Iron Oxide Nanoparticles for Photosynthetic Recovery in Iron-Deficient ‘Micro-Tom’ Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Condition
2.2. Plant Species
2.3. Preparation and Functionalization of Iron Oxide Nanoparticles
2.4. Functionalization of Iron Oxide Nanoparticles
2.5. Treatment Application and Experimental Design
2.6. Chlorophyll a Fluorescence and Gas Exchange
2.7. Quantification of Hydrogen Peroxide
2.8. Lipid Peroxidation
2.9. Statistical Evaluation
2.10. Heatmap
3. Results
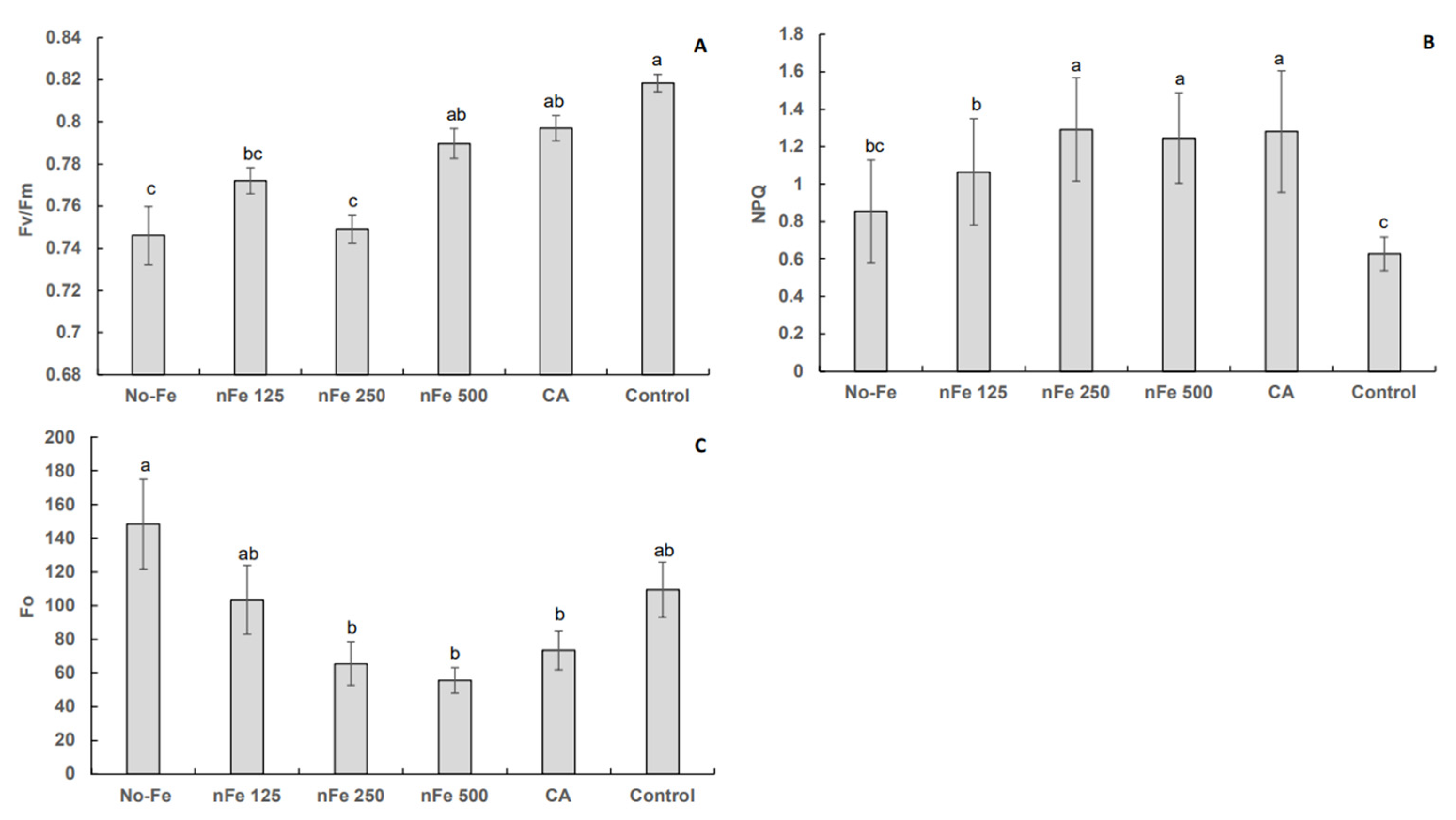
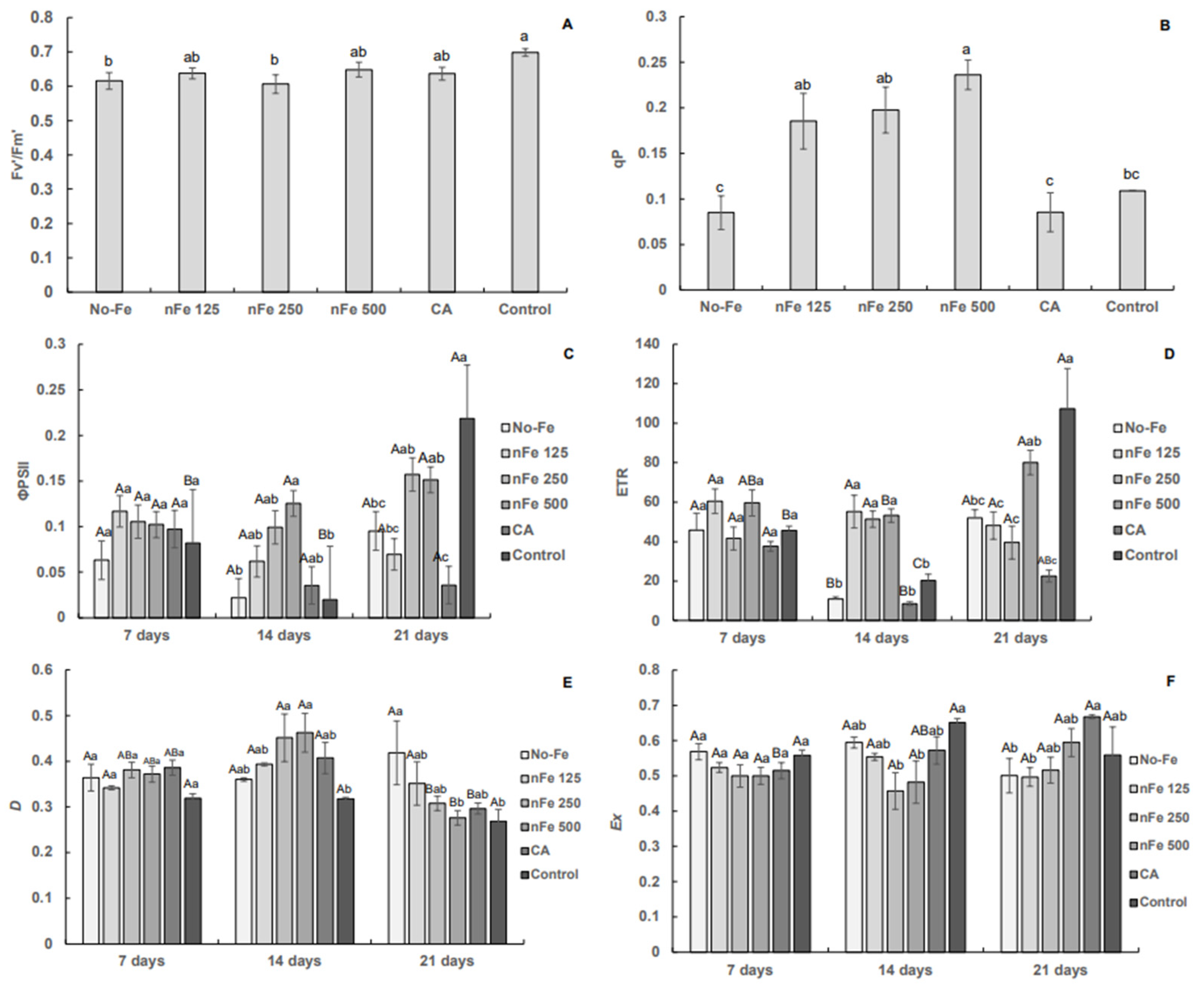
3.1. Chlorophyll a Fluorescence
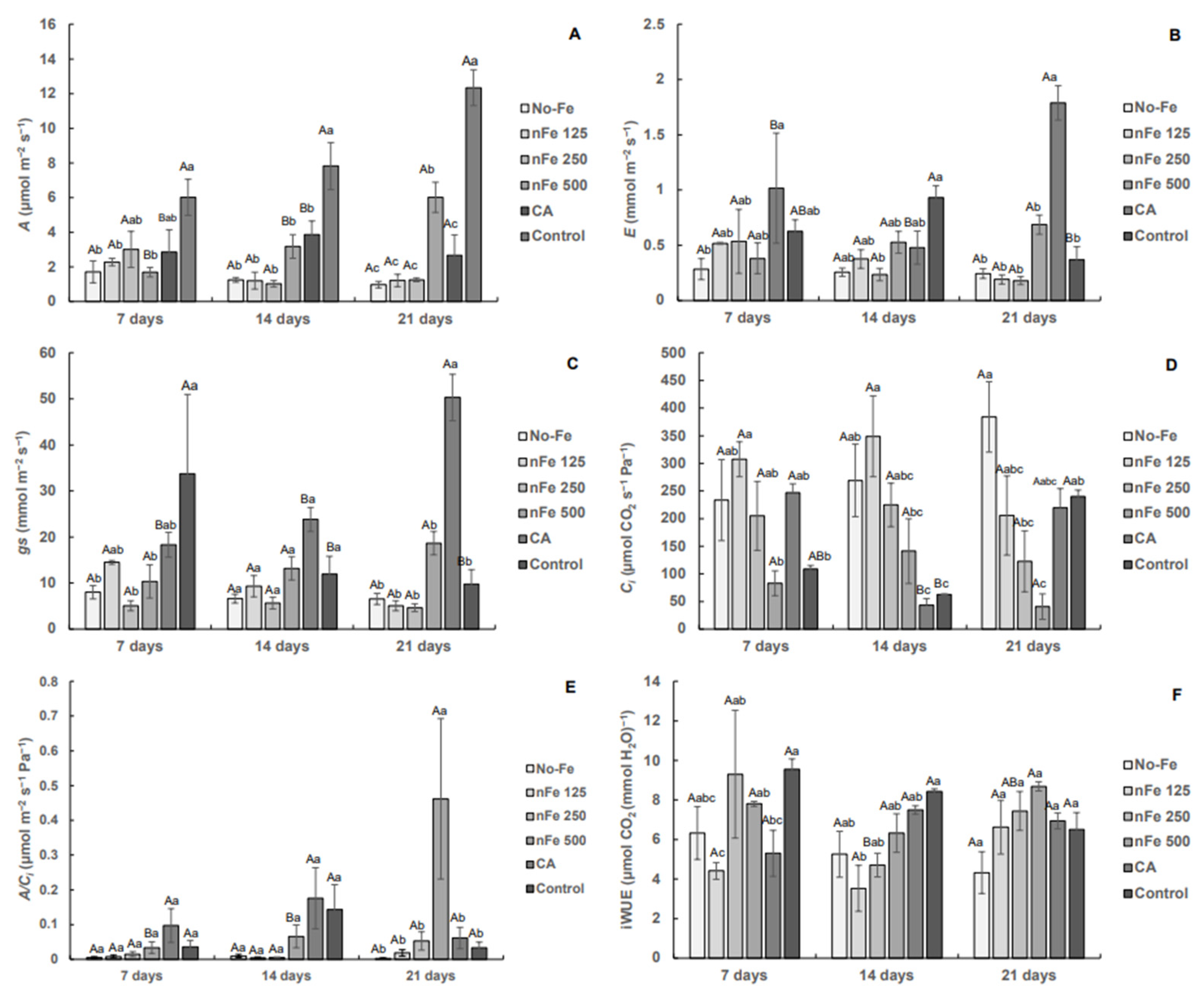
3.2. Gas Exchange
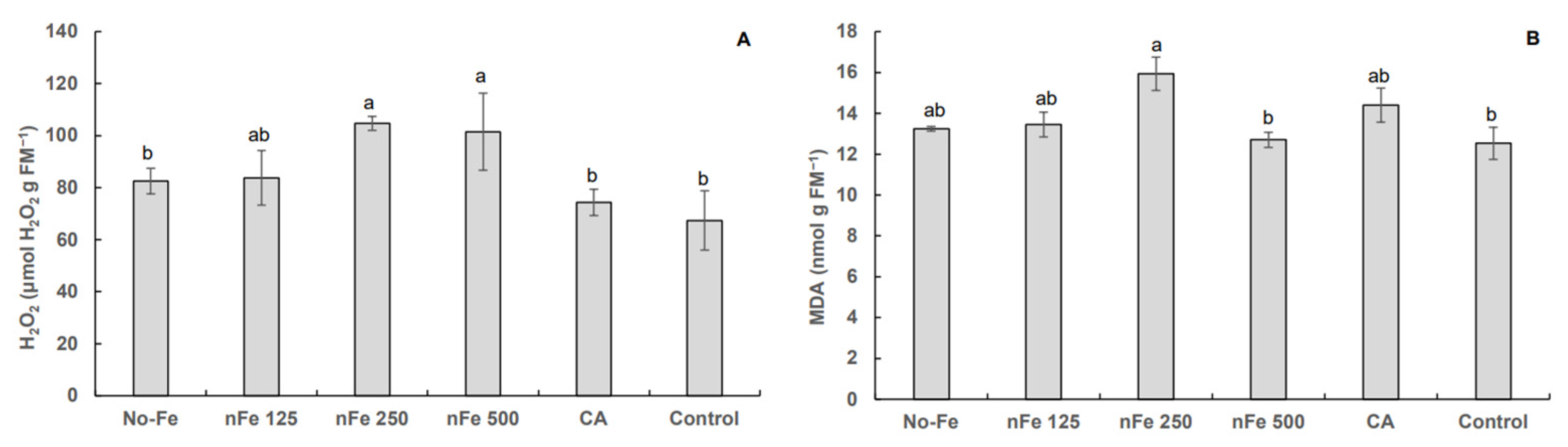
3.3. Hydrogen Peroxide and Lipid Peroxidation
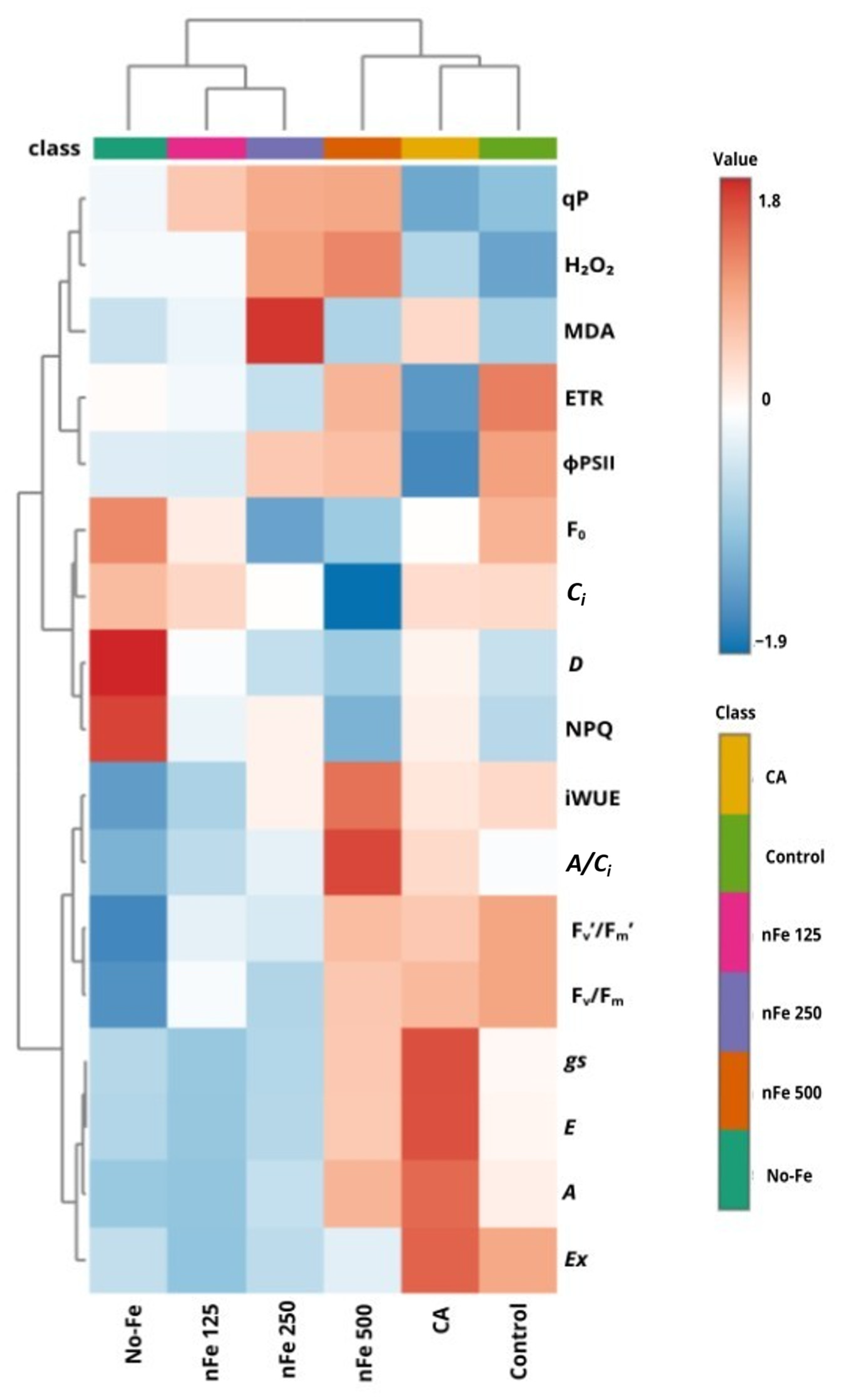
3.4. Heatmap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal nanoparticles in agriculture: A review of possible use. Coatigns 2022, 12, 1586. [Google Scholar] [CrossRef]
- Aithal, P.S.; Aithal, S. Opportunities and challenges for green and eco-friendly nanotechnology in twenty-first century. In Sustainable Nanotechnology: Strategies, Products, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; Chapter 3; pp. 31–50. [Google Scholar] [CrossRef]
- El-Kalliny, A.S.; Abdel-Wahed, M.S.; El-Zahhar, A.A.; Hamza, I.A.; Gad-Allah, T.A. Nanomaterials: A review of emerging contaminants with potential health or environmental impact. Discov. Nano 2023, 18, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhou, Q.; Tao, Z.; Ouyang, S. Magnetic iron-based nanoparticles biogeochemical behavior in soil-plant system: A critical review. Sci. Total Environ. 2023, 90, 166643. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Van Aken, B. Gene expression changes in plants and microorganisms exposed to nanomaterials. Curr. Opin. Biotechnol. 2015, 33, 206–219. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Ashraf, U. Nanoparticle application and abiotic-stress tolerance in plants. In Plant Life Under Changing Environment: Responses and Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 627–641. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Movahedi, A.; Taghimolaee, M.; Ghayebpanah, S.; Monaiemi, F.; Hanbari, M.; Farahzadi, H.N. A Review of the Effect of Nanoparticles on Reactive Oxygen Species Production in Different Plants. J. Eng. Appl. Res. 2024, 1, 131–163. [Google Scholar]
- Fang, G.D.; Zhou, D.M.; Dionysiou, D.D. Superoxide mediated production of hydroxyl radicals by magnetite nanoparticles: Demonstration in the degradation of 2-chlorobiphenyl. J. Hazard. Mater. 2013, 250–251, 68–75. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Mazarji, M.; Shende, S.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Chaplygin, V.; Singh, A.; Jatav, H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 2020, 65, 137–143. [Google Scholar] [CrossRef]
- Yang, X.; Alidoust, D.; Wang, C. Effects of iron oxide nanoparticles on the mineral composition and growth of soybean (Glycine max L.) plants. Acta Physiol. Plant 2020, 42, 128. [Google Scholar] [CrossRef]
- Hu, J.; Xianyu, Y. When nano meets plants: A review on the interplay between nanoparticles and plants. Nano Today 2021, 38, 101143. [Google Scholar] [CrossRef]
- Martí, E.; Gisbert, C.; Bishop, G.J.; Dixon, M.S.; García-Martínez, J.L. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef]
- Parrotta, L.; Aloisi, I.; Faleri, C.; Romi, M.; Del Duca, S.; Cai, G. Chronic heat stress affects the photosynthetic apparatus of Solanum lycopersicum L. cv Micro-Tom. Plant Physiol. Biochem. 2020, 154, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Von Caemmerer, S.; Farquhar, G.D. Some Relationships between the Biochemistry of Photosynthesis and the Gas Exchange of Leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-Induced Changes in Antioxidant Enzymes Activities in Fronds of Duckweed (Lemna Minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021; 49, W388–W396. [Google Scholar]
- Cakmak, I.; Rengel, Z.; White, P.J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: London, UK, 2022; cap. 7; pp. 283–293. [Google Scholar]
- Kim, J.H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.S. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Env. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Hideg, E.; Kalai, T.; Hideg, K.; Vass, I. Do oxidative stress conditions impairing photosynthesis in the light manifest as photoinhibition? In Philosophical Transactions of the Royal Society B: Biological Sciences. R. Soc. 2000, 355, 1511–1516. [Google Scholar]
- Knaapen, A.M.; Borm, P.J.A.; Albrecht, C.; Schins, R.P.F. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer, 2004; 109, 799–809. [Google Scholar] [CrossRef]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of the wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef] [PubMed]
- Barzotto, G.R.; Cardoso, C.P.; Jorge, L.G.; Campos, F.G.; Boaro, C.S.F. Hydrogen peroxide signal photosynthetic acclimation of Solanum lycopersicum L. cv Micro-Tom under water deficit. Sci. Rep. 2023, 13, 13059. [Google Scholar] [CrossRef] [PubMed]
- López-Millán, A.F.; Morales, F.; Gogorcena, Y.; Abadía, A.; Abadía, J. Metabolic responses in iron deficient tomato plants. J. Plant Physiol. 2009, 166, 375–384. [Google Scholar] [CrossRef]
- Cohen, I.; Knopf, J.A.; Irihimovitch, V.; Shapira, M. A proposed mechanism for the inhibitory effects of oxidative stress on Rubisco assembly and its subunit expression. Plant Physiol. 2005, 137, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, H.; Maali-Amir, R.; Zeinali, H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 2015, 62, 779–787. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Metal and metal oxide nanoparticles-induced reactive oxygen species: Phytotoxicity and detoxification mechanisms in plant cell. Plant Physiol. Biochem. 2024, 213, 108847. [Google Scholar] [CrossRef]
- Abdalbagemohammedabdalsadeg, S.; Xiao, B.-L.; Ma, X.-X.; Li, Y.-Y.; Wei, J.-S.; Moosavi-Movahedi, A.A.; Yousefi, R.; Hong, J. Catalase immobilization: Current knowledge, key insights, applications, and future prospects—A review. Int. J. Biol. Macromol. 2024, 276, 133941. [Google Scholar] [CrossRef] [PubMed]
- Melicher, P.; Dvorák, P.; Krasylenko, Y.; Shapiguzov, A.; Kangasjärvi, J.; Šamaj, J.; Takác, T. Arabidopsis Iron Superoxide Dismutase FSD1 Protects Against Methyl Viologen-Induced Oxidative Stress in a Copper-Dependent Manner. Front. Plant Sci. 2022, 13, 823561. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yu, J.; Cheng, Y.; Ruan, M.; Wang, R.; Ye, Q.; Zhou, G.; Li, Z.; Yao, Z.; Yang, Y.; et al. The SOD Gene Family in Tomato: Identification, Phylogenetic Relationships, and Expression Patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gama, J.P.S.; Campos, F.G.; Riccardi, C.d.S.; Boaro, C.S.F. Iron Oxide Nanoparticles for Photosynthetic Recovery in Iron-Deficient ‘Micro-Tom’ Tomato Plants. Environments 2025, 12, 346. https://doi.org/10.3390/environments12100346
Gama JPS, Campos FG, Riccardi CdS, Boaro CSF. Iron Oxide Nanoparticles for Photosynthetic Recovery in Iron-Deficient ‘Micro-Tom’ Tomato Plants. Environments. 2025; 12(10):346. https://doi.org/10.3390/environments12100346
Chicago/Turabian StyleGama, João Pedro Sampaio, Felipe Girotto Campos, Carla dos Santos Riccardi, and Carmen Sílvia Fernandes Boaro. 2025. "Iron Oxide Nanoparticles for Photosynthetic Recovery in Iron-Deficient ‘Micro-Tom’ Tomato Plants" Environments 12, no. 10: 346. https://doi.org/10.3390/environments12100346
APA StyleGama, J. P. S., Campos, F. G., Riccardi, C. d. S., & Boaro, C. S. F. (2025). Iron Oxide Nanoparticles for Photosynthetic Recovery in Iron-Deficient ‘Micro-Tom’ Tomato Plants. Environments, 12(10), 346. https://doi.org/10.3390/environments12100346






