Bioaccumulation Patterns in Different Tissues of Twelve Species of Elasmobranchs from the Tyrrhenian and Ionian Sea (Calabria, Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Trace Elements Analysis
2.3. Statistical Analysis
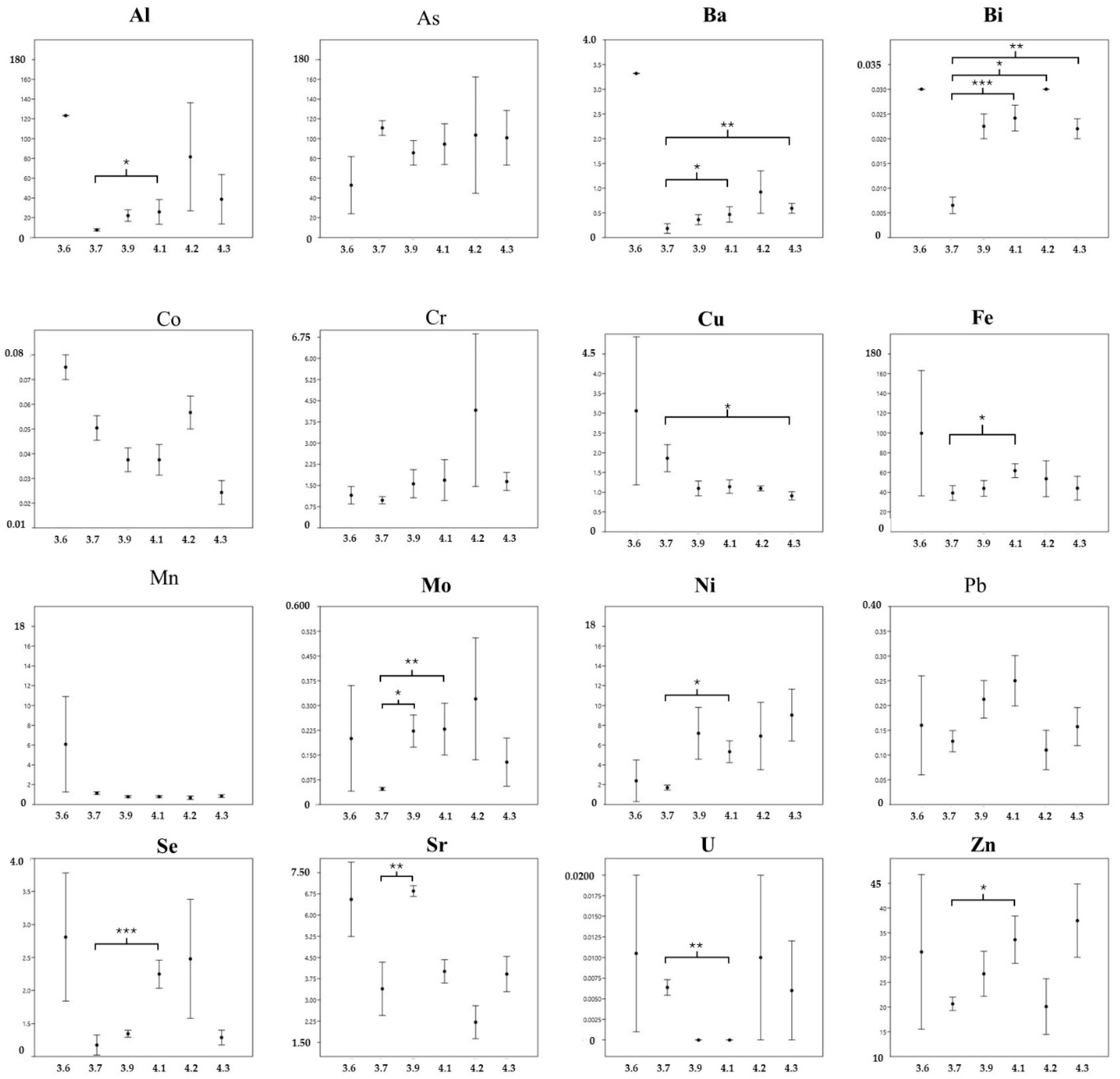
3. Results and Discussion
3.1. Muscle Tissue
3.2. Comparison Among Tissues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consales, G.; Marsili, L. Assessment of the Conservation Status of Chondrichthyans: Underestimation of the Pollution Threat. Eur. Zool. J. 2021, 88, 165–180. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Interspecific Variation in Total Arsenic Body Concentrations in Elasmobranch Fish from the Mediterranean Sea. Mar. Pollut. Bull. 2004, 48, 1145–1149. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G. Mercury Accumulation and Speciation in Muscle Tissue of Different Species of Sharks from Mediterranean Sea, Italy. Bull. Environ. Contam. Toxicol. 2002, 68, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M. Potential Human Health Risks from Metals (Hg, Cd, and Pb) and Polychlorinated Biphenyls (PCBs) via Seafood Consumption: Estimation of Target Hazard Quotients (THQs) and Toxic Equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.M.F.; Nunes, M.; Marchand, P.; Le Bizec, B.; Mendes, S.; Correia, J.P.S.; Lemos, M.F.L.; Novais, S.C. Blue Sharks (Prionace glauca) as Bioindicators of Pollution and Health in the Atlantic Ocean: Contamination Levels and Biochemical Stress Responses. Sci. Total Environ. 2016, 563–564, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Barrera-García, A.; O’Hara, T.; Galván-Magaña, F.; Méndez-Rodríguez, L.C.; Castellini, J.M.; Zenteno-Savín, T. Trace Elements and Oxidative Stress Indicators in the Liver and Kidney of the Blue Shark (Prionace glauca). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2013, 165, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Canli, M.; Atli, G. The Relationships between Heavy Metal (Cd, Cr, Cu, Fe, Pb, Zn) Levels and the Size of Six Mediterranean Fish Species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- De Boeck, G.; Eyckmans, M.; Lardon, I.; Bobbaers, R.; Sinha, A.K.; Blust, R. Metal Accumulation and Metallothionein Induction in the Spotted Dogfish Scyliorhinus canicula. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Jeffree, R.A.; Warnau, M.; Teyssié, J.-L.; Markich, S.J. Comparison of the Bioaccumulation from Seawater and Depuration of Heavy Metals and Radionuclides in the Spotted Dogfish Scyliorhinus canicula (Chondrichthys) and the Turbot Psetta maxima (Actinopterygii: Teleostei). Sci. Total Environ. 2006, 368, 839–852. [Google Scholar] [CrossRef]
- Lopez, S.A.; Abarca, N.L.; Meléndez, R.C. Heavy Metal Concentrations of Two Highly Migratory Sharks ( Prionace glauca and Isurus oxyrinchus ) in the Southeastern Pacific Waters: Comments on Public Health and Conservation. Trop. Conserv. Sci. 2013, 6, 126–137. [Google Scholar] [CrossRef]
- Merly, L.; Lange, L.; Meÿer, M.; Hewitt, A.M.; Koen, P.; Fischer, C.; Muller, J.; Schilack, V.; Wentzel, M.; Hammerschlag, N. Blood Plasma Levels of Heavy Metals and Trace Elements in White Sharks (Carcharodon carcharias) and Potential Health Consequences. Mar. Pollut. Bull. 2019, 142, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Barrera-García, A.; O’Hara, T.; Galván-Magaña, F.; Méndez-Rodríguez, L.C.; Castellini, J.M.; Zenteno-Savín, T. Oxidative Stress Indicators and Trace Elements in the Blue Shark (Prionace glauca) off the East Coast of the Mexican Pacific Ocean. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 59–66. [Google Scholar] [CrossRef]
- Marcovecchio, J.E.; Moreno, V.J.; Pérez, A. Metal Accumulation in Tissues of Sharks from the Bahía Blanca Estuary, Argentina. Mar. Environ. Res. 1991, 31, 263–274. [Google Scholar] [CrossRef]
- Vas, P. Trace Metal Levels in Sharks from British and Atlantic Waters. Mar. Pollut. Bull. 1991, 22, 67–72. [Google Scholar] [CrossRef]
- Torres, P.; Da Cunha, R.T.; Maia, R.; Dos Santos Rodrigues, A. Trophic Ecology and Bioindicator Potential of the North Atlantic Tope Shark. Sci. Total Environ. 2014, 481, 574–581. [Google Scholar] [CrossRef]
- Torres, P.; Tristão Da Cunha, R.; Micaelo, C.; Rodrigues, A.D.S. Bioaccumulation of Metals and PCBs in Raja clavata. Sci. Total Environ. 2016, 573, 1021–1030. [Google Scholar] [CrossRef]
- McKinney, M.A.; Dean, K.; Hussey, N.E.; Cliff, G.; Wintner, S.P.; Dudley, S.F.J.; Zungu, M.P.; Fisk, A.T. Global versus Local Causes and Health Implications of High Mercury Concentrations in Sharks from the East Coast of South Africa. Sci. Total Environ. 2016, 541, 176–183. [Google Scholar] [CrossRef]
- Bevacqua, L.; Reinero, F.R.; Becerril-García, E.E.; Elorriaga-Verplancken, F.R.; Juaristi-Videgaray, D.; Micarelli, P.; Galván-Magaña, F.; Curiel-Godoy, P.; Giglio, G.; Tripepi, S.; et al. Trace Elements and Isotopes Analyses on Historical Samples of White Sharks from the Mediterranean Sea. Eur. Zool. J. 2021, 88, 132–141. [Google Scholar] [CrossRef]
- Pethybridge, H.; Cossa, D.; Butler, E.C.V. Mercury in 16 Demersal Sharks from Southeast Australia: Biotic and Abiotic Sources of Variation and Consumer Health Implications. Mar. Environ. Res. 2010, 69, 18–26. [Google Scholar] [CrossRef]
- Matulik, A.G.; Kerstetter, D.W.; Hammerschlag, N.; Divoll, T.; Hammerschmidt, C.R.; Evers, D.C. Bioaccumulation and Biomagnification of Mercury and Methylmercury in Four Sympatric Coastal Sharks in a Protected Subtropical Lagoon. Mar. Pollut. Bull. 2017, 116, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bendall, V.A.; Barber, J.L.; Papachlimitzou, A.; Bolam, T.; Warford, L.; Hetherington, S.J.; Silva, J.F.; McCully, S.R.; Losada, S.; Maes, T.; et al. Organohalogen Contaminants and Trace Metals in North-East Atlantic Porbeagle Shark (Lamna nasus). Mar. Pollut. Bull. 2014, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Kutil, N.J.; Malek, A.J.; Collie, J.S. Mercury Bioaccumulation in Cartilaginous Fishes from Southern New England Coastal Waters: Contamination from a Trophic Ecology and Human Health Perspective. Mar. Environ. Res. 2014, 99, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Reinero, F.R.; Cerra, M.C.; Faggio, C.; Leonetti, F.L.; Micarelli, P.; Giglio, G.; Sperone, E.; Barca, D.; Imbrogno, S. Contamination by Trace Elements and Oxidative Stress in the Skeletal Muscle of Scyliorhinus canicula from the Central Tyrrhenian Sea. Antioxidants 2023, 12, 524. [Google Scholar] [CrossRef]
- Storelli, M.M.; Cuttone, G.; Marcotrigiano, G.O. Distribution of Trace Elements in the Tissues of Smooth Hound Mustelus mustelus (Linnaeus, 1758) from the Southern–Eastern Waters of Mediterranean Sea (Italy). Environ. Monit. Assess. 2011, 174, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Reinero, F.R.; Milazzo, C.; Minervino, M.; Marchio, C.; Filice, M.; Bevacqua, L.; Giglio, G.; Leonetti, F.L.; Micarelli, P.; Tripepi, S.; et al. Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 3 December 2024).
- Sperone, E.; Parise, G.; Leone, A.; Milazzo, C.; Circosta, V.; Santoro, G.; Paolillo, G.; Micarelli, P.; Tripepi, S. Spatiotemporal Patterns of Distribution of Large Predatory Sharks in Calabria (Central Mediterranean, Southern Italy). Acta Adriat. 2012, 53, 13–24. [Google Scholar]
- Ebert, D.A.; Sarah, F. Sharks of the World: A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021; Volume 19. [Google Scholar]
- Gallo, S.; Nania, G.; Caruso, V.; Zicarelli, G.; Leonetti, F.L.; Giglio, G.; Fedele, G.; Romano, C.; Bottaro, M.; Mangoni, O.; et al. Bioaccumulation of Trace Elements in the Muscle of the Blackmouth Catshark Galeus melastomus from Mediterranean Waters. Biology 2023, 12, 951. [Google Scholar] [CrossRef]
- De Donato, C.; Barca, D.; Milazzo, C.; Santoro, R.; Giglio, G.; Tripepi, S.; Sperone, E. Is Trace Element Concentration Correlated to Parasite Abundance? A Case Study in a Population of the Green Frog Pelophylax synkl. hispanicus from the Neto River (Calabria, Southern Italy). Parasitol. Res. 2017, 116, 1745–1753. [Google Scholar] [CrossRef]
- Cortes, E. Standardized Diet Compositions and Trophic Levels of Sharks. ICES J. Mar. Sci. 1999, 56, 707–717. [Google Scholar] [CrossRef]
- Jacobsen, I.P.; Bennett, M.B. A Comparative Analysis of Feeding and Trophic Level Ecology in Stingrays (Rajiformes; Myliobatoidei) and Electric Rays (Rajiformes: Torpedinoidei). PLoS ONE 2013, 8, e71348. [Google Scholar] [CrossRef] [PubMed]
- Gaion, A.; Scuderi, A.; Sartori, D.; Pellegrini, D.; Ligas, A. Trace Metals in Tissues of Galeus melastomus Rafinesque, 1810 from the Northern Tyrrhenian Sea (NW Mediterranean). Acta Adriat. 2016, 57, 165–172. [Google Scholar]
- Roubie, E.; Karavoltsos, S.; Sakellari, A.; Katsikatsos, N.; Dassenakis, M.; Megalofonou, P. Trace Metals Distribution in Tissues of 10 Different Shark Species from the Eastern Mediterranean Sea. Fishes 2024, 9, 77. [Google Scholar] [CrossRef]
- Hornung, H.; Krom, M.D.; Cohen, Y.; Bernhard, M. Trace Metal Content in Deep-Water Sharks from the Eastern Mediterranean Sea. Mar. Biol. 1993, 115, 331–338. [Google Scholar] [CrossRef]
- LeBlanc, P.J.; Jackson, A.L. Arsenic in Marine Fish and Invertebrates. Mar. Pollut. Bull. 1973, 4, 88–90. [Google Scholar] [CrossRef]
- Giovos, I.; Brundo, M.V.; Doumpas, N.; Kazlari, Z.; Loukovitis, D.; Moutopoulos, D.K.; Spyridopoulou, R.N.A.; Papadopoulou, A.; Papapetrou, M.; Tiralongo, F. Trace Elements in Edible Tissues of Elasmobranchs from the North Aegean Sea (Eastern Mediterranean) and Potential Risks from Consumption. Mar. Pollut. Bull. 2022, 184, 114129. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Tristão Da Cunha, R.; Rodrigues, A.D.S. Mid-Atlantic Elasmobranchs: Suitable Metal Scouts? Mar. Pollut. Bull. 2017, 117, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Davis, R.A.; Rocha, R.C.C.; Saint’Pierre, T.D.; Adams, D.H. Metal Concentrations and Metallothionein Metal Detoxification in Blue Sharks, Prionace glauca L. from the Western North Atlantic Ocean. J. Trace Elem. Med. Biol. 2021, 68, 126813. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Berlanga, S.; Calatayud-Pavía, C.E.; Cruz-Ramírez, A.; Soto-Jiménez, M.F.; Liñán-Cabello, M.A. Trace Elements in Muscle Tissue of Three Commercial Shark Species: Prionace glauca, Carcharhinus falciformis, and Alopias pelagicus off the Manzanillo, Colima Coast, Mexico. Environ. Sci. Pollut. Res. 2021, 28, 22679–22692. [Google Scholar] [CrossRef]
- Mille, T.; Cresson, P.; Chouvelon, T.; Bustamante, P.; Brach-Papa, C.; Bruzac, S.; Rozuel, E.; Bouchoucha, M. Trace Metal Concentrations in the Muscle of Seven Marine Species: Comparison between the Gulf of Lions (North-West Mediterranean Sea) and the Bay of Biscay (North-East Atlantic Ocean). Mar. Pollut. Bull. 2018, 135, 9–16. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Aubail, A.; Hussey, N.E.; Heithaus, M.R.; Caurant, F.; Bustamante, P. Plasticity of Trophic Interactions among Sharks from the Oceanic South-Western Indian Ocean Revealed by Stable Isotope and Mercury Analyses. Deep Sea Res. Part Oceanogr. Res. Pap. 2015, 96, 49–58. [Google Scholar] [CrossRef]
- Nussey, G.; van Vuren, J.; du Preez, H.H. Bioaccumulation of Chromium, Manganese, Nickel and Lead in the Tissues of the Moggel, Labeo umbratus (Cyprinidae), from Witbank Dam, Mpumalanga. Water SA 2000, 26, 269–284. [Google Scholar]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Interactions between Cadmium and Zinc in the Organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]




| Code | Species | TRL | Ecology | Year | Location | Basin | Source |
|---|---|---|---|---|---|---|---|
| #4 | C. granulosus | 4.1 | D | 2017 | Paola (CS) | Tyrrhenian Sea | Stranded |
| #2 | D. licha | 4.1 | D | 2008 | Vibo marina (VV) | Tyrrhenian Sea | Fishing activity |
| #13 | D. licha | 4.1 | D | 2008 | Vibo marina (VV) | Tyrrhenian Sea | Fishing activity |
| #16 | D. licha | 4.1 | D | 2015 | Schiavonea (CS) | Ionian Sea | Fishing activity |
| #23 | D. licha | 4.1 | D | 2014 | Montepaone lido (CZ) | Ionian Sea | Fishing activity |
| #30 | D. licha | 4.1 | D | 2012 | Crotone (KR) | Ionian Sea | Stranded |
| GG1 | G. galeus | 4.2 | D | 2013 | Pellaro (RC) | Ionian Sea | Fishing activity |
| GMF1 | G. melastomus | 3.7 | D | 2010 | Fiumefreddo Bruzio (CS) | Tyrrhenian Sea | Fishing activity |
| GMF2 | G. melastomus | 3.7 | D | 2010 | Fiumefreddo Bruzio (CS) | Tyrrhenian Sea | Fishing activity |
| GMF3 | G. melastomus | 3.7 | D | 2010 | Fiumefreddo Bruzio (CS) | Tyrrhenian Sea | Fishing activity |
| GMF4 | G. melastomus | 3.7 | D | 2010 | Fiumefreddo Bruzio (CS) | Tyrrhenian Sea | Fishing activity |
| GMF5 | G. melastomus | 3.7 | D | 2010 | Fiumefreddo Bruzio (CS) | Tyrrhenian Sea | Fishing activity |
| GM001 | G. melastomus * | 3.7 | D | 2020 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM023 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM024 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM026 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM032 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM033 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM002 | G. melastomus * | 3.7 | D | 2020 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM003 | G. melastomus * | 3.7 | D | 2020 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM006 | G. melastomus * | 3.7 | D | 2020 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM031 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM052 | G. melastomus * | 3.7 | D | 2021 | Golfo di Sant’Eufemia (CZ) | Tyrrhenian Sea | Fishing activity |
| GM121 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM122 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM123 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM124 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM125 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM166 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM126 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM128 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| GM167 | G. melastomus * | 3.7 | D | 2021 | Golfo di Taranto (CS) | Ionian Sea | Fishing activity |
| #5 | H. griseus | 4.3 | D | 2015 | Crotone (KR) | Ionian Sea | Fishing activity |
| #25 | H. griseus | 4.3 | D | 2013 | Torretta di Crucoli (KR) | Ionian Sea | Fishing activity |
| #26 | H. griseus | 4.3 | D | 2015 | Crotone (KR) | Ionian Sea | Fishing activity |
| #28 | H. griseus | 4.3 | D | 2015 | Corigliano Calabro (CS) | Ionian Sea | Stranded |
| HGF1 | H. griseus | 4.3 | D | 2012 | Torretta di Crucoli (KR) | Ionian Sea | Fishing activity |
| HGF2 | H. griseus | 4.3 | D | 2016 | Cetraro (CS) | Tyrrhenian Sea | Stranded |
| #8 | H. perlo | 4.2 | D | 2012 | Bovalino (RC) | Ionian Sea | Stranded |
| #27 | I. oxyrinchus | 4.3 | P | 2012 | Soverato (CZ) | Ionian Sea | Fishing activity |
| #6 | P. glauca | 4.1 | P | 2018 | Schiavonea (CS) | Ionian Sea | Stranded |
| #20 | P. glauca | 4.1 | P | 2014 | Bianco (RC) | Ionian Sea | Stranded |
| #21 | P. glauca | 4.1 | P | 2014 | Villa San Giovanni (RC) | Tyrrhenian Sea | Stranded |
| #22 | P. glauca | 4.1 | P | 2014 | Cirò marina (KR) | Ionian Sea | Fishing activity |
| #24 | P. glauca | 4.1 | P | 2013 | Punta Alice (KR) | Ionian Sea | Stranded |
| #29 | P. glauca | 4.1 | P | 2015 | Sibari (CS) | Ionian Sea | Stranded |
| #7 | P. violacea | 3.6 | D | 2013 | Cirò marina (KR) | Ionian Sea | Fishing activity |
| #9 | S. acanthias | 3.9 | D | 2012 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| #10 | S. acanthias | 3.9 | D | 2012 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| #11 | S. acanthias | 3.9 | D | 2012 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| #12 | S. acanthias | 3.9 | D | 2012 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| SCF1 | S. canicula | 3.6 | D | 2010 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| #15 | T. torpedo | 4.2 | D | 2011 | Cetraro (CS) | Tyrrhenian Sea | Fishing activity |
| G. melastomus | D. licha | P. glauca | S. acanthias | H. griseus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dev. | n | Mean | St. Dev. | n | Mean | St. Dev. | n | Mean | St. Dev. | n | Mean | St. Dev. | n | Max Value | |
| Al27 | 7.652 | 4.672 | 25 | 13.540 | 4.700 | 5 | 35.997 | 56.761 | 6 | 22.150 | 10.114 | 4 | 45.693 | 53.702 | 6 | 45.693 |
| As75 | 118.714 | 35.833 | 25 | 91.166 | 62.984 | 5 | 76.130 | 55.327 | 6 | 85.630 | 21.468 | 4 | 80.710 | 53.134 | 6 | 118.714 |
| Ba138 | 0.183 | 0.429 | 25 | 0.392 | 0.100 | 5 | 0.523 | 0.725 | 6 | 0.360 | 0.175 | 4 | 0.628 | 0.204 | 6 | 0.628 |
| Bi209 | 0.007 | 0.007 | 25 | 0.026 | 0.012 | 5 | 0.022 | 0.004 | 6 | 0.023 | 0.004 | 4 | 0.023 | 0.004 | 6 | 0.026 |
| Co59 | 0.044 | 0.019 | 25 | 0.024 | 0.010 | 5 | 0.047 | 0.022 | 6 | 0.038 | 0.008 | 4 | 0.033 | 0.008 | 6 | 0.047 |
| Cr52 | 0.925 | 0.663 | 25 | 1.152 | 0.450 | 5 | 0.820 | 0.261 | 6 | 1.558 | 0.863 | 4 | 1.468 | 0.498 | 6 | 1.558 |
| Cu63 | 1.959 | 1.829 | 25 | 0.850 | 0.130 | 5 | 1.400 | 0.690 | 6 | 1.098 | 0.323 | 4 | 1.015 | 0.148 | 6 | 1.959 |
| Fe57 | 41.901 | 39.630 | 25 | 66.738 | 17.379 | 5 | 53.237 | 24.760 | 6 | 43.858 | 13.662 | 4 | 59.068 | 30.931 | 6 | 66.738 |
| Mn55 | 0.986 | 0.438 | 25 | 0.836 | 0.232 | 5 | 0.747 | 0.411 | 6 | 0.780 | 0.168 | 4 | 0.945 | 0.411 | 6 | 0.986 |
| Mo98 | 0.048 | 0.023 | 25 | 0.246 | 0.312 | 5 | 0.143 | 0.128 | 6 | 0.223 | 0.084 | 4 | 0.195 | 0.211 | 6 | 0.246 |
| Ni60 | 1.991 | 1.177 | 25 | 6.242 | 4.056 | 5 | 3.817 | 2.636 | 6 | 7.188 | 4.541 | 4 | 13.180 | 3.856 | 6 | 13.180 |
| Pb208 | 0.141 | 0.109 | 25 | 0.372 | 0.169 | 5 | 0.165 | 0.110 | 6 | 0.213 | 0.066 | 4 | 0.215 | 0.069 | 6 | 0.372 |
| Se82 | 0.939 | 0.177 | 25 | 2.238 | 0.585 | 5 | 2.010 | 0.533 | 6 | 1.345 | 0.092 | 4 | 1.383 | 0.134 | 6 | 2.238 |
| Sr88 | 2.513 | 0.953 | 25 | 4.044 | 1.183 | 5 | 4.092 | 1.602 | 6 | 6.853 | 0.329 | 4 | 4.823 | 0.954 | 6 | 6.853 |
| U238 | 0.007 | 0.005 | 25 | bdl | bdl | 5 | bdl | bdl | 6 | bdl | bdl | 4 | 0.008 | 0.013 | 6 | 0.008 |
| Zn64 | 21.529 | 5.847 | 25 | 29.322 | 9.602 | 5 | 37.890 | 19.714 | 6 | 26.710 | 7.853 | 4 | 48.370 | 16.910 | 6 | 48.370 |
| G. melastomus | D. licha | P. glauca | S. acanthias | H. griseus | |
|---|---|---|---|---|---|
| Al27 | 2.219 | 3.927 | 10.439 | 6.424 | 13.251 |
| As75 | 34.427 | 26.438 | 22.078 | 24.833 | 23.406 |
| Ba138 | 0.053 | 0.114 | 0.152 | 0.104 | 0.182 |
| Bi209 | 0.002 | 0.008 | 0.006 | 0.007 | 0.007 |
| Co59 | 0.013 | 0.007 | 0.014 | 0.011 | 0.009 |
| Cr52 | 0.268 | 0.334 | 0.238 | 0.452 | 0.426 |
| Cu63 | 0.568 | 0.247 | 0.406 | 0.318 | 0.294 |
| Fe57 | 12.151 | 19.354 | 15.439 | 12.719 | 17.130 |
| Mn55 | 0.286 | 0.242 | 0.217 | 0.226 | 0.274 |
| Mo98 | 0.014 | 0.071 | 0.042 | 0.065 | 0.057 |
| Ni60 | 0.577 | 1.810 | 1.107 | 2.084 | 3.822 |
| Pb208 | 0.041 | 0.108 | 0.048 | 0.062 | 0.062 |
| Se82 | 0.272 | 0.649 | 0.583 | 0.390 | 0.401 |
| Sr88 | 0.729 | 1.173 | 1.187 | 1.987 | 1.399 |
| U238 | 0.002 | 0.000 | 0.000 | 0.000 | 0.002 |
| Zn64 | 6.243 | 8.503 | 10.988 | 7.746 | 14.027 |
| Muscle | Skin | Brain | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dev. | n | Mean | St. Dev. | n | Mean | St. Dev. | n | Max Value | |
| As75 | 79.350 | 15.082 | 5 | 41.420 | 20.212 | 3 | 75.325 | 25.255 | 2 | 79.350 |
| Cd112 | 0.054 | 0.034 | 5 | 0.120 | 0.099 | 3 | 0.150 | 0.060 | 2 | 0.150 |
| Co59 | 0.076 | 0.026 | 5 | 0.227 | 0.021 | 3 | 0.220 | 0.040 | 2 | 0.227 |
| Cr52 | 1.176 | 0.358 | 5 | 1.297 | 0.333 | 3 | 1.380 | 0.510 | 2 | 1.380 |
| Cu63 | 1.466 | 0.583 | 5 | 2.593 | 0.827 | 3 | 10.645 | 0.245 | 2 | 10.645 |
| Fe57 | 28.794 | 17.084 | 5 | 359.057 | 71.277 | 3 | 67.950 | 14.520 | 2 | 359.057 |
| Mn55 | 1.758 | 0.963 | 5 | 13.443 | 2.989 | 3 | 2.700 | 0.850 | 2 | 13.443 |
| Mo98 | 0.046 | 0.021 | 5 | 0.063 | 0.005 | 3 | 0.130 | 0.080 | 2 | 0.130 |
| Ni60 | 0.576 | 0.264 | 5 | 1.603 | 0.454 | 3 | 0.605 | 0.455 | 2 | 1.603 |
| Pb208 | 0.075 | 0.060 | 5 | 3.317 | 3.426 | 3 | 0.215 | 0.135 | 2 | 3.317 |
| Se82 | 2.112 | 1.245 | 5 | 2.437 | 1.177 | 3 | 2.185 | 0.715 | 2 | 2.437 |
| Sn | 4.990 | 1.352 | 5 | 57.493 | 60.993 | 3 | 12.340 | 6.860 | 2 | 57.493 |
| Sr88 | 6.912 | 9.346 | 5 | 330.537 | 71.845 | 3 | 7.500 | 2.680 | 2 | 330.537 |
| U238 | 0.003 | 0.002 | 5 | 0.020 | 0.006 | 3 | 0.009 | 0.007 | 2 | 0.020 |
| Zn64 | 17.074 | 7.870 | 5 | 49.940 | 8.841 | 3 | 33.660 | 0.160 | 2 | 49.940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, S.; Leonetti, F.L.; Reinero, F.R.; Micarelli, P.; Passarelli, L.; Giglio, G.; Milazzo, C.; Imbrogno, S.; Barca, D.; Bottaro, M.; et al. Bioaccumulation Patterns in Different Tissues of Twelve Species of Elasmobranchs from the Tyrrhenian and Ionian Sea (Calabria, Southern Italy). Environments 2025, 12, 12. https://doi.org/10.3390/environments12010012
Gallo S, Leonetti FL, Reinero FR, Micarelli P, Passarelli L, Giglio G, Milazzo C, Imbrogno S, Barca D, Bottaro M, et al. Bioaccumulation Patterns in Different Tissues of Twelve Species of Elasmobranchs from the Tyrrhenian and Ionian Sea (Calabria, Southern Italy). Environments. 2025; 12(1):12. https://doi.org/10.3390/environments12010012
Chicago/Turabian StyleGallo, Samira, Francesco Luigi Leonetti, Francesca Romana Reinero, Primo Micarelli, Luigi Passarelli, Gianni Giglio, Concetta Milazzo, Sandra Imbrogno, Donatella Barca, Massimiliano Bottaro, and et al. 2025. "Bioaccumulation Patterns in Different Tissues of Twelve Species of Elasmobranchs from the Tyrrhenian and Ionian Sea (Calabria, Southern Italy)" Environments 12, no. 1: 12. https://doi.org/10.3390/environments12010012
APA StyleGallo, S., Leonetti, F. L., Reinero, F. R., Micarelli, P., Passarelli, L., Giglio, G., Milazzo, C., Imbrogno, S., Barca, D., Bottaro, M., & Sperone, E. (2025). Bioaccumulation Patterns in Different Tissues of Twelve Species of Elasmobranchs from the Tyrrhenian and Ionian Sea (Calabria, Southern Italy). Environments, 12(1), 12. https://doi.org/10.3390/environments12010012










