Comprehensive Assessment of Soil Health: The Cornell Framework; Cornell University: Ithaca, NY, USA, 2017. [Google Scholar]
- Kchaou, R.; Baccar, R.; Bouzid, J.; Rejeb, S. The impact of sewage sludge and compost on winter triticale. Environ. Sci. Pollut. Res. 2018, 25, 18314–18319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soni, R.; Soni, S.K. From waste to wealth: Exploring modern composting innovations and compost valorization. J. Mater. Cycles Waste Manag. 2024, 26, 20–48. [Google Scholar] [CrossRef]
- Hernández, F.d.C.C.; Castillo, G.A.; Viveros, G.S. Trichoderma spp., una alternativa para la agricultura sostenible: Una revisión. Rev. Colomb. Biotecnol. 2023, 25, 62–76. [Google Scholar] [CrossRef]
- Companioni González, B.; Domínguez Arizmendi, G.; García Velasco, R.; Companioni González, B.; Domínguez Arizmendi, G.; García Velasco, R. Trichoderma: Su potencial en el desarrollo sostenible de la agricultura. Biotecnol. Veg. 2019, 19, 237–248. [Google Scholar]
- Siddiqui, Y.; Meon, S.; Ismail, M.R.; Ali, A. Trichoderma-fortified compost extracts for the control of choanephora wet rot in okra production. Crop Prot. 2008, 27, 385–390. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A.; Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A. Trichoderma: Importancia Agrícola, Biotecnológica, y sistemas de Fermentación para Producir Biomasa y Enzimas de Interés Industrial. Chil. J. Agric. Amp; Anim. Sci. 2019, 35, 98–112. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Bhuiyan, M.K.A.; Siddique, S.S.; Rahman, M.A.; Rubayet, M.T. Integration of Trichoderma harzianum with organic amendments for controlling major soil-borne diseases in chickpea. Egypt. J. Agric. Res. 2024, 102, 67–78. [Google Scholar] [CrossRef]
- Rahman, R.; Bhuiyan, M.K.A.; Khan, M.A.A.; Hossain, M.M.; Rubayet, M.T. Trichoderma-fortified compost in controlling diseases and increasing yield of tomato. Int. J. Environ. Agric. Biotechnol. 2024, 9. [Google Scholar] [CrossRef]
- Ferreira, N.C.d.F.; Ramos, M.L.G.; Gatto, A. Use of Trichoderma in the Production of Forest Seedlings. Microorganisms 2024, 12, 237. [Google Scholar] [CrossRef]
- Boudjabi, S.; Kribaa, M.; Chenchouni, H. Sewage sludge fertilization alleviates drought stress and improves physiological adaptation and yield performances in Durum Wheat (Triticum durum): A double-edged sword. J. King Saud Univ. Sci. 2019, 31, 336–344. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tong, L.; Lv, Y. Sustainable Agriculture Practices: Utilizing Composted Sludge Fertilizer for Improved Crop Yield and Soil Health. Agronomy 2024, 14, 756. [Google Scholar] [CrossRef]
- Wydro, U.; Jankowska, M.; Wołejko, E.; Kondzior, P.; Łozowicka, B.; Kaczyński, P.; Rodziewicz, J.; Janczukowicz, W.; Pietryczuk, A.; Cudowski, A.; et al. Changes in Soil Biological Properties after Sewage Sludge and Pesticide Application in Wheat Cultivation. Appl. Sci. 2022, 12, 11452. [Google Scholar] [CrossRef]
- Namdari, M.; Soleimani, M.; Mirghaffari, N.; Kharrazi, S.M. Effect of biological sewage sludge and its derived biochar on accumulation of potentially toxic elements by corn (Zea mays L.). Sci. Rep. 2024, 14, 5985. [Google Scholar] [CrossRef] [PubMed]
- Appels, R.; Eversole, K.; Feuillet, C.; Gallagher, D. (Eds.) The Wheat Genome, Compendium of Plant Genomes; Springer International Publishing: Cham, Switerland, 2024. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Que, Y.; Yu, D.; Wang, H. The influence of rhizosphere soil fungal diversity and complex community structure on wheat root rot disease. PeerJ 2021, 9, e12601. [Google Scholar] [CrossRef]
- Sudha, A.; Praveen, V.; Selva Amala, A.; Ramalakshmi, A.; Ramjegathesh, R.; Anitta Fanish, S.; Vimal, S. Expedition of Trichoderma formulations: Production to storage in India—A review. J. Environ. Biol. 2024, 45, 363–371. [Google Scholar] [CrossRef]
- Huang, B.; Lv, X.; Zheng, H.; Yu, H.; Zhang, Y.; Zhang, C.; Wang, J. Microbial organic fertilizer prepared by co-composting of Trichoderma dregs mitigates dissemination of resistance, virulence genes, and bacterial pathogens in soil and rhizosphere. Environ. Res. 2024, 241, 117718. [Google Scholar] [CrossRef]
- Arshad, U.; Altaf, M.T.; Liaqat, W.; Ali, M.; Shah, M.N.; Jabran, M.; Ali, M.A. Biochar: Black Gold for Sustainable Agriculture and Fortification Against Plant Pathogens—A Review. J. Crop Health 2024, 76, 385–396. [Google Scholar] [CrossRef]
- Hafez, M.; Gourlie, R.; Telfer, M.; Schatz, N.; Turkington, T.K.; Beres, B.; Aboukhaddour, R. Diversity of Fusarium spp. Associated with Wheat Node and Grain in Representative Sites Across the Western Canadian Prairies. Phytopathology 2022, 112, 1003–1015. [Google Scholar] [CrossRef]
- Rocher, F.; Dou, S.; Philippe, G.; Martin, M.-L.; Label, P.; Langin, T.; Bonhomme, L. Integrative systems biology of wheat susceptibility to Fusarium graminearum uncovers a conserved gene regulatory network and identifies master regulators targeted by fungal core effectors. BMC Biol. 2024, 22, 53. [Google Scholar] [CrossRef]
- Junior, A.; Guo, M. Efficacy of sewage sludge derived biochar on enhancing soil health and crop productivity in strongly acidic soil. Front. Soil Sci. 2023, 3, 1066547. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Grobelak, A.; Placek, A.; Grosser, A.; Singh, B.R.; Almås, Å.R.; Napora, A.; Kacprzak, M. Effects of single sewage sludge application on soil phytoremediation. J. Clean. Prod. Sustain. Dev. Energy Water Environ. Syst. 2017, 155, 189–197. [Google Scholar] [CrossRef]
- Jalali Mohsen Imanifard, A.; Jalali, M. Heavy metals accumulation in wheat (Triticum aestivum L.) roots and shoots grown in calcareous soils treated with non-spiked and spiked sewage sludge. Environ. Sci. Pollut. Res. 2023, 30, 20862–20873. [Google Scholar] [CrossRef] [PubMed]
- Lasmini, S.A.; Edy, N.; Yunus, M.; Nasir, B.H.; Khasanah, N.; Lasmini, S.A.; Edy, N.; Yunus, M.; Nasir, B.H.; Khasanah, N. Effect of the Combined Application of Manure Compost and Trichoderma sp. On Production Parameters and Stem rot Disease Incidence of Shallot. Chil. J. Agric. Amp. Anim. Sci. 2022, 38, 335–344. [Google Scholar] [CrossRef]
- Talukdar, P.; Siddiqa, M.s.t.M.; Masum, M.d.M.I.; Habibullah, A.B.M.; Bhuiyan, M.d.K.A. Effect of Trichoderma Fortified Compost on Disease Suppression, Growth and Yield of Chickpea. IJEAB 2017, 2, 831–839. [Google Scholar] [CrossRef]
- Andriguetto, K.V.; Waimer, G.C.; Silva Dos Santos, B.M.; Turchetto, F.; Griebeler, A.M.; Araujo, M.M. The Influence of Fertilizer Type and Trichoderma harzianum Inoculation on the Growth and Physiology of Young Plants of Cordia americana. Floresta 2024, 54, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Ma, L.; Zhang, Y.; Zhao, W.; Zhao, B.; Zhang, J. Effect of wheat cultivars with different resistance to Fusarium head blight on rhizosphere Fusarium graminearum abundance and microbial community composition. Plant Soil 2020, 448, 383–397. [Google Scholar] [CrossRef]

| Control Treatments | Description |
|---|---|
| Q + I0 | Quero soil without inoculum |
| V + I0 | Villacañas soil without inoculum |
| CV + I0 | 1/3 composted sludge + 2/3 Villacañas soil without inoculum |
| CQ + I0 | 1/3 composted sludge + 2/3 Quero soil without inoculum |
| Studied Treatments | Description |
| V + I1 | Villacañas soil with inoculum I1 |
| V + I2 | Villacañas soil with inoculum I2 |
| CV + I1 | 1/3 composted sludge + 2/3 Villacañas soil with inoculum I1 |
| CV + I2 | 1/3 composted sludge + 2/3 Villacañas soil with inoculum I2 |
| Q + I1 | Quero soil with inoculum I1 |
| Q + I2 | Quero soil with inoculum I2 |
| CQ + I1 | 1/3 composted sludge + 2/3 Quero soil with inoculum I1 |
| CQ + I2 | 1/3 composted sludge + 2/3 Quero soil with inoculum I2 |
| N (%) | P2O5 (%) | K2O (%) | Organic Matter (%) |
|---|---|---|---|
| 3.57 | 3.12 | 0.89 | 6.10 |
| CV | CQ | ||||
|---|---|---|---|---|---|
| Parameter | Units | Result | Remarks | Result | Remarks |
| Sand | % | 47.4 | Loam texture (USDA) | 65.3 | Loam texture (USDA) |
| Loam | % | 34.1 | 19.0 | ||
| Clay | % | 19.0 | 16.0 | ||
| pH (extract 1:2.5) | 7.8 | Basic | 7.9 | Basic | |
| Electrical Conductivity: EC (saturated paste) | µS cm−1 | 7600 | Saline | 9.150 | Very saline |
| Chlorides | ppm | 48 | 46 | ||
| Sulfates | mg gypsum/100 g soil | 503 | 592 | ||
| Organic matter * | % | 3.03 | High | 4.22 | High |
| Total Nitrogen | % | 0.43 | Very high | 0.37 | High |
| C/N ratio | 4.1 | High nitrogen release | 6.6 | High nitrogen release | |
| Nitric nitrogen | ppm | 1.0 | Very low | 62.0 | Moderate |
| Assimilable phosphorus | ppm | 346.0 | Very high | 449.0 | Very high |
| Total carbonates | % | 44.3 | Very high | 21.9 | High |
| Active limestone * | % | 13.3 | Potential nutritional problems | 5.7 | |
| Assimilable potassium | meq/100 g | 2.53 | Very high | 1.72 | Very high |
| Assimilable sodium | meq/100 g | 2.39 | Very high | 2.13 | Very high |
| Assimilable calcium | meq/100 g | 16.11 | Very high | 14.31 | Very high |
| Assimilable magnesium | meq/100 g | 2.23 | Normal | 2.03 | Very high |
| K/mg ratio | 1.1 | Possible Mg deficiencies | 0.8 | Possible Mg deficiencies | |
| Ca/mg ratio | 7.224 | 7.05 | |||
| Cation Exchange Capacity (CEC) | meq/100 g | 17.14 | Medium–normal | 12.12 | Moderate–normal |
| Assimilable iron | ppm | 14.81 | 12.62 | ||
| Assimilable zinc | ppm | 13.71 | 13.43 | ||
| Assimilable copper | ppm | 2.48 | 2.61 | ||
| Assimilable manganese | ppm | 28.27 | 22.26 | ||
| Assimilable boron | ppm | 2.06 | 4.15 | ||
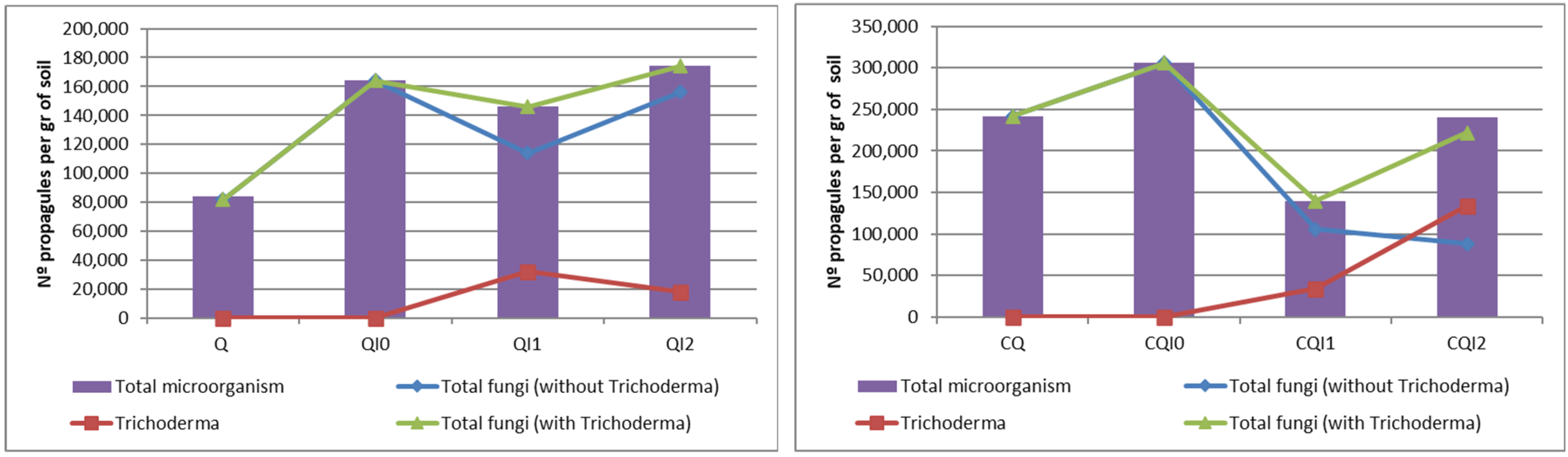
| Microorganism | Q | V | CQ | CV | QI | VI | CQI | CVI |
|---|---|---|---|---|---|---|---|---|
| Alternaria | 0 | 0 | 2000 | 0 | 0 | 0 | 0 | 2000 |
| Aspergillus | 14,000 | 4000 | 40,000 | 0 | 0 | 2000 | 54,000 | 0 |
| Candida | 0 | 8000 | 4000 | 4000 | 0 | 0 | 0 | 0 |
| Chirosporium | 0 | 0 | 2000 | 4000 | 0 | 0 | 0 | 0 |
| Cladosporium | 0 | 2000 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fusarium type 1 | 4000 | 0 | 38,000 | 4000 | 0 | 0 | 4000 | 0 |
| Fusarium type 2 | 0 | 0 | 0 | 4000 | 0 | 0 | 0 | 4000 |
| Gliocadium | 0 | 0 | 0 | 2000 | 0 | 0 | 4000 | 0 |
| Helminthosporium | 0 | 0 | 0 | 0 | 0 | 0 | 2000 | 0 |
| Yeast | 0 | 0 | 2000 | 0 | 0 | 0 | 0 | 0 |
| Mucor | 0 | 26,000 | 2000 | 10,000 | 2000 | 4000 | 0 | 4000 |
| Oomycetus type 1 | 6000 | 4000 | 2000 | 10,000 | 4000 | 4000 | 0 | 0 |
| Oomycetus type 2 | 4000 | 6000 | 0 | 4000 | 4000 | 16,000 | 0 | 8000 |
| Other oomycetes distinct from each other | 0 | 0 | 0 | 0 | 0 | 6000 | 2000 | 0 |
| Paecylomices | 0 | 2000 | 0 | 0 | 0 | 0 | 2000 | 0 |
| Penicillium | 16,000 | 10,000 | 22,000 | 80,000 | 0 | 6000 | 8000 | 2000 |
| Periconiella | 0 | 0 | 0 | 2000 | 0 | 0 | 0 | 2000 |
| Phymatotrichum | 0 | 0 | 24,000 | 12,000 | 0 | 0 | 10,000 | 0 |
| Rhizopus | 26,000 | 10,000 | 0 | 24,000 | 92,000 | 12,000 | 0 | 8000 |
| Stigmine | 0 | 6000 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thielaviopsis | 0 | 2000 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tieghemiomyces | 2000 | 12,000 | 10,000 | 24,000 | 0 | 4000 | 4000 | 8000 |
| Non-sporulating type 1 | 10,000 | 0 | 16,000 | 0 | 4000 | 0 | 12,000 | 0 |
| Non-sporulating type 2 | 0 | 4000 | 52,000 | 26,000 | 12,000 | 18,000 | 10,000 | 26,000 |
| Non-sporulating other fungi | 0 | 2000 | 26,000 | 4000 | 0 | 0 | 16,000 | 4000 |
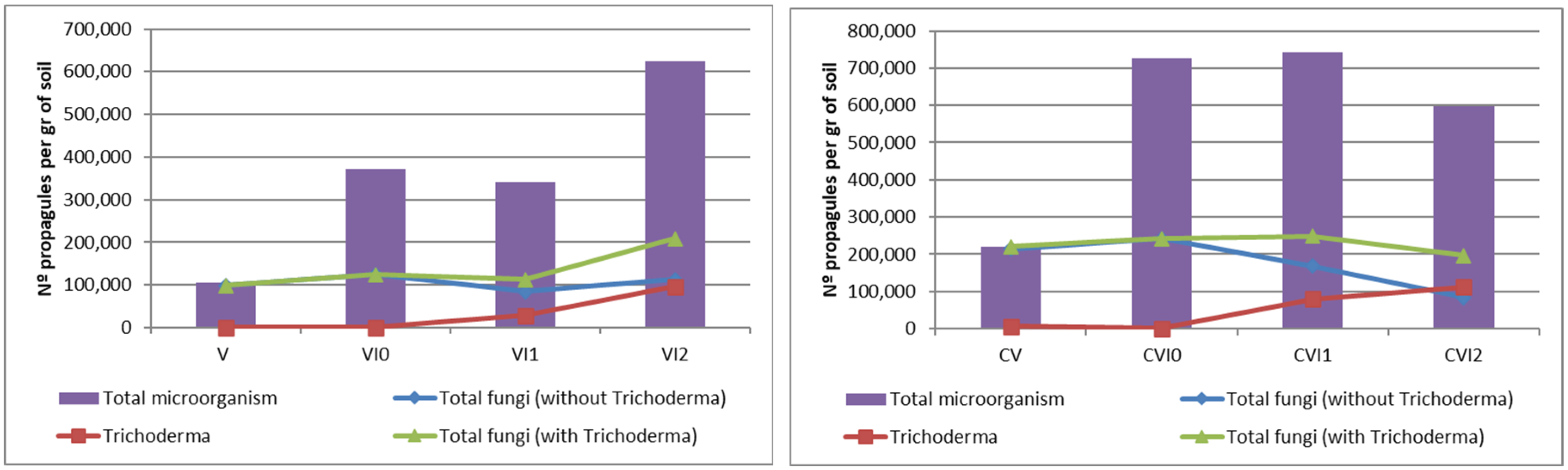
| Total Fungi (not including Trichoderma) | 82,000 | 98,000 | 242,000 | 214,000 | 118,000 | 74,000 | 128,000 | 68,000 |
| Trichoderma | 0 | 0 | 0 | 6000 | 6000 | 30,000 | 0 | 108,000 |
| Total Fungi (including Trichoderma) | 82,000 | 98,000 | 242,000 | 220,000 | 124,000 | 104,000 | 128,000 | 176,000 |
| Bacteria | 2000 | 8000 | 0 | 0 | 0 | 2000 | 2000 | 2000 |
| Total Microorganism | 84,000 | 106,000 | 242,000 | 220,000 | 124,000 | 106,000 | 130,000 | 178,000 |
| p-Values | |
|---|---|
| Additive (A) | 0.0306 * |
| Inoculum (B) | 0.7515 |
| Soil (C) | 0.4863 |
| Interactions | |
| AB | 0.4026 |
| AC | 0.4863 |
| BC | 0.9038 |
| ABC | 0.2416 |
| Treatment | Number of Plants Per Pot | Number of Spikes | Total Weight of Spikes (g) | Average Weight of a Spike (g) | Root Weight (g) | Shoot Weight (g) | Shoot/Root Ratio |
|---|---|---|---|---|---|---|---|
| VI0 | 9.0 ab | 2.7 ab | 0.15 a | 0.04 ab | 0.61 c | 1.30 ab | 3.30 abc |
| VI1 | 5.0 ab | 5.0 b | 0.37 a | 0.07 abc | 0.16 abc | 0.76 ab | 4.70 abc |
| VI2 | 8.7 ab | 3.3 ab | 0.22 a | 0.04 ab | 0.50 abc | 1.20 ab | 2.58 ab |
| CVI0 | 9.0 ab | 2.3 ab | 0.20 a | 0.07 abc | 0.24 abc | 1.87 b | 7.37 cde |
| CVI1 | 4.7 a | 3.3 ab | 0.30 a | 0.09 bc | 0.14 ab | 1.50 ab | 10.37 of |
| CVI2 | 6.0 ab | 1.3 a | 0.07 a | 0.03 ab | 0.08 a | 0.92 ab | 11.47 e |
| QI0 | 5.0 a | 4.3 ab | 0.31 a | 0.08 bc | 0.48 bc | 0.92 ab | 2.09 a |
| QI1 | 7.3 ab | 3.3 ab | 0.21 a | 0.07 abc | 0.40 bc | 1.07 ab | 2.76 ab |
| QI2 | 9.6 b | 3.3 ab | 0.27 a | 0.04 ab | 0.42 abc | 1.26 ab | 3.03 ab |
| CQI0 | 5.0 a | 1.5 a | 0.05 a | 0.02 a | 0.07 a | 0.48 a | 6.36 abcd |
| CQI1 | 5.3 ab | 1.3 a | 0.06 a | 0.03 ab | 0.21 ab | 0.77 ab | 3.46 abc |
| CQI2 | 6.3 ab | 3.0 ab | 0.31 a | 0.10 c | 0.27 abc | 1.80 b | 6.78 bcd |
| Number of Plants Per Pot | Total Root Weight | Number of Spikes Per Pot | Total Shoot Weight | Total Spikes Weight | Shoot/Root Ratio | |
|---|---|---|---|---|---|---|
| Additive (A) | 0.1273 | 0.0010 * | 0.00261 * | 0.5766 | 0.2004 | 0.0000 * |
| Inoculum (B) | 0.2125 | 0.5401 | 0.6626 | 0.6309 | 0.7584 | 0.5154 |
| Soil (C) | 0.4731 | 0.9658 | 0.6395 | 0.3156 | 0.7858 | 0.0106 * |
| Interactions | ||||||
| AB | 0.4197 | 0.4476 | 0.8776 | 0.9798 | 0.9354 | 0.4281 |
| AC | 0.7187 | 0.7111 | 0.7546 | 0.3930 | 0.6117 | 0.0447 * |
| BC | 0.0515 | 0.3521 | 0.2474 | 0.0700 | 0.1334 | 0.4243 |
| ABC | 0.9568 | 0.5904 | 0.4031 | 0.1586 | 0.3467 | 0.2907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañas, P.; De las Heras, J. Composted Sludge and Trichoderma harzianum T-22 as a Dual Strategy to Enhance Wheat Growth and Soil Microbial Diversity. Environments 2025, 12, 145. https://doi.org/10.3390/environments12050145
Mañas P, De las Heras J. Composted Sludge and Trichoderma harzianum T-22 as a Dual Strategy to Enhance Wheat Growth and Soil Microbial Diversity. Environments. 2025; 12(5):145. https://doi.org/10.3390/environments12050145
Chicago/Turabian StyleMañas, Pilar, and Jorge De las Heras. 2025. "Composted Sludge and Trichoderma harzianum T-22 as a Dual Strategy to Enhance Wheat Growth and Soil Microbial Diversity" Environments 12, no. 5: 145. https://doi.org/10.3390/environments12050145
APA StyleMañas, P., & De las Heras, J. (2025). Composted Sludge and Trichoderma harzianum T-22 as a Dual Strategy to Enhance Wheat Growth and Soil Microbial Diversity. Environments, 12(5), 145. https://doi.org/10.3390/environments12050145








