Understanding the Origin of Wet Deposition Black Carbon in North America During the Fall Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. NADP National Trend Network (NTN) Samples
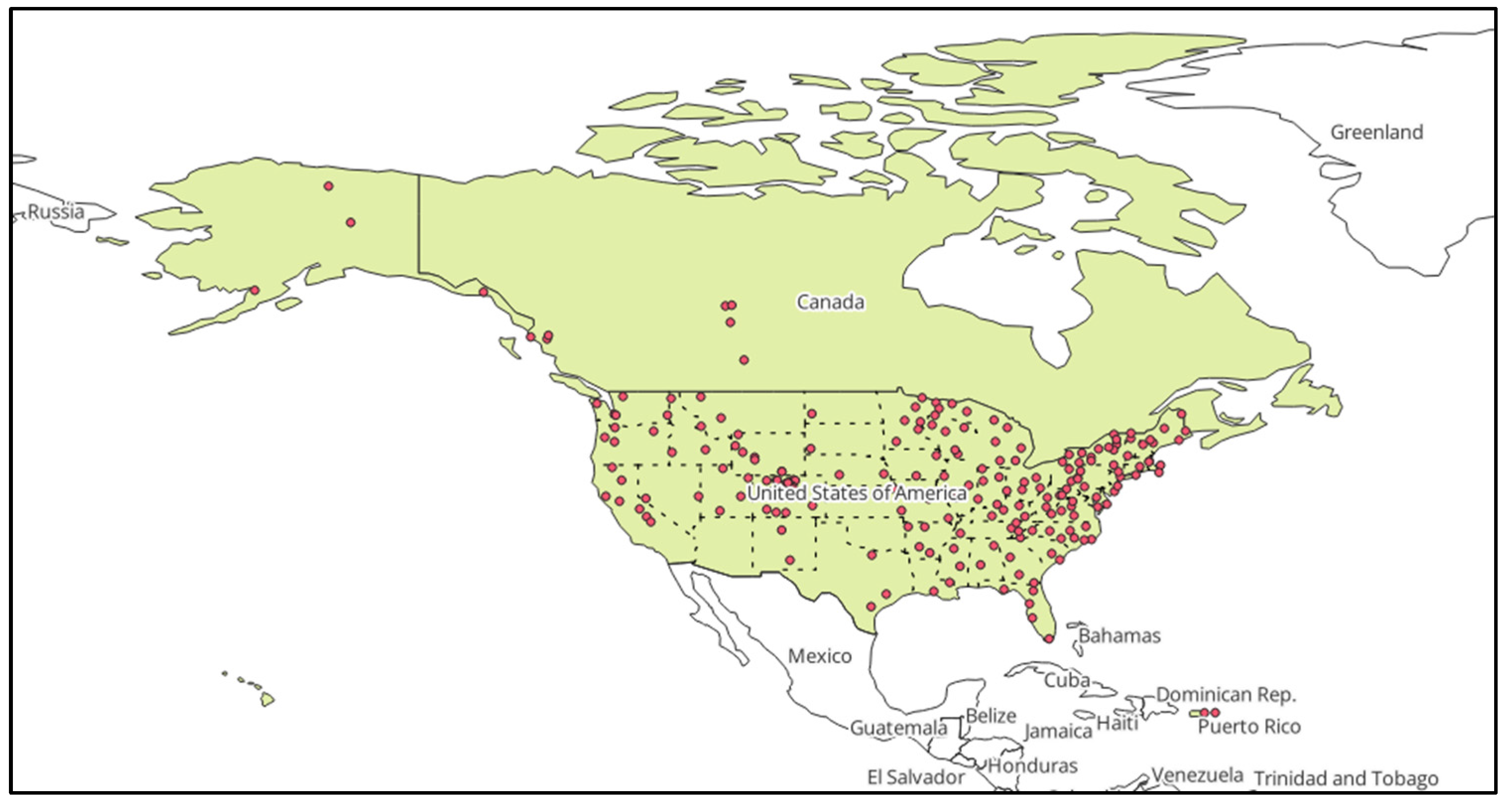
2.1.1. Sampling Sites
2.1.2. Wet Deposition Sample Collection Method and Shipment
2.1.3. NADP National Trend Network (NTN) Sampling Container Preparation
2.2. NADP Measurements
2.3. Black Carbon (BC) and Water-Soluble Organic Carbon (WSOC) Analyses
2.3.1. Sample Preparation
2.3.2. Cleaning Procedures for Labware and Glass Microfiber Filters
2.3.3. Black Carbon (BC) Analysis
2.3.4. Water-Soluble Organic Carbon (WSOC) Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.4.1. NTN Supply Quality Control (QC)
2.4.2. Analysis of rBC
2.4.3. Analysis of WSOC
2.5. Statistical Analysis
3. Results and Discussion
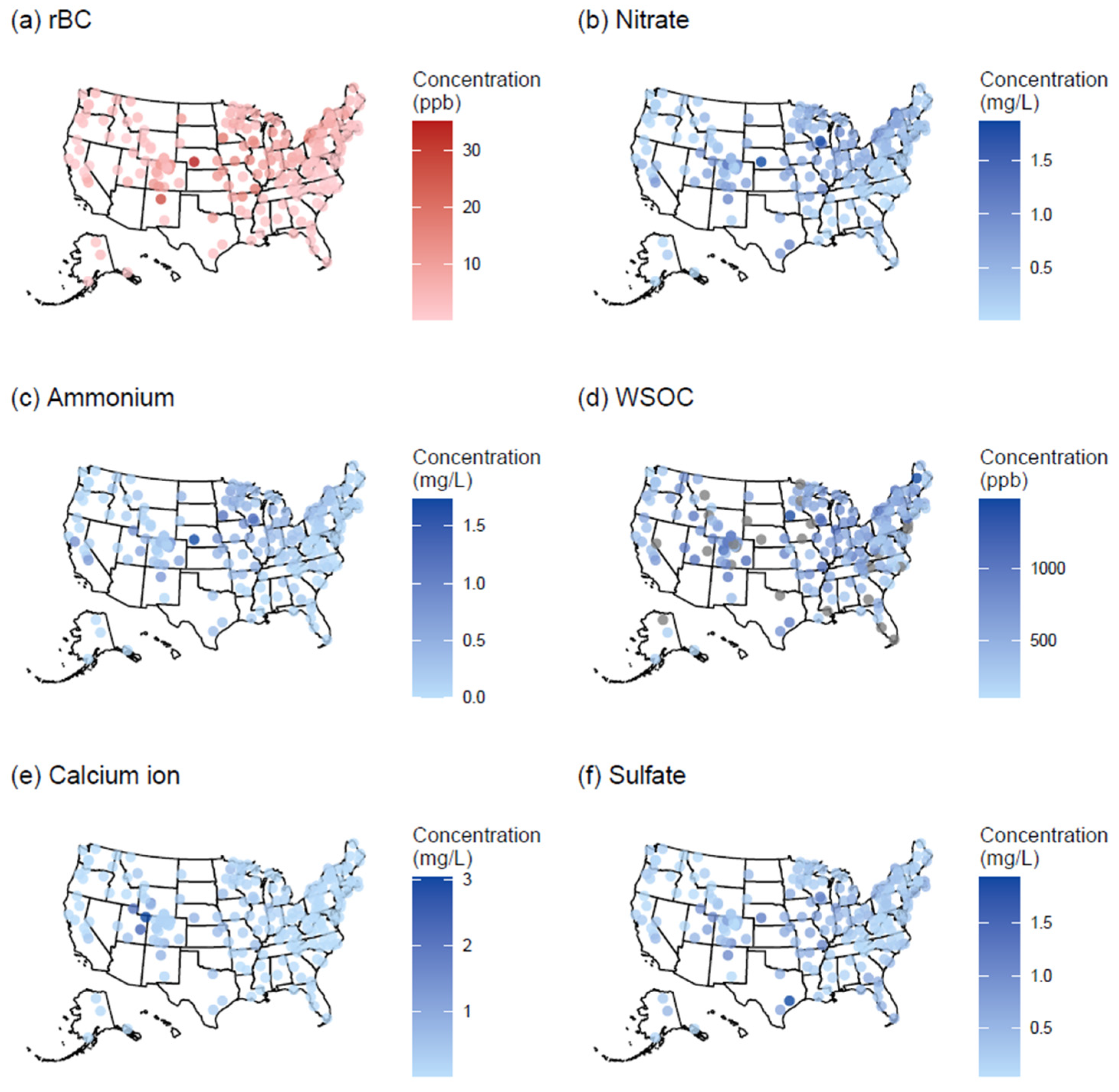
3.1. Results of NADP Measurements
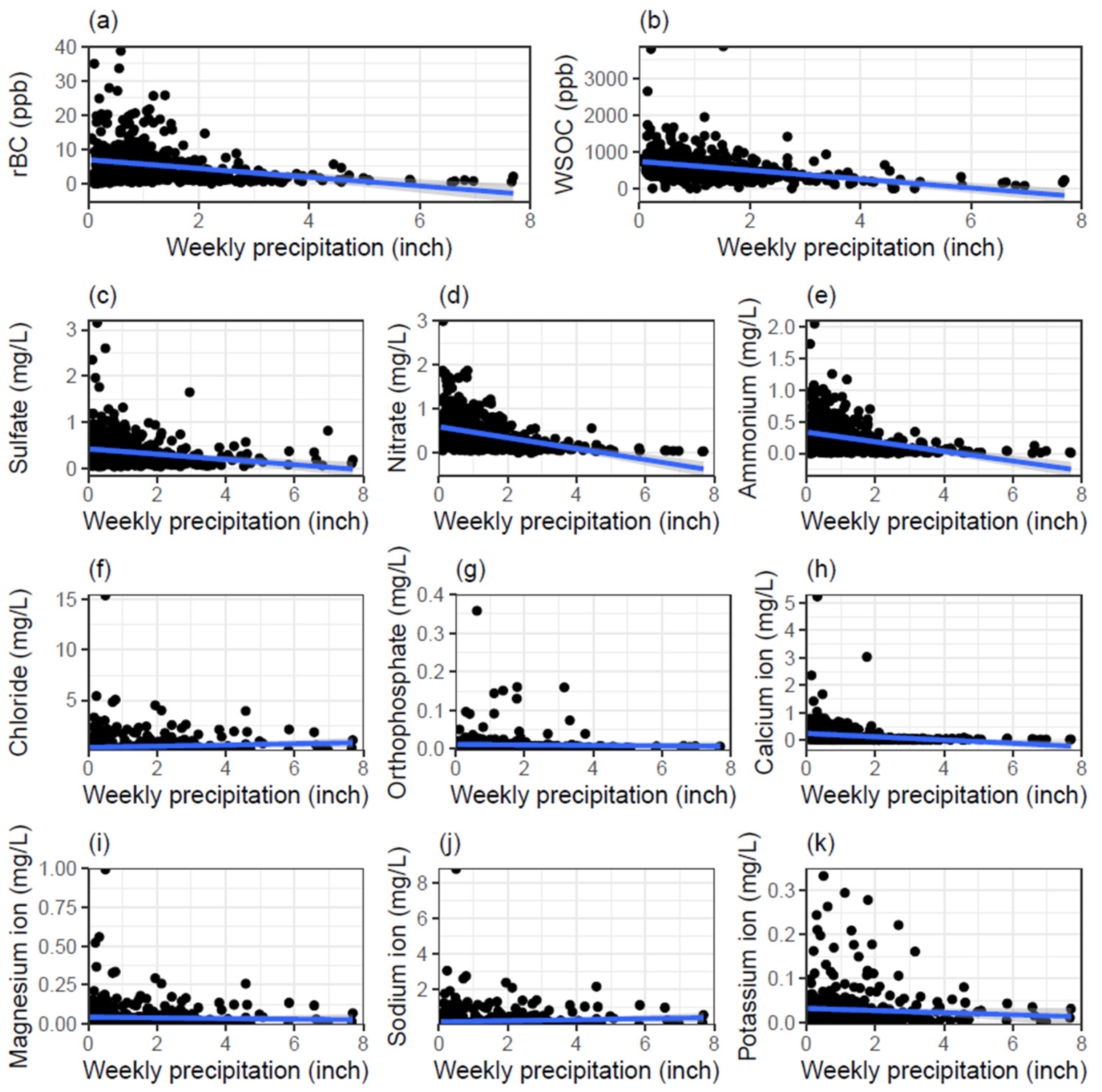
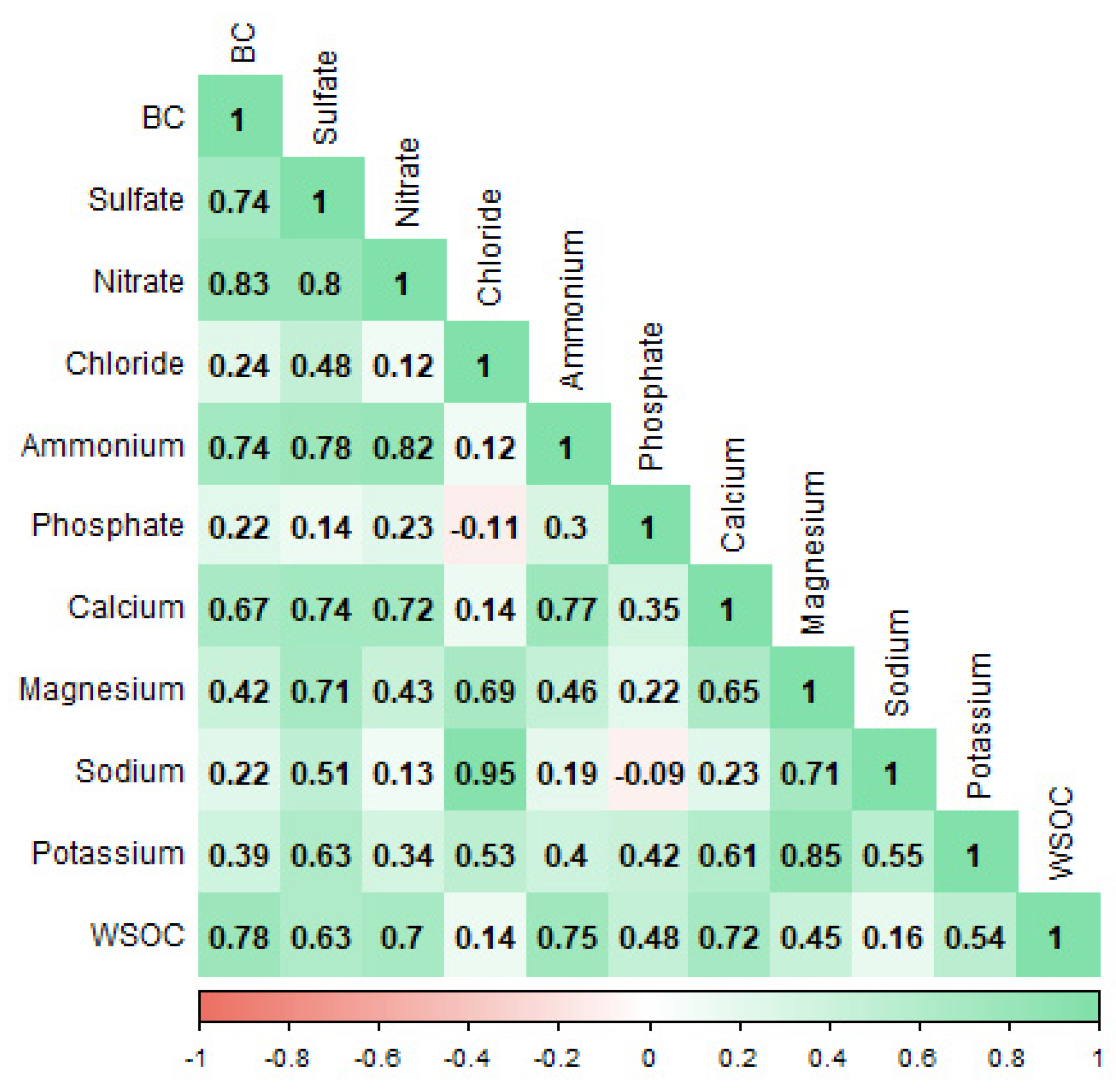
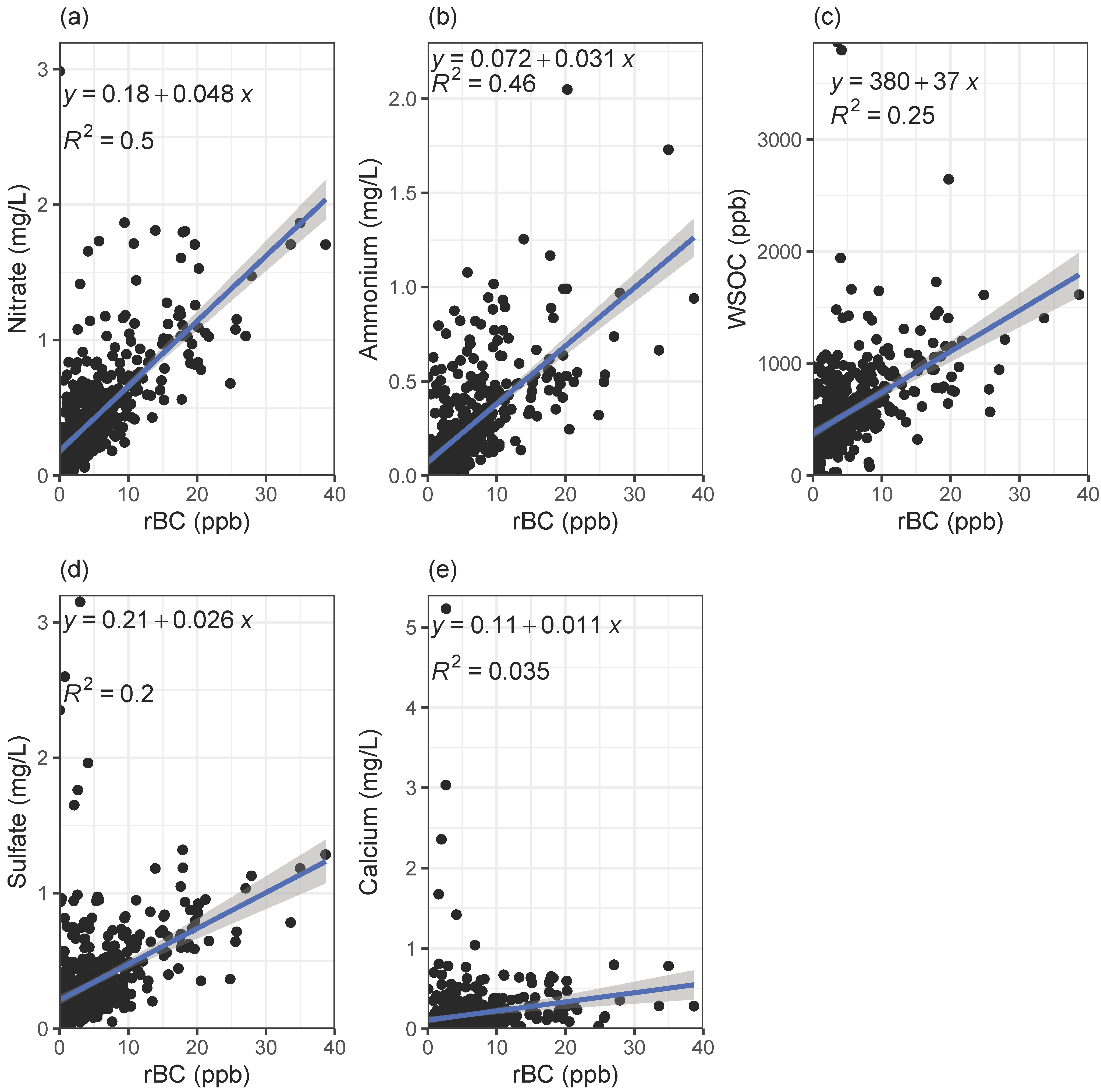
3.2. Correlations with Water-Soluble Ions and WSOC
3.3. Source Identification of BC in Wet Deposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Massling, A.; Nielsen, I.E.; Kristensen, D.; Christensen, J.H.; Sørensen, L.L.; Jensen, B.; Nguyen, Q.T.; Nøjgaard, J.K.; Glasius, M.; Skov, H. Atmospheric Black Carbon and Sulfate Concentrations in Northeast Greenland. Atmos. Chem. Phys. 2015, 15, 9681–9692. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, W.; Fu, J.; Luo, X.; Hung, D.L.S.; Xu, M.; Li, X. Impact of Flash Boiling Spray on Soot Generation of a Rich Fuel–Air Mixture under Various Ambient Pressures. Combust. Flame 2024, 263, 113388. [Google Scholar] [CrossRef]
- Kong, Y.; Zhi, G.; Jin, W.; Zhang, Y.; Shen, Y.; Li, Z.; Sun, J.; Ren, Y. A Review of Quantification Methods for Light Absorption Enhancement of Black Carbon Aerosol. Sci. Total Environ. 2024, 924, 171539. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Nazarenko, L. Soot Climate Forcing via Snow and Ice Albedos. Proc. Natl. Acad. Sci. USA 2004, 101, 423–428. [Google Scholar] [CrossRef]
- Booth, B.; Bellouin, N. Black Carbon and Atmospheric Feedbacks. Nature 2015, 519, 167–168. [Google Scholar] [CrossRef]
- Ramanathan, V.; Chung, C.; Kim, D.; Bettge, T.; Buja, L.; Kiehl, J.T.; Washington, W.M.; Fu, Q.; Sikka, D.R.; Wild, M. Atmospheric Brown Clouds: Impacts on South Asian Climate and Hydrological Cycle. Proc. Natl. Acad. Sci. USA 2005, 102, 5326–5333. [Google Scholar] [CrossRef]
- Tiwari, S.; Dumka, U.C.; Hopke, P.K.; Tunved, P.; Srivastava, A.K.; Bisht, D.S.; Chakrabarty, R.K. Atmospheric Heating Due to Black Carbon Aerosol during the Summer Monsoon Period over Ballia: A Rural Environment over Indo-Gangetic Plain. Atmos. Res. 2016, 178–179, 393–400. [Google Scholar] [CrossRef]
- Wei, C.; Wang, M.H.; Fu, Q.Y.; Dai, C.; Huang, R.; Bao, Q. Temporal Characteristics and Potential Sources of Black Carbon in Megacity Shanghai, China. J. Geophys. Res. Atmos. 2020, 125, e2019JD031827. [Google Scholar] [CrossRef]
- Debnath, S.; Govardhan, G.; Jat, R.; Kalita, G.; Yadav, P.; Jena, C.; Kumar, R.; Ghude, S.D. Black Carbon Emissions and Its Impact on the Monsoon Rainfall Patterns over the Indian Subcontinent: Insights into Localized Warming Effects. Atmos. Environ. X 2024, 22, 100257. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lamarque, J.-F.; Flanner, M.G.; Jiao, C.; Shindell, D.T.; Berntsen, T.; Bisiaux, M.M.; Cao, J.; Collins, W.J.; Curran, M.; et al. Evaluation of Preindustrial to Present-Day Black Carbon and Its Albedo Forcing from Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmos. Chem. Phys. 2013, 13, 2607–2634. [Google Scholar] [CrossRef]
- Kumar, R.R.; Soni, V.K.; Jain, M.K. Evaluation of Spatial and Temporal Heterogeneity of Black Carbon Aerosol Mass Concentration over India Using Three Year Measurements from IMD BC Observation Network. Sci. Total Environ. 2020, 723, 138060. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z. Short-Term Effects of Controlling Fossil-Fuel Soot, Biofuel Soot and Gases, and Methane on Climate, Arctic Ice, and Air Pollution Health. J. Geophys. Res. Atmos. 2010, 115, D14209. [Google Scholar] [CrossRef]
- Fierce, L.; Riemer, N.; Bond, T.C. Explaining Variance in Black Carbon’s Aging Timescale. Atmos. Chem. Phys. 2015, 15, 3173–3191. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Liu, J.; Yi, K.; Xiang, S.; Hu, X.; Wang, Y.; Tao, S.; Ban-Weiss, G. Influence of Cloud Microphysical Processes on Black Carbon Wet Removal, Global Distributions, and Radiative Forcing. Atmos. Chem. Phys. 2019, 19, 1587–1603. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Xiao, C.; Gu, Y.; Jin, X.; Wang, P.; Zhao, L. The Pollution Characterization of Black Carbon Aerosols in the Southwest Suburb of Beijing from 2013 to 2019. Atmos. Pollut. Res. 2023, 14, 101669. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Cheng, Y.; Geng, G.; Hong, C.; Li, H.; Li, X.; Tong, D.; Wu, N.; Zhang, X.; et al. Modeling the Aging Process of Black Carbon during Atmospheric Transport Using a New Approach: A Case Study in Beijing. Atmos. Chem. Phys. 2019, 19, 9663–9680. [Google Scholar] [CrossRef]
- Liu, J.; Fan, S.; Horowitz, L.W.; Levy II, H. Evaluation of Factors Controlling Long-Range Transport of Black Carbon to the Arctic. J. Geophys. Res. Atmos. 2011, 116, D04307. [Google Scholar] [CrossRef]
- Nazeer Hussain, S.; Chakradhar Rao, T.; Balakrishnaiah, G.; Rama Gopal, K.; Raja Obul Reddy, K.; Siva Kumar Reddy, N.; Lokeswara Reddy, T.; Pavan Kumari, S.; Ramanjaneya Reddy, P.; Ramakrishna Reddy, R. Investigation of Black Carbon Aerosols and Their Characteristics over Tropical Urban and Semi-Arid Rural Environments in Peninsular India. J. Atmos. Sol. Terr. Phys. 2018, 167, 48–57. [Google Scholar] [CrossRef]
- Kajino, M.; Aikawa, M. A Model Validation Study of the Washout/Rainout Contribution of Sulfate and Nitrate in Wet Deposition Compared with Precipitation Chemistry Data in Japan. Atmos. Environ. 2015, 117, 124–134. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Schwarz, J.P.; Wang, X. Lifecycle of Light-Absorbing Carbonaceous Aerosols in the Atmosphere. npj Clim. Atmos. Sci. 2020, 3, 40. [Google Scholar] [CrossRef]
- Williams, M.A.; Kumar, T.V.L.; Rao, D.N. Characterizing Black Carbon Aerosols in Relation to Atmospheric Boundary Layer Height during Wet Removal Processes over a Semi Urban Location. J. Atmos. Sol. Terr. Phys. 2019, 182, 165–176. [Google Scholar] [CrossRef]
- Lei, S.; Ge, B.; Liu, H.; Quan, J.; Xu, D.; Zhang, Y.; Yao, W.; Lei, L.; Tian, Y.; Liao, Q.; et al. Refractory Black Carbon Aerosols in Rainwater in the Summer of 2019 in Beijing: Mass Concentration, Size Distribution and Wet Scavenging Ratio. J. Environ. Sci. 2023, 132, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ohata, S.; Moteki, N.; Mori, T.; Koike, M.; Kondo, Y. A Key Process Controlling the Wet Removal of Aerosols: New Observational Evidence. Sci. Rep. 2016, 6, 34113. [Google Scholar] [CrossRef] [PubMed]
- Ekman, A.M.L.; Wang, C.; Wilson, J.; Ström, J. Explicit Simulations of Aerosol Physics in a Cloud-Resolving Model: A Sensitivity Study Based on an Observed Convective Cloud. Atmos. Chem. Phys. 2004, 4, 773–791. [Google Scholar] [CrossRef]
- Barrett, T.E.; Ponette-González, A.G.; Rindy, J.E.; Weathers, K.C. Wet Deposition of Black Carbon: A Synthesis. Atmos. Environ. 2019, 213, 558–567. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, C.; Chen, H.; Nizkorodov, S.A.; Chen, J.; Yang, X. Size Distribution and Mixing State of Black Carbon Particles during a Heavy Air Pollution Episode in Shanghai. Atmos. Chem. Phys. 2016, 16, 5399–5411. [Google Scholar] [CrossRef]
- Sricharoenvech, P.; Edwards, R.; Yaşar, M.; Gay, D.; Schauer, J.J. Investigation of Black Carbon Wet Deposition to the United States from National Atmospheric Deposition Network Samples. Aerosol Air Qual. Res. 2024, 24, 230089. [Google Scholar] [CrossRef]
- Torres, A.; Bond, T.C.; Lehmann, C.M.B.; Subramanian, R.; Hadley, O.L. Measuring Organic Carbon and Black Carbon in Rainwater: Evaluation of Methods. Aerosol Sci. Technol. 2014, 48, 239–250. [Google Scholar] [CrossRef]
- National Centers for Environmental Information (NCEI). November 2020 Wildfires Report | National Centers for Environmental Information (NCEI). Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/fire/202011 (accessed on 25 December 2022).
- Chang, Y.; Zhang, Y.; Tian, C.; Zhang, S.; Ma, X.; Cao, F.; Liu, X.; Zhang, W.; Kuhn, T.; Lehmann, M.F. Nitrogen Isotope Fractionation during Gas-to-Particle Conversion of NOx to NO3− in the Atmosphere—Implications for Isotope-Based NOx Source Apportionment. Atmos. Chem. Phys. 2018, 18, 11647–11661. [Google Scholar] [CrossRef]
- Li, Z.; Walters, W.W.; Hastings, M.G.; Song, L.; Huang, S.; Zhu, F.; Liu, D.; Shi, G.; Li, Y.; Fang, Y. Atmospheric Nitrate Formation Pathways in Urban and Rural Atmosphere of Northeast China: Implications for Complicated Anthropogenic Effects. Environ. Pollut. 2022, 296, 118752. [Google Scholar] [CrossRef] [PubMed]
- Piña, A.J.; Schumacher, R.S.; Denning, A.S.; Faulkner, W.B.; Baron, J.S.; Ham, J.; Ojima, D.S.; Collett, J.L. Reducing Wet Ammonium Deposition in Rocky Mountain National Park: The Development and Evaluation of A Pilot Early Warning System for Agricultural Operations in Eastern Colorado. Environ. Manag. 2019, 64, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Sokolik, I.N.; Soja, A.J.; DeMott, P.J.; Winker, D. Progress and Challenges in Quantifying Wildfire Smoke Emissions, Their Properties, Transport, and Atmospheric Impacts. J. Geophys. Res. Atmos. 2019, 124, 13005–13025. [Google Scholar] [CrossRef]
- Yuan, C.; Zheng, J.; Ma, Y.; Jiang, Y.; Li, Y.; Wang, Z. Significant Restructuring and Light Absorption Enhancement of Black Carbon Particles by Ammonium Nitrate Coating. Environ. Pollut. 2020, 262, 114172. [Google Scholar] [CrossRef]
- Le Roy, E.; Walters, W.W.; Joyce, E.E.; Hastings, M.G. Sources of Ammonium in Seasonal Wet Deposition at a Coastal New England City. Atmos. Environ. 2021, 260, 118557. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.; Whitehead, J.; Young, D.; Flynn, M.; Coe, H.; McFiggans, G.; Fleming, Z.L.; Bandy, B. Ambient Black Carbon Particle Hygroscopic Properties Controlled by Mixing State and Composition. Atmos. Chem. Phys. 2013, 13, 2015–2029. [Google Scholar] [CrossRef]
- Niu, X.; Pu, W.; Fu, P.; Chen, Y.; Xing, Y.; Wu, D.; Chen, Z.; Shi, T.; Zhou, Y.; Wen, H.; et al. Fluorescence Characteristics, Absorption Properties, and Radiative Effects of Water-Soluble Organic Carbon in Seasonal Snow across Northeastern China. Atmos. Chem. Phys. 2022, 22, 14075–14094. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Peltier, R.E.; Brock, C.A.; de Gouw, J.A.; Holloway, J.S.; Warneke, C.; Wollny, A.G.; Weber, R.J. Airborne Measurements of Carbonaceous Aerosol Soluble in Water over Northeastern United States: Method Development and an Investigation into Water-Soluble Organic Carbon Sources. J. Geophys. Res. Atmos. 2006, 111, D23S46. [Google Scholar] [CrossRef]
- Kirillova, E.N.; Andersson, A.; Sheesley, R.J.; Kruså, M.; Praveen, P.S.; Budhavant, K.; Safai, P.D.; Rao, P.S.P.; Gustafsson, Ö. 13C- and 14C-Based Study of Sources and Atmospheric Processing of Water-Soluble Organic Carbon (WSOC) in South Asian Aerosols. J. Geophys. Res. Atmos. 2013, 118, 614–626. [Google Scholar] [CrossRef]
- Wu, G.; Fu, P.; Ram, K.; Song, J.; Chen, Q.; Kawamura, K.; Wan, X.; Kang, S.; Wang, X.; Laskin, A.; et al. Fluorescence Characteristics of Water-Soluble Organic Carbon in Atmospheric Aerosol☆. Environ. Pollut. 2021, 268, 115906. [Google Scholar] [CrossRef]
- Willey, J.D.; Kieber, R.J.; Eyman, M.S.; Avery, G.B., Jr. Rainwater Dissolved Organic Carbon: Concentrations and Global Flux. Glob. Biogeochem. Cycles 2000, 14, 139–148. [Google Scholar] [CrossRef]
- Popovicheva, O.; Kistler, M.; Kireeva, E.; Persiantseva, N.; Timofeev, M.; Kopeikin, V.; Kasper-Giebl, A. Physicochemical Characterization of Smoke Aerosol during Large-Scale Wildfires: Extreme Event of August 2010 in Moscow. Atmos. Environ. 2014, 96, 405–414. [Google Scholar] [CrossRef]
- Sparks, T.L.; Wagner, J. Composition of Particulate Matter during a Wildfire Smoke Episode in an Urban Area. Aerosol Sci. Technol. 2021, 55, 734–747. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Smith, S.J.; Easter, R.; Ma, P.-L.; Qian, Y.; Yu, H.; Li, C.; Rasch, P.J. Global Source Attribution of Sulfate Concentration and Direct and Indirect Radiative Forcing. Atmos. Chem. Phys. 2017, 17, 8903–8922. [Google Scholar] [CrossRef]
- Carboni, E.; Mather, T.A.; Schmidt, A.; Grainger, R.G.; Pfeffer, M.A.; Ialongo, I.; Theys, N. Satellite-Derived Sulfur Dioxide (SO2) Emissions from the 2014–2015 Holuhraun Eruption (Iceland). Atmos. Chem. Phys. 2019, 19, 4851–4862. [Google Scholar] [CrossRef]
- Jalava, P.I.; Salonen, R.O.; Hälinen, A.I.; Penttinen, P.; Pennanen, A.S.; Sillanpää, M.; Sandell, E.; Hillamo, R.; Hirvonen, M.-R. In Vitro Inflammatory and Cytotoxic Effects of Size-Segregated Particulate Samples Collected during Long-Range Transport of Wildfire Smoke to Helsinki. Toxicol. Appl. Pharmacol. 2006, 215, 341–353. [Google Scholar] [CrossRef]
- Brahney, J.; Ballantyne, A.P.; Sievers, C.; Neff, J.C. Increasing Ca2+ Deposition in the Western US: The Role of Mineral Aerosols. Aeolian Res. 2013, 10, 77–87. [Google Scholar] [CrossRef]
- Pachon, J.E.; Weber, R.J.; Zhang, X.; Mulholland, J.A.; Russell, A.G. Revising the Use of Potassium (K) in the Source Apportionment of PM2.5. Atmos. Pollut. Res. 2013, 4, 14–21. [Google Scholar] [CrossRef]
- Rincón-Riveros, J.M.; Rincón-Caro, M.A.; Sullivan, A.P.; Mendez-Espinosa, J.F.; Belalcazar, L.C.; Quirama Aguilar, M.; Morales Betancourt, R. Long-Term Brown Carbon and Smoke Tracer Observations in Bogotá, Colombia: Association with Medium-Range Transport of Biomass Burning Plumes. Atmos. Chem. Phys. 2020, 20, 7459–7472. [Google Scholar] [CrossRef]
- Sillanpää, M.; Saarikoski, S.; Hillamo, R.; Pennanen, A.; Makkonen, U.; Spolnik, Z.; Van Grieken, R.; Koskentalo, T.; Salonen, R.O. Chemical Composition, Mass Size Distribution and Source Analysis of Long-Range Transported Wildfire Smokes in Helsinki. Sci. Total Environ. 2005, 350, 119–135. [Google Scholar] [CrossRef]
- Saarnio, K.; Aurela, M.; Timonen, H.; Saarikoski, S.; Teinilä, K.; Mäkelä, T.; Sofiev, M.; Koskinen, J.; Aalto, P.P.; Kulmala, M.; et al. Chemical Composition of Fine Particles in Fresh Smoke Plumes from Boreal Wild-Land Fires in Europe. Sci. Total Environ. 2010, 408, 2527–2542. [Google Scholar] [CrossRef]
- Gao, J.; Dong, S.; Yu, H.; Peng, X.; Wang, W.; Shi, G.; Han, B.; Wei, Y.; Feng, Y. Source Apportionment for Online Dataset at a Megacity in China Using a New PTT-PMF Model. Atmos. Environ. 2020, 229, 117457. [Google Scholar] [CrossRef]

| Parameter (Unit) | Minimum | 25th Percentile | Median | Mean | 75th Percentile | Maximum |
|---|---|---|---|---|---|---|
| rBC (ppb) | 0.000 * | 1.583 | 3.415 | 5.302 | 7.040 | 38.660 |
| Sulfate (mg/L) | 0.0374 | 0.1412 | 0.2531 | 0.3506 | 0.4625 | 3.1507 |
| Nitrate (mg/L) | 0.0000 * | 0.1693 | 0.3162 | 0.4342 | 0.5844 | 2.9849 |
| Chloride (mg/L) | 0.0075 | 0.0390 | 0.0863 | 0.3909 | 0.2730 | 15.4262 |
| Ammonium (mg/L) | 0.00000 * | 0.00569 | 0.1447 | 0.2350 | 0.3317 | 2.0489 |
| Orthophosphate (mg/L) | 0.0023 | 0.0038 | 0.0064 | 0.0109 | 0.0097 | 0.3581 |
| Calcium (mg/L) | 0.0073 | 0.0348 | 0.0701 | 0.1666 | 0.1808 | 5.2310 |
| Magnesium (mg/L) | 0.0008 | 0.0081 | 0.0197 | 0.0390 | 0.0408 | 0.9958 |
| Sodium (mg/L) | 0.0028 | 0.0230 | 0.0612 | 0.2211 | 0.1862 | 8.7850 |
| Potassium (mg/L) | 0.0019 | 0.0095 | 0.0172 | 0.0294 | 0.0318 | 0.3337 |
| WSOC (ppb) ** | 0.0 * | 314.8 | 470.3 | 569.7 | 751.4 | 3869.3 |
| Factor | Variance Explained (%) | Determining Species | Potential Sources |
|---|---|---|---|
| 1 | 28.27 | NO3−, rBC, NH4+, SO42−, WSOC | Secondary aerosols; incomplete combustion of biomass |
| 2 | 27.86 | Na, Mg, Cl | Sea salts |
| 3 | 14.77 | PO43−, K+ | Mineral dust; fertilizers |
| 4 | 11.39 | Ca2+ | Crustal derived |
| 5 | 8.58 | WSOC | Biomass burning; secondary aerosols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sricharoenvech, P.; Edwards, R.; Yaşar, M.; Gay, D.A.; Schauer, J. Understanding the Origin of Wet Deposition Black Carbon in North America During the Fall Season. Environments 2025, 12, 58. https://doi.org/10.3390/environments12020058
Sricharoenvech P, Edwards R, Yaşar M, Gay DA, Schauer J. Understanding the Origin of Wet Deposition Black Carbon in North America During the Fall Season. Environments. 2025; 12(2):58. https://doi.org/10.3390/environments12020058
Chicago/Turabian StyleSricharoenvech, Piyaporn, Ross Edwards, Müge Yaşar, David A. Gay, and James Schauer. 2025. "Understanding the Origin of Wet Deposition Black Carbon in North America During the Fall Season" Environments 12, no. 2: 58. https://doi.org/10.3390/environments12020058
APA StyleSricharoenvech, P., Edwards, R., Yaşar, M., Gay, D. A., & Schauer, J. (2025). Understanding the Origin of Wet Deposition Black Carbon in North America During the Fall Season. Environments, 12(2), 58. https://doi.org/10.3390/environments12020058






