The Fate of the Cyanotoxin Dihydroanatoxin-a in Drinking Water Treatment Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
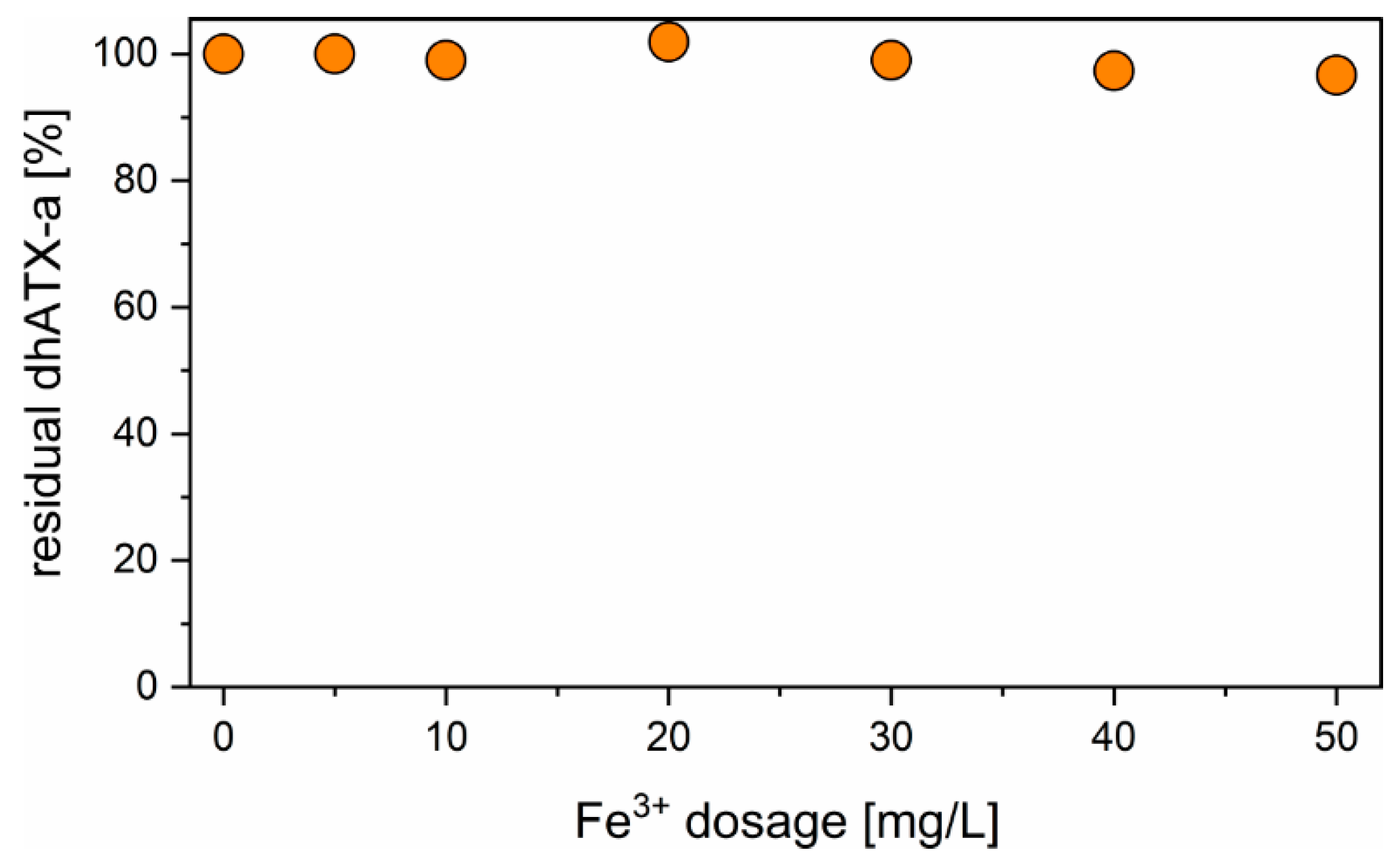
2.2. Flocculation Experiments
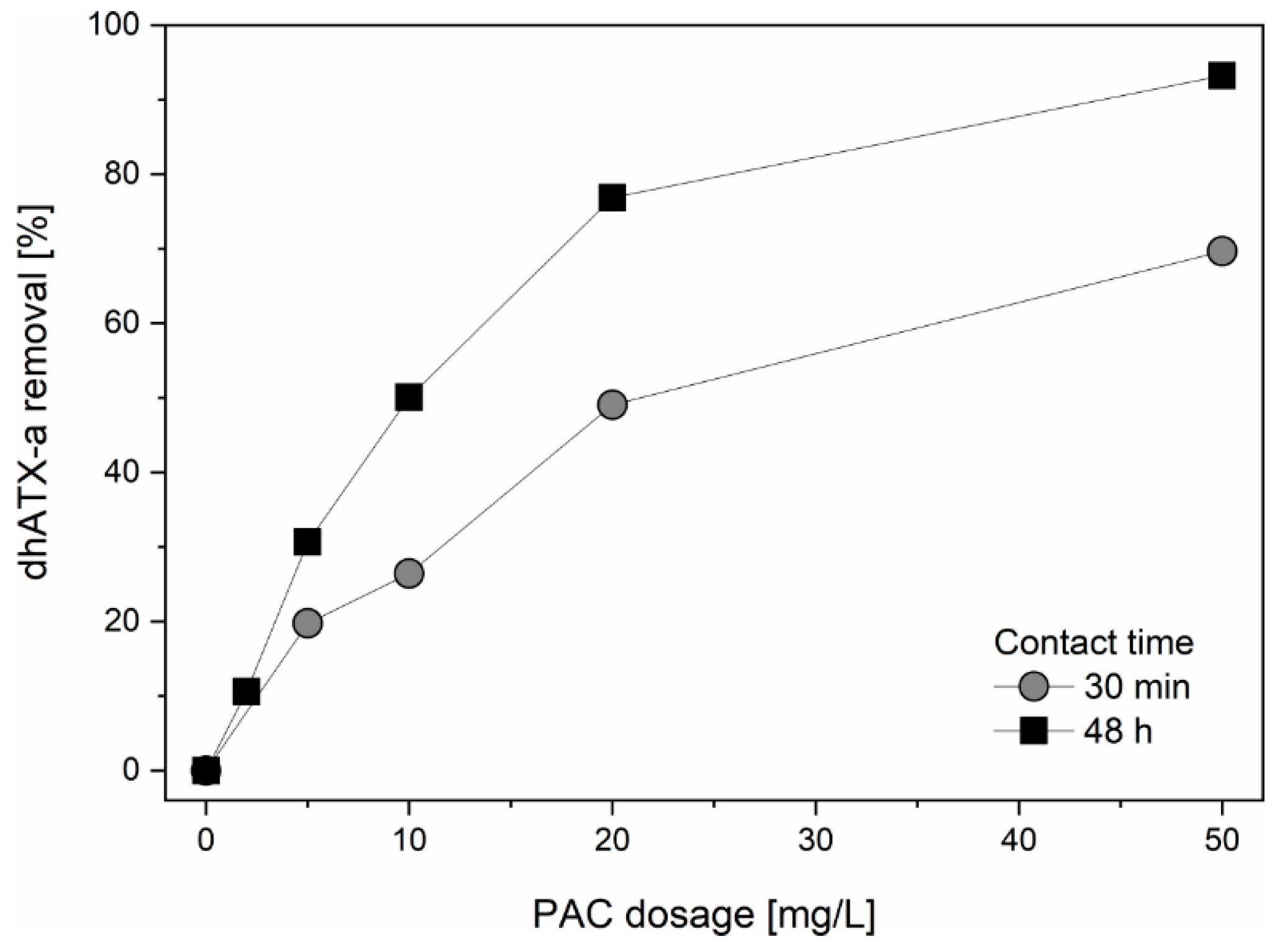
2.3. Adsorption Experiments
2.4. Ozonation Experiments
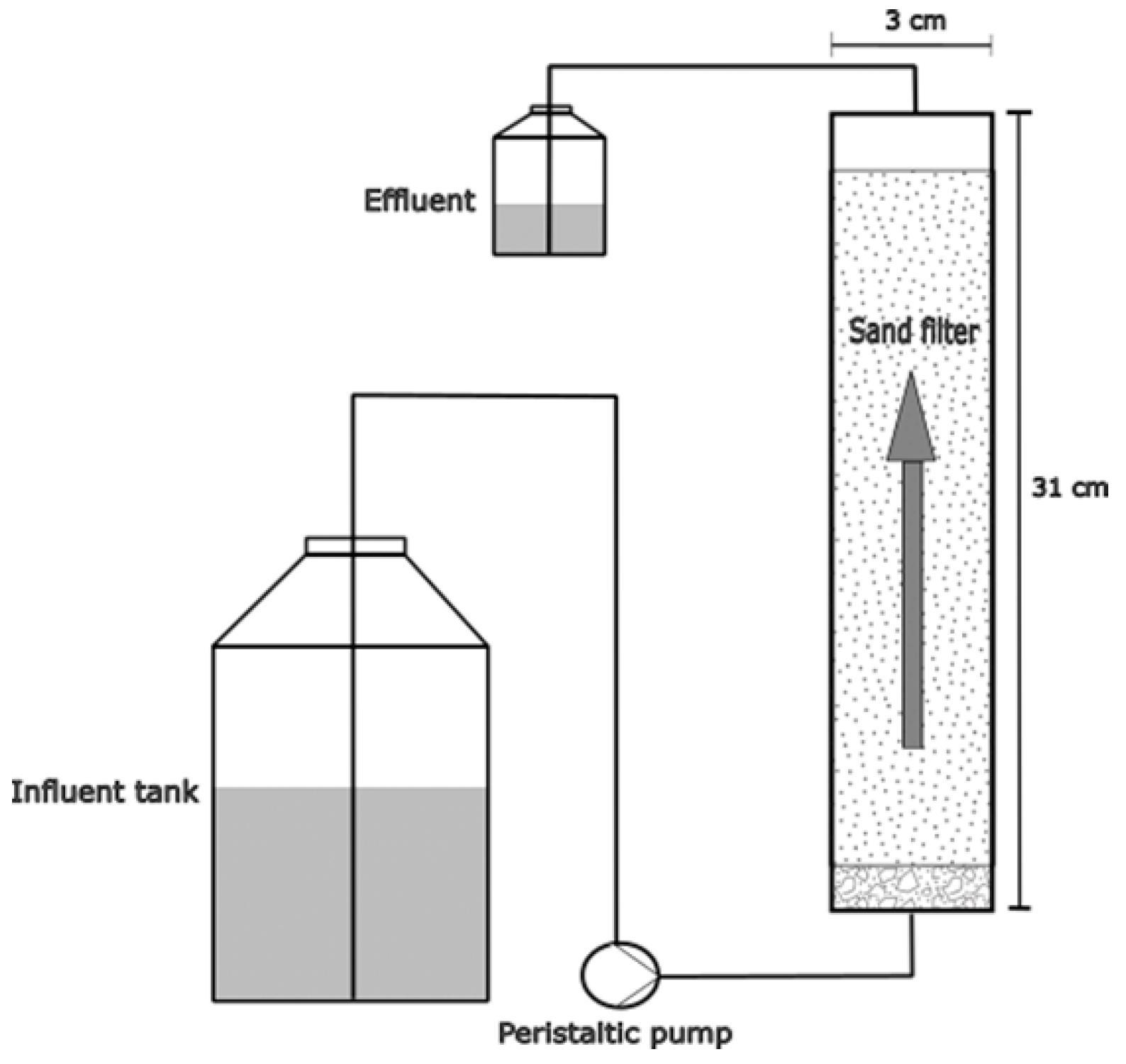
2.5. Sand Filtration Experiments
2.6. Chlorination Experiments
2.7. Analyses
3. Results and Discussion
3.1. Flocculation
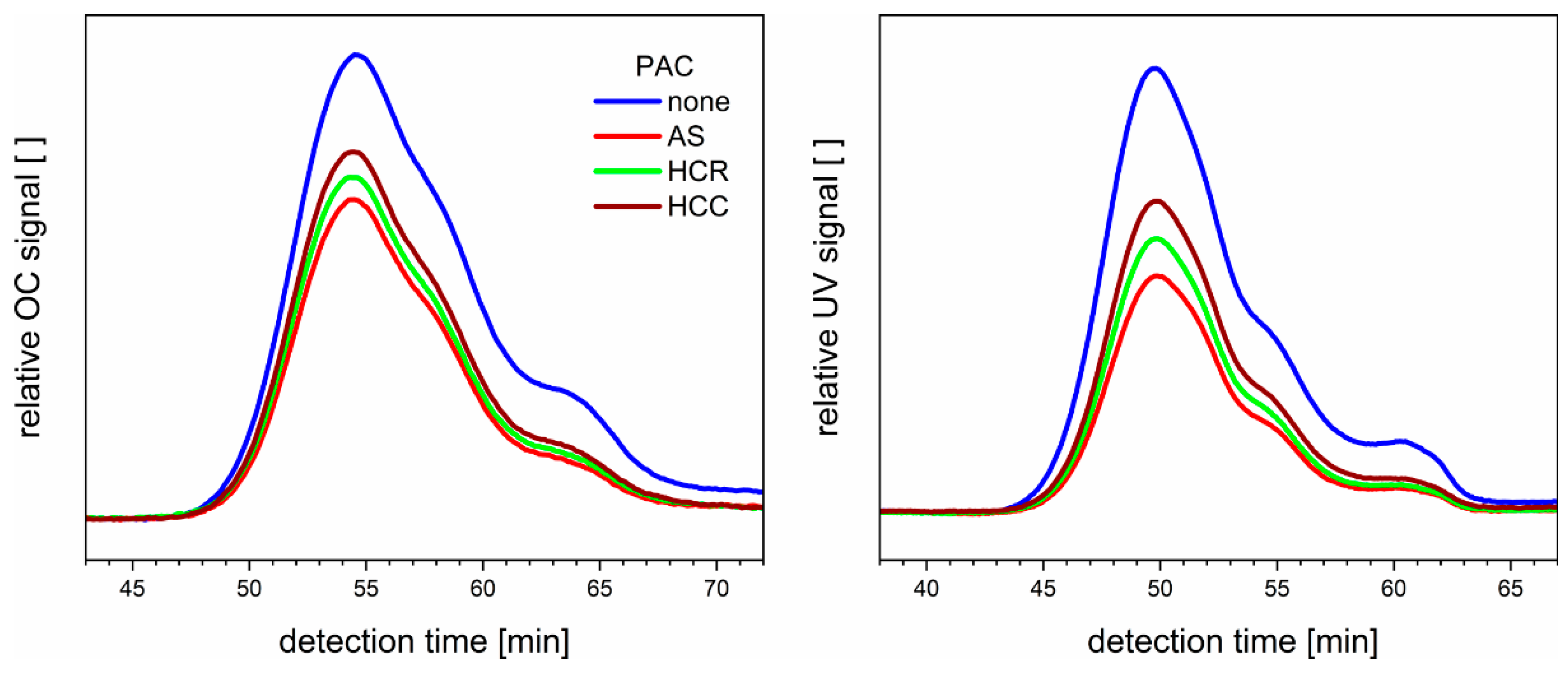
3.2. Adsorption onto Activated Carbon
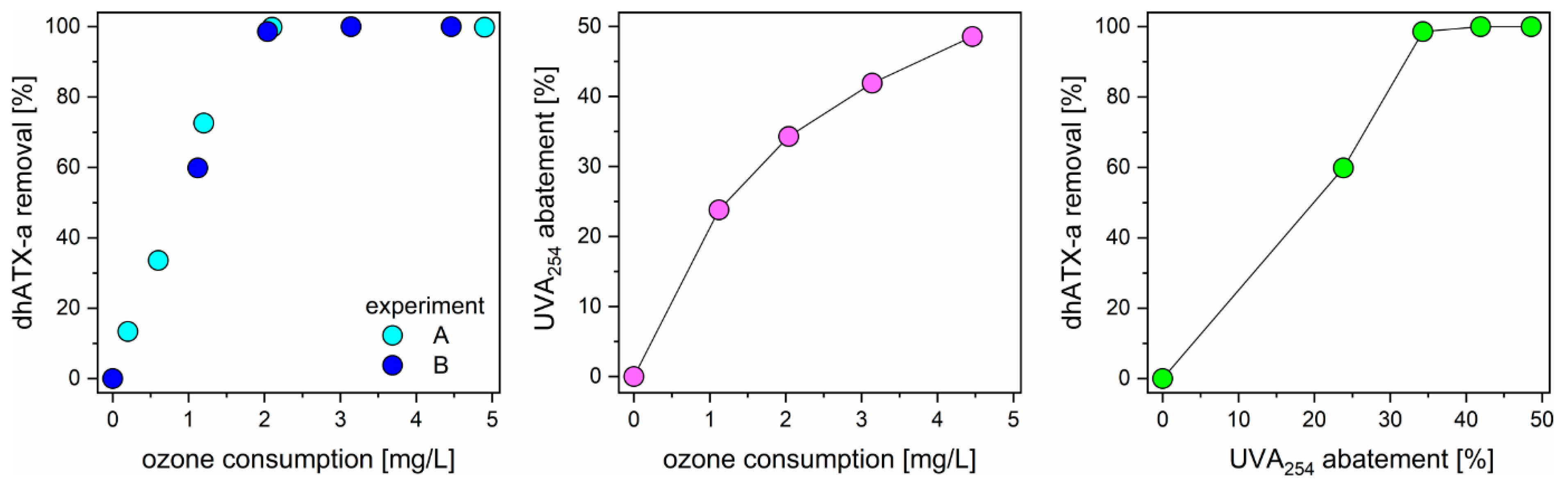
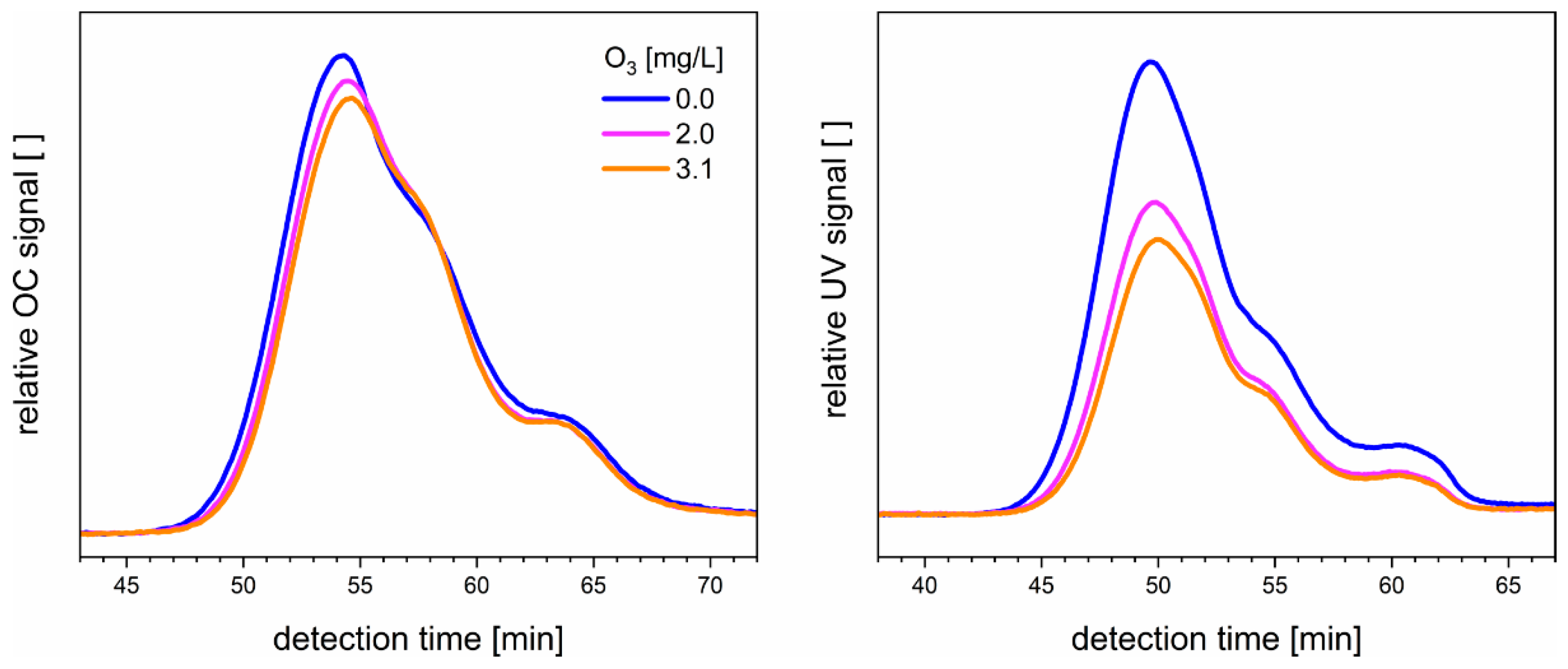
3.3. Ozonation
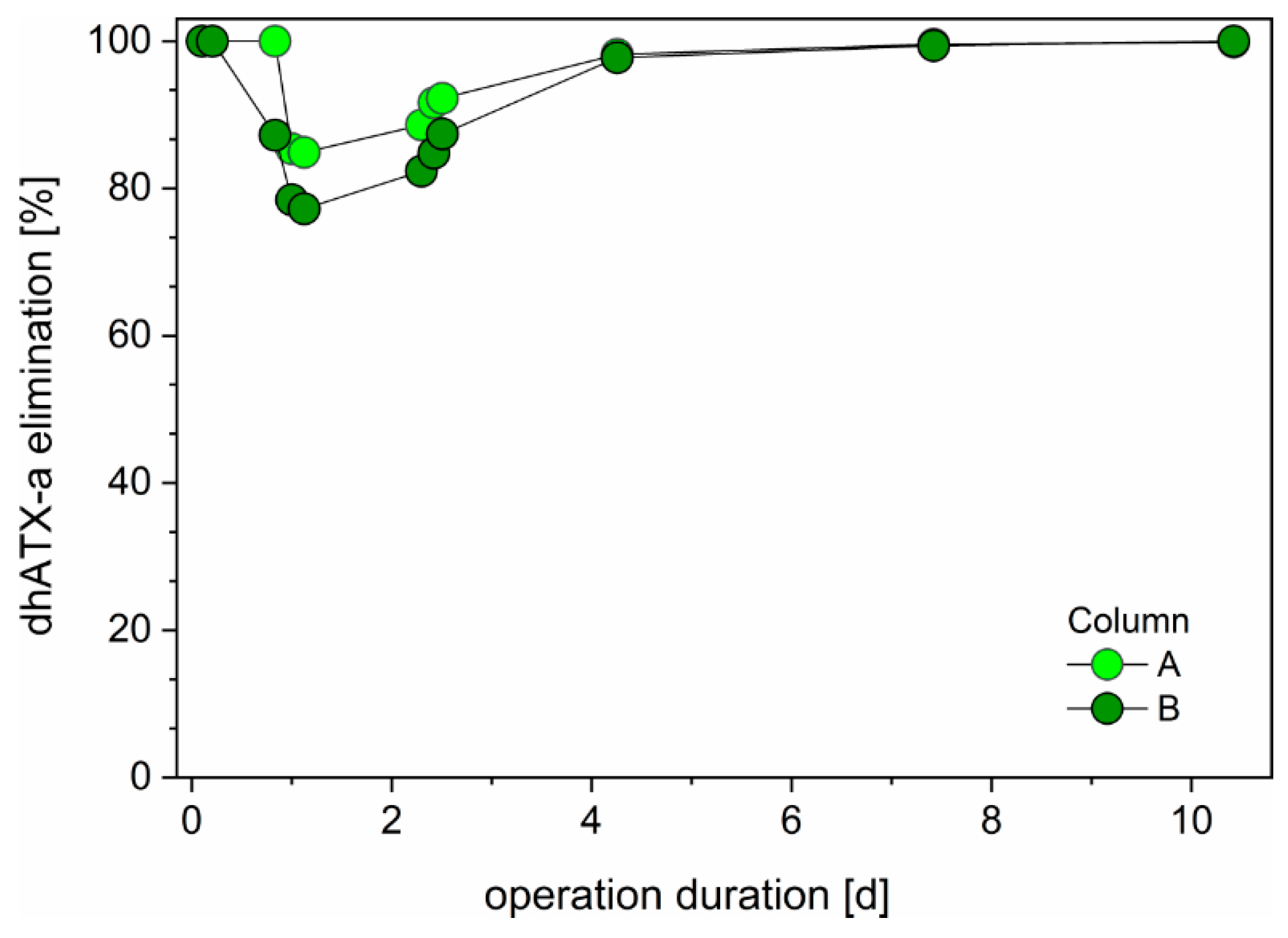
3.4. Slow Sand Filtration
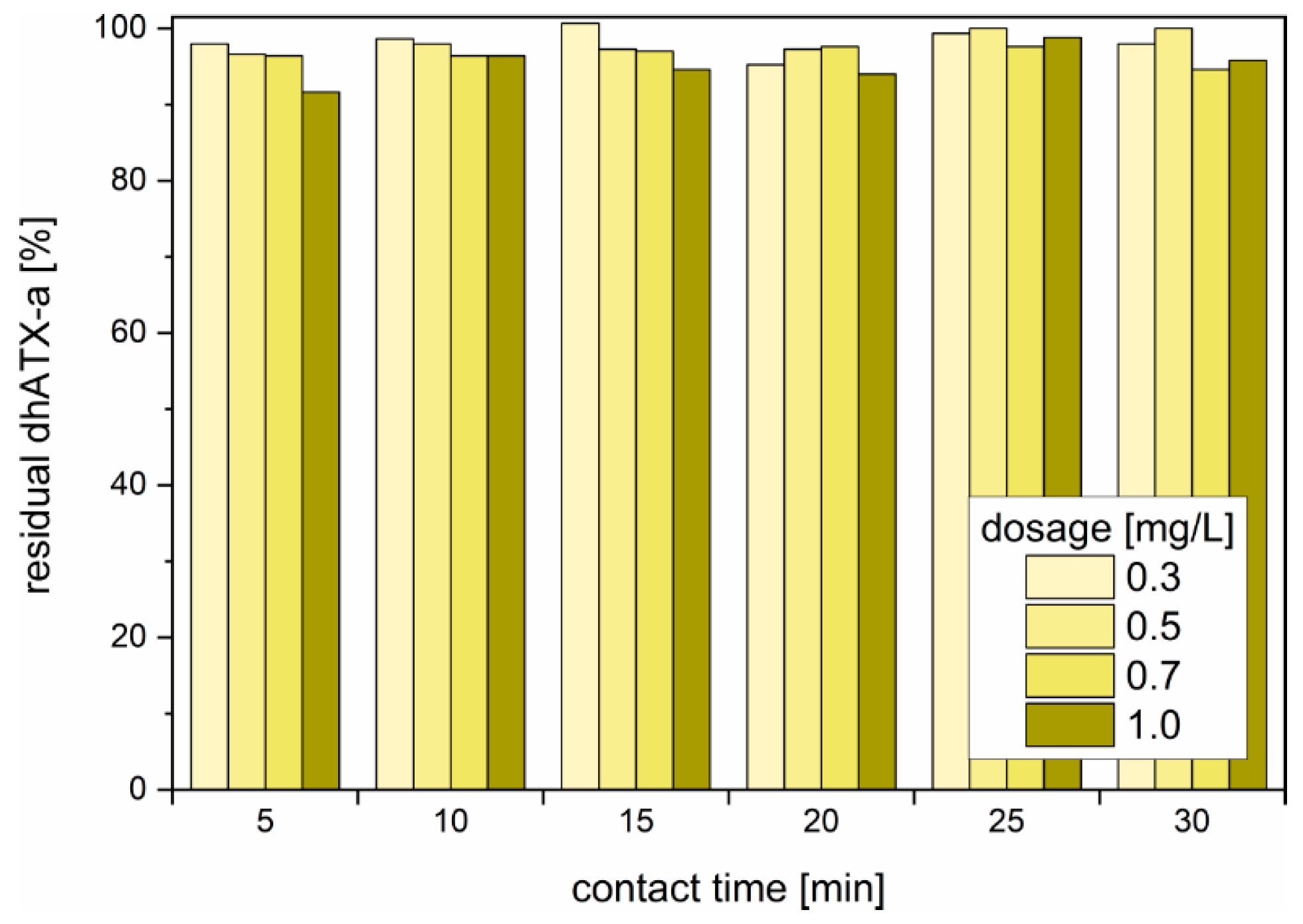
3.5. Chlorination
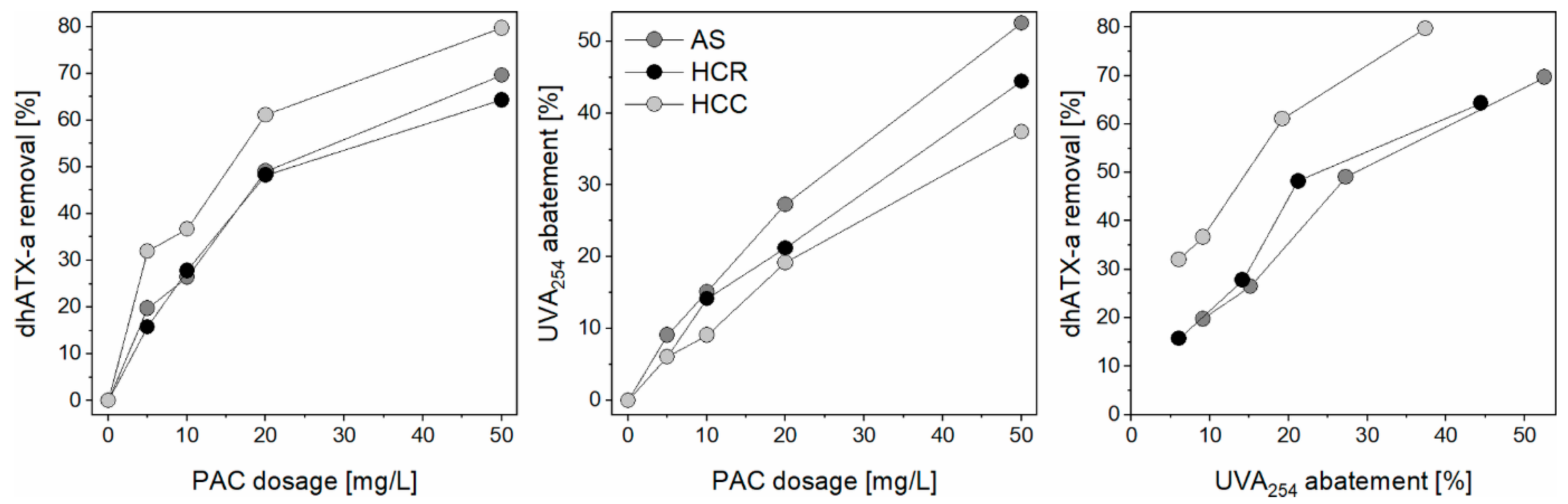
3.6. Comparison
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| dhATX-a | Dihydroanatoxin-a |
| ATX-a | Anatoxin-a |
| HATX-a | Hydroanatoxin-a |
| MC | Microcystins |
| CYN | Cylindrospermopsin |
| PAC | Powdered activated carbon |
| DOC | Dissolved organic carbon |
| UVA254 | UV light absorption at 254 nm wavelength |
| HPLC-MS/MS | High-performance liquid chromatography/tandem mass spectrometry |
| LC-OCD-UVD | Liquid chromatography with organic carbon and UV detections |
References
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water, 2nd ed.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Catherine, Q.; Susanna, W.; Isidora, E.-S.; Mark, H.; Aurélie, V.; Jean-François, H. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Testai, M. Anatoxin-a and analogues. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Stevens, D.K.; Krieger, R.I. Stability studies on the cyanobacterial nicotinic alkaloid snatoxin-A. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Cohen, M.; Chapuis-Hugon, F.; Pichon, V.; Mazmouz, R.; Méjean, A.; Ploux, O. Synthesis, configuration assignment, and simultaneous quantification by liquid chromatography coupled to tandem mass spectrometry, of dihydroanatoxin-a and dihydrohomoanatoxin-a together with the parent toxins, in axenic cyanobacterial strains and in environmental samples. Toxicon 2012, 60, 1404–1414. [Google Scholar] [PubMed]
- Fastner, J.; Teikari, J.; Hoffmann, A.; Köhler, A.; Hoppe, S.; Dittmann, E.; Welker, M. Cyanotoxins associated with macrophytes in Berlin (Germany) water bodies—Occurrence and risk assessment. Sci. Total Environ. 2023, 858, 159433. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Nicholson, B. Water treatment options for dissolved cyanotoxins. J. Water Supply Res. Technol.-Aqua 2004, 53, 227–239. [Google Scholar] [CrossRef]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass Occurrence of Anatoxin-a- and Dihydroanatoxin-a-Producing Tychonema sp. in Mesotrophic Reservoir Mandichosee (River Lech, Germany) as a Cause of Neurotoxicosis in Dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef]
- Pietsch, J.; Bornmann, K.; Schmidt, W. Relevance of Intra- and Extracellular Cyanotoxins for Drinking Water Treatment. Acta Hydrochim. Hydrobiol. 2002, 30, 7–15. [Google Scholar] [CrossRef]
- de Lucena-Silva, D.; Molozzi, J.; dos Santos Severiano, J.; Becker, V.; de Lucena Barbosa, J.E. Removal efficiency of phosphorus, cyanobacteria and cyanotoxins by the “flock & sink” mitigation technique in semi-arid eutrophic waters. Water Res. 2019, 159, 262–273. [Google Scholar] [CrossRef]
- Wood, S.A.; Holland, P.T.; MacKenzie, L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere 2011, 82, 888–894. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G. Removal of microcystin variants with powdered activated carbon. Water Supply 2002, 2, 201–207. [Google Scholar] [CrossRef]
- Vlad, S.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Removal of the cyanotoxin anatoxin-a by drinking water treatment processes: A review. J. Water Health 2014, 12, 601–617. [Google Scholar] [CrossRef]
- Newcombe, G.; Ho, L.; Neto, J.C. Controlling cyanotoxin occurrence—Drinking water treatment. In Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Klitzke, S.; Beusch, C.; Fastner, J. Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Res. 2011, 45, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Lahti, K.; Sivonen, K.; Niemelä, S.I. Biodegradability and adsorption on lake sediments of cyanobacterial hepatotoxins and anatoxin-a. Lett. Appl. Microbiol. 1994, 19, 423–428. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Elnour, R.O.; Alamri, S.; Hashem, M. Biodegradation of the cyanobacterial toxin anatoxin-a by a Bacillus subtilis strain isolated from a eutrophic lake in Saudi Arabia. Arch. Microbiol. 2024, 206, 348. [Google Scholar] [CrossRef] [PubMed]
- Schumann, P.; Muschket, M.; Dittmann, D.; Rabe, L.; Reemtsma, T.; Jekel, M.; Ruhl, A.S. Is adsorption onto activated carbon a feasible drinking water treatment option for persistent and mobile substances? Water Res. 2023, 235, 119861. [Google Scholar] [CrossRef]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Filter, J.; Zhiteneva, V.; Vick, C.; Ruhl, A.S.; Jekel, M.; Hübner, U.; Drewes, J.E. Varying attenuation of trace organic chemicals in natural treatment systems—A review of key influential factors. Chemosphere 2021, 274, 129774. [Google Scholar] [CrossRef]
- Willson, V.A. Determination of available chlorine in hypochlorite solutions by direct titration with sodium thiosulfate. Ind. Eng. Chem. Anal. Ed. 1935, 7, 44–45. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the Main Disinfection Processes for Wastewater and Drinking Water Treatment Plants. Sustainability 2018, 10, 86. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography—organic carbon detection—organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef]
- Hitzfeld, B.C.; Höger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar] [CrossRef]
- Keijola, A.M.; Himberg, K.; Esala, A.L.; Sivonen, K.; Hiis-Virta, L. Removal of cyanobacterial toxins in water treatment processes: Laboratory and pilot-scale experiments. Toxic. Assess. 1988, 3, 643–656. [Google Scholar] [CrossRef]
- Mouchet, P.; Bonnélye, V. Solving algae problems: French expertise and world-wide applications. J. Water Supply Res. Technol.-Aqua 1998, 47, 125–141. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Zietzschmann, F.; Hilbrandt, I.; Meinel, F.; Altmann, J.; Sperlich, A.; Jekel, M. Targeted testing of activated carbons for advanced wastewater treatment. Chem. Eng. J. 2014, 257, 184–190. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Altmann, J.; Ruhl, A.S.; Dunnbier, U.; Dommisch, I.; Sperlich, A.; Meinel, F.; Jekel, M. Estimating organic micro-pollutant removal potential of activated carbons using UV absorption and carbon characteristics. Water Res. 2014, 56, 48–55. [Google Scholar] [CrossRef]
- Schneider, F.; Ruhl, A.S.; Hübner, U.; Jekel, M. Removal of Residual Dissolved Ozone with Manganese Dioxide for Process Control with UV254. Ozone Sci. Eng. 2016, 38, 79–85. [Google Scholar] [CrossRef]
- Filter, J.; Kopp, M.G.V.; Ruhl, A.S.; Jekel, M. Influence of low oxygen concentrations on biological transformations of trace organic chemicals in sand filter systems. Chemosphere 2023, 335, 139069. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, S.; Apelt, S.; Weiler, C.; Fastner, J.; Chorus, I. Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments—The role of sediment preconditioning and DOM composition. Toxicon 2010, 55, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Clément, M.; Thomas, O. State of the art on cyanotoxins in water and their behaviour towards chlorine. Toxicon 2010, 55, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Onstad, G.D.; Kull, T.P.J.; Metcalf, J.S.; Acero, J.L.; von Gunten, U. Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Res. 2007, 41, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Ruhl, A.S.; Sauter, D.; Pohl, J.; Jekel, M. How to dose powdered activated carbon in deep bed filtration for efficient micropollutant removal. Water Res. 2015, 78, 9–17. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Elimination [%] | Dosage [mg/L] | Time | Limitations |
|---|---|---|---|---|
| flocculation | 0 | up to 50 | ca. 20 min | ineffective for dissolved toxins |
| adsorption onto PAC | 75–93 | 20–50 | >30 min | PAC separation needed, competition with DOC |
| ozonation | >95 | 2 mg/L consumption | ca. 10 min | ozone consumption by DOC, oxidation products |
| slow sand filtration | >95 | no | <6 h | adaptation time required |
| chlorination | 0 | up to 1 | ineffective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolatimehr, A.; Fastner, J.; Ruhl, A.S. The Fate of the Cyanotoxin Dihydroanatoxin-a in Drinking Water Treatment Processes. Environments 2025, 12, 52. https://doi.org/10.3390/environments12020052
Dolatimehr A, Fastner J, Ruhl AS. The Fate of the Cyanotoxin Dihydroanatoxin-a in Drinking Water Treatment Processes. Environments. 2025; 12(2):52. https://doi.org/10.3390/environments12020052
Chicago/Turabian StyleDolatimehr, Armin, Jutta Fastner, and Aki Sebastian Ruhl. 2025. "The Fate of the Cyanotoxin Dihydroanatoxin-a in Drinking Water Treatment Processes" Environments 12, no. 2: 52. https://doi.org/10.3390/environments12020052
APA StyleDolatimehr, A., Fastner, J., & Ruhl, A. S. (2025). The Fate of the Cyanotoxin Dihydroanatoxin-a in Drinking Water Treatment Processes. Environments, 12(2), 52. https://doi.org/10.3390/environments12020052






