Simulating Gibberellic Acid Effect on Pasture Yield on Naturally Deposited and Fixed Area Urine
Abstract
:1. Introduction
2. Materials and Methods
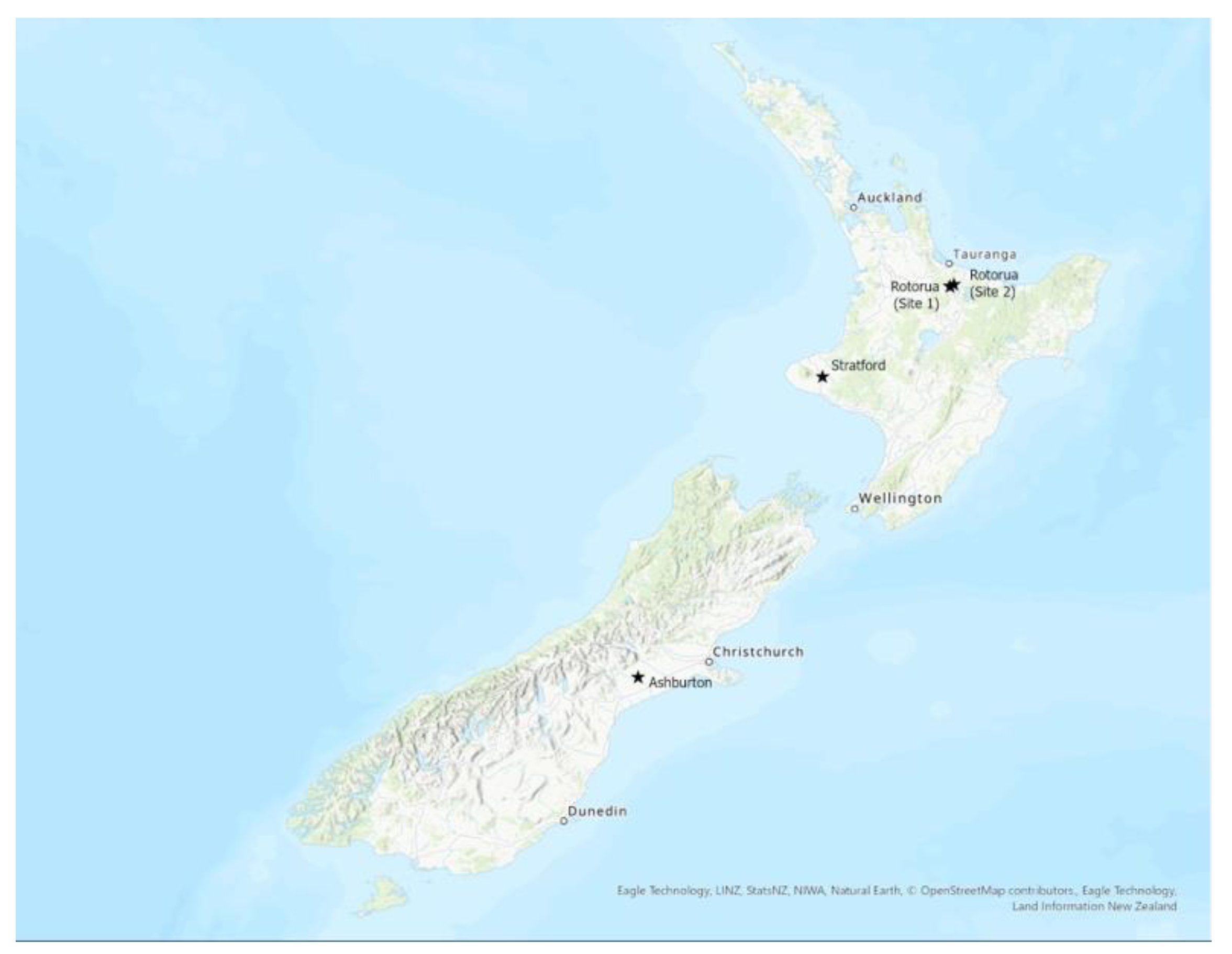
2.1. Sites and Soils
2.2. Experimental Design and Treatments
2.3. Herbage Sampling
2.4. Soil Sampling and Analysis
2.5. Model Description
2.6. Simulation
2.6.1. APSIM-MET
2.6.2. APSIM-Soil
2.6.3. Model Evaluation
2.7. Statistical Analysis
3. Results
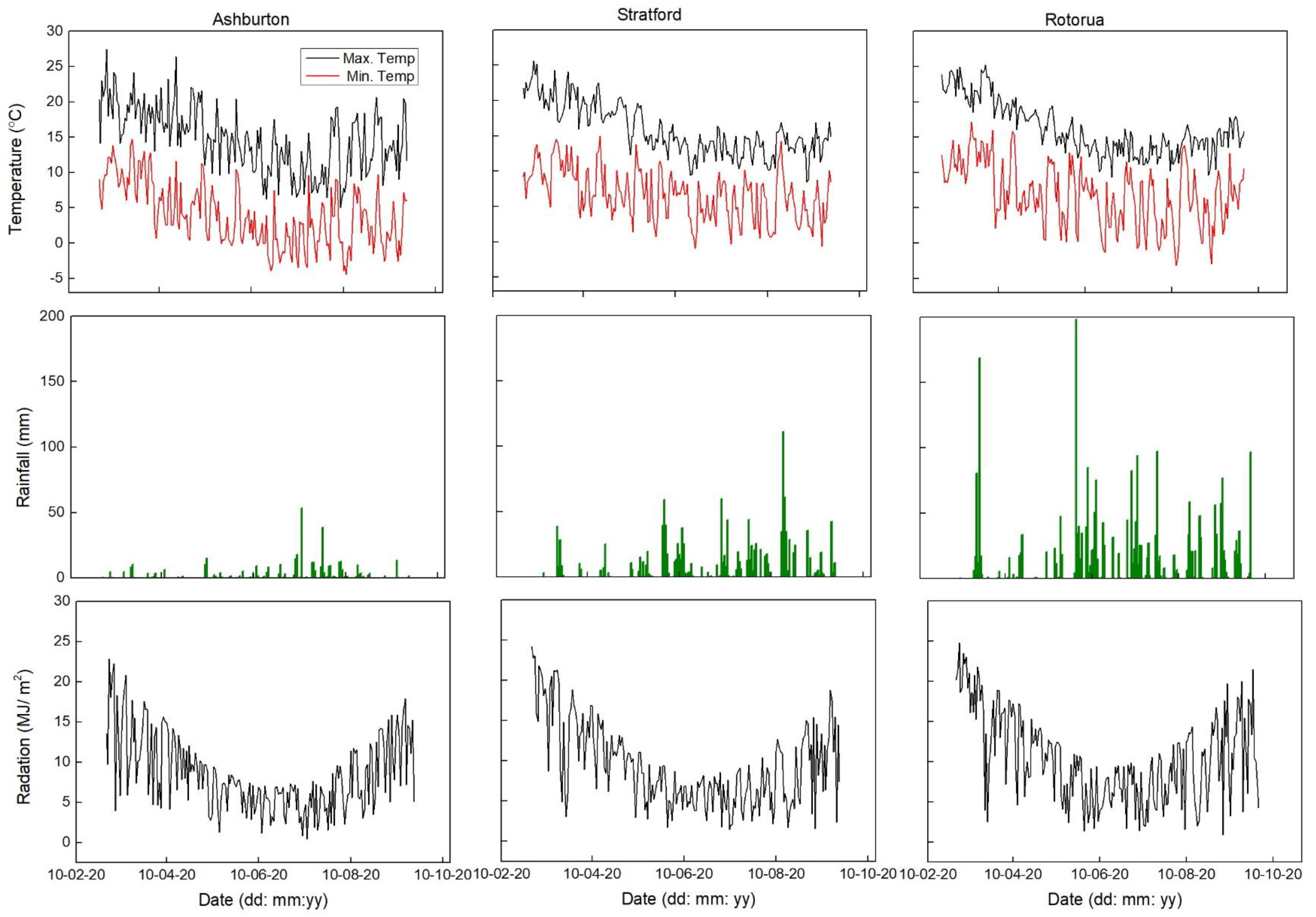
3.1. Soil Moisture
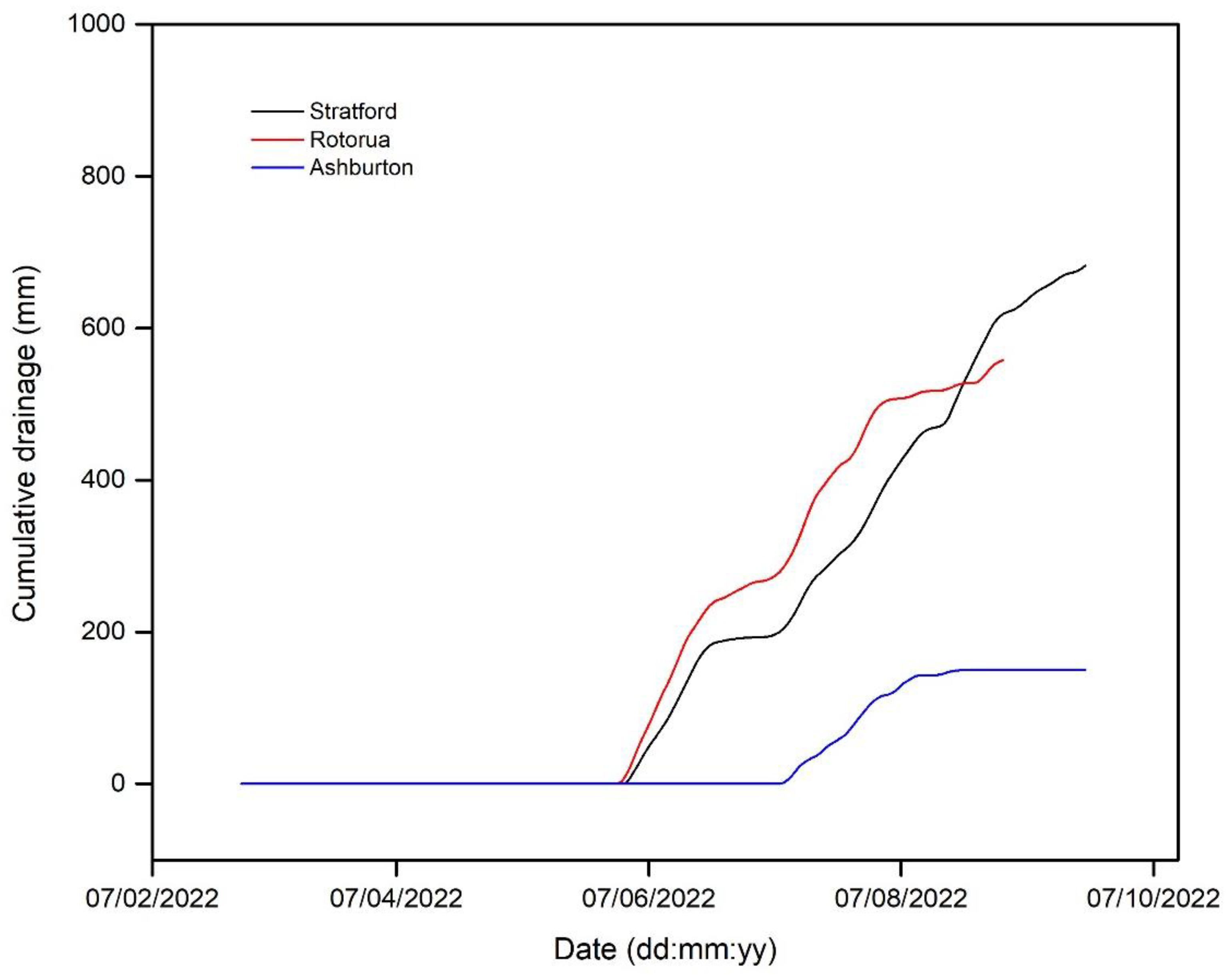
3.2. Drainage
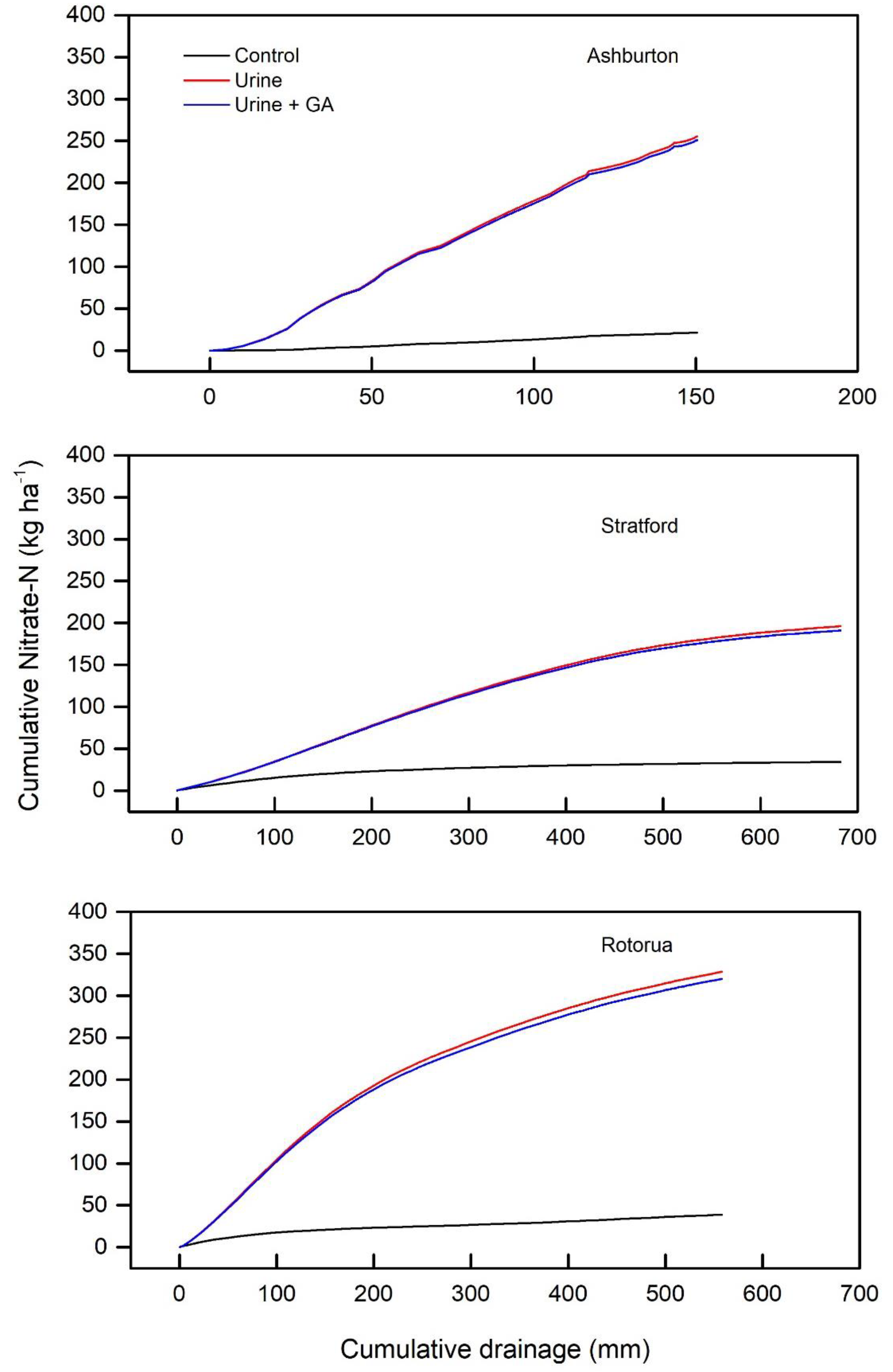
3.3. Drainage vs. Nitrate-N Leaching
3.4. Pasture DM Yield
4. Discussion
4.1. Nitrate-N Leaching
4.2. Herbage DM Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vibart, R.E.; Vogeler, I.; Dodd, M.; Koolaard, J. Simple versus Diverse Temperate Pastures: Aspects of Soil–Plant–Animal Interrelationships Central to Nitrogen Leaching Losses. Agron. J. 2016, 108, 2174–2188. [Google Scholar] [CrossRef]
- Ministry for the Environment. Nitrogen Cap Guidance for Dairy Farms; Ministry for the Environment: Wellington, New Zealand, 2021; pp. 6–10.
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M.A. The challenge of the urine patch for managing nitrogen in grazed pasture systems. In Adv. Agron. 2015, 129, 229–292. [Google Scholar]
- Bishop, P.; Jeyakumar, P. A comparison of three nitrate leaching mitigation treatments with dicyandiamide using lysimeters. N. Z. J. Agric. Res. 2022, 65, 547–560. [Google Scholar] [CrossRef]
- Woods, R.R.; Cameron, K.C.; Edwards, G.R.; Di, H.J.; Clough, T.J. Reducing nitrogen leaching losses in grazed dairy systems using an Italian ryegrass-plantain-white clover forage mix. Grass Forage Sci. 2018, 73, 878–887. [Google Scholar] [CrossRef]
- Parsons, A.J.; Rasmussen, S.; Liu, Q.; Xue, H.; Ball, C.; Shaw, C. Plant growth—Resource or strategy limited: Insights from responses to gibberellin. Grass Forage Sci. 2013, 68, 577–588. [Google Scholar] [CrossRef]
- Woods, R.R.; Cameron, K.C.; Edwards, G.R.; Di, H.J.; Clough, T.J. Effects of forage type and gibberellic acid on nitrate leaching losses. Soil Use Manag. 2016, 32, 565–572. [Google Scholar] [CrossRef]
- Bahri, H.; Annabi, M.; Cheikh M’Hamed, H.; Frija, A. Assessing the long-term impact of conservation agriculture on wheat-based systems in Tunisia using APSIM simulations under a climate change context. Sci. Total Environ. 2019, 692, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Bosi, C.; Sentelhas, P.C.; Huth, N.I.; Pezzopane, J.R.M.; Andreucci, M.P.; Santos, P.M. APSIM-Tropical Pasture: A model for simulating perennial tropical grass growth and its parameterisation for palisade grass (Brachiaria brizantha). Agric. Syst. 2020, 184, 102917. [Google Scholar] [CrossRef]
- Briak, H.; Kebede, F. Wheat (Triticum aestivum) adaptability evaluation in a semi-arid region of Central Morocco using APSIM model. Sci. Rep. 2021, 11, 23173. [Google Scholar] [CrossRef] [PubMed]
- Matse, D.T.; Jeyakumar, P.; Bishop, P.; Anderson, C.W.N. Nitrate Leaching Mitigation Options in Two Dairy Pastoral Soils and Climatic Conditions in New Zealand. Plants 2022, 11, 2430. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, L.C. Methods for chemicalanalysis of soils. N. Z. Soil Bur. Sci. Rep. 1987, 80, 72–76. [Google Scholar]
- Chimonyo, V.G.P.; Modi, A.T.; Mabhaudhi, T. Simulating yield and water use of a sorghum–cowpea intercrop using APSIM. Agric. Water Manag. 2016, 177, 317–328. [Google Scholar] [CrossRef]
- Ebrahimi-Mollabashi, E.; Huth, N.I.; Holzwoth, D.P.; Ordóñez, R.A.; Hatfield, J.L.; Huber, I.; Castellano, M.J.; Archontoulis, S.V. Enhancing APSIM to simulate excessive moisture effects on root growth. Field Crops Res. 2019, 236, 58–67. [Google Scholar] [CrossRef]
- Yang, X.; Brown, H.E.; Teixeira, E.I.; Moot, D.J. Development of a lucerne model in APSIM next generation: 1 phenology and morphology of genotypes with different fall dormancies. Eur. J. Agron. 2021, 130, 126372. [Google Scholar] [CrossRef]
- Matse, D.T.; Jeyakumar, P.; Bishop, P.; Anderson, C.W.N. Nitrification rate in dairy cattle urine patches can be inhibited by changing soil bioavailable Cu concentration. Environ. Pollut. 2023, 320, 121107. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.A.; Akoumianakis, K.A.; Olympios, C.M.; Passam, H.C. The effect of the time and mode of application of gibberellic acid and inhibitors of gibberellin biosynthesis on the dormancy of potato tubers grown from true potato seed. J. Sci. Food Agric. 2007, 87, 1973–1979. [Google Scholar] [CrossRef]
- O’Leary, G.J.; Liu, D.L.; Ma, Y.; Li, F.Y.; McCaskill, M.; Conyers, M.; Dalal, R.; Reeves, S.; Page, K.; Dang, Y.P.; et al. Modelling soil organic carbon 1. Performance of APSIM crop and pasture modules against long-term experimental data. Geoderma 2016, 264, 227–237. [Google Scholar] [CrossRef]
- Watt, L.J.; Bell, L.W.; Pembleton, K.G. A forage brassica simulation model using APSIM: Model calibration and validation across multiple environments. Eur. J. Agron. 2022, 137, 126517. [Google Scholar] [CrossRef]


| No. | Treatments | Urine Application Rate (kg N ha−1) | Replicates |
|---|---|---|---|
| 1. | Control | Nil urine (only water) | 5 |
| 2. | Urine | 600 kg N ha−1 | 5 |
| 3. | Urine + ProGibb® SG (GA) at 8 g h−1 | 600 kg N ha−1 | 5 |
| Experimental Site | Application and Harvesting Dates | ||
|---|---|---|---|
| Application | Harvesting | ||
| Ashburton | 1st | 9 March 2022 | 11 April 2022 |
| 2nd | 11 April 2022 | 13 June 2022 | |
| 3rd | 10 May 2022 | 13 September 2022 | |
| Stratford | 1st | 1 April 2022 | 1 May 2022 |
| 2nd | 1 May 2022 | 20 June 2022 | |
| 3rd | 3 June 2022 | 4 September 2022 | |
| 1st | 5 April 2022 | 1 May 2022 | |
| Rotorua | 2nd | 4 May 2022 | 28 June 2022 |
| 3rd | 5 June 2022 | 28 September 2022 | |
| Soil Layer (cm) | BD (g cm−3) | SAT (mm mm−1) | DUL (mm mm−1) | LL15 (mm mm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ashb | Strat | Roto | Ash | Strat | Roto | Ashb | Strat | Roto | Ashb | Strat | Roto | |
| 0–10 | 1.01 | 0.67 | 0.85 | 0.62 | 0.61 | 0.56 | 0.32 | 0.48 | 0.28 | 0.15 | 0.31 | 0.08 |
| 10–20 | 1.35 | 0.81 | 0.85 | 0.45 | 0.61 | 0.56 | 0.29 | 0.48 | 0.28 | 0.18 | 0.26 | 0.08 |
| 20–40 | 1.11 | 0.75 | 0.85 | 0.34 | 0.57 | 0.50 | 0.29 | 0.45 | 0.18 | 0.14 | 0.30 | 0.05 |
| 40–55 | 0.77 | 1.01 | 0.85 | 0.20 | 0.56 | 0.50 | 0.16 | 0.44 | 0.18 | 0.10 | 0.28 | 0.05 |
| 55–75 | 0.60 | 1.04 | 0.85 | 0.18 | 0.55 | 0.50 | 0.10 | 0.43 | 0.18 | 0.06 | 0.28 | 0.05 |
| 75–100 | 0.48 | 1.05 | 0.85 | 0.19 | 0.55 | 0.50 | 0.06 | 0.43 | 0.18 | 0.02 | 0.28 | 0.05 |
| Soil Layer (cm) | OC (%) | Soil C/N Ratio (g g−1) | Initial Soil N (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ashb | Strat | Roto | Ash | Strat | Roto | Ashb | Strat | Roto | |
| 0–10 | 4.8 | 12.3 | 7.30 | 10 | 15 | 10 | 4.5 | 5.0 | 21.0 |
| 10–20 | 4.8 | 12.3 | 7.30 | 10 | 15 | 10 | 3.0 | 5.0 | 11.0 |
| 20–40 | 4.8 | 4.0 | 5.00 | 10 | 15 | 10 | 3.0 | 5.0 | 6.0 |
| 40–55 | 0.8 | 3.1 | 3.43 | 10 | 15 | 10 | 3.0 | 5.0 | 4.0 |
| 55–75 | 0.6 | 2.8 | 3.07 | 10 | 15 | 10 | 3.0 | 5.0 | 4.0 |
| 75–100 | 0.5 | 2.7 | 2.82 | 10 | 15 | 10 | 3.0 | 5.0 | 4.0 |
| Treatment | Ashburton | Stratford | Rotorua |
|---|---|---|---|
| R2 | R2 | R2 | |
| Control | 0.9064 | 0.8562 | 0.9948 |
| Urine | 0.9384 | 0.9950 | 0.9845 |
| Urine + GA | 0.8920 | 0.9585 | 0.9660 |
| Treatments | Ashburton | Stratford | Rotorua | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 11 April 2022 | 13 June 2022 | 13 September 2022 | 1 May 2022 | 20 June 2022 | 4 September 2022 | 1 May 2022 | 28 June 2022 | 28 September 2022 | |
| Control | 49 | 255 | 53 | 72 | 90 | 49 | 85 | 180 | 57 |
| Urine | 493 | 218 | 29 | 345 | 87 | 67 | 254 | 119 | 75 |
| Urine-Control | 445 | −37 | −24 | 273 | −3 | 18 | 169 | −61 | 18 |
| Treatments | Ashburton | Stratford | Rotorua | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 9 March 2022 | 11 April 2022 | 10 May 2022 | 1 April 2022 | 1 May 2022 | 3 June 2022 | 4 April 2022 | 4 May 2022 | 5 June 2022 | |
| Control | 40 | 49 | 109 | 80 | 62 | 119 | 101 | 87 | 213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matse, D.T.; Avendano, F.; Bishop, P.; Jeyakumar, P.; Bates, G. Simulating Gibberellic Acid Effect on Pasture Yield on Naturally Deposited and Fixed Area Urine. Environments 2023, 10, 112. https://doi.org/10.3390/environments10070112
Matse DT, Avendano F, Bishop P, Jeyakumar P, Bates G. Simulating Gibberellic Acid Effect on Pasture Yield on Naturally Deposited and Fixed Area Urine. Environments. 2023; 10(7):112. https://doi.org/10.3390/environments10070112
Chicago/Turabian StyleMatse, Dumsane Themba, Fernando Avendano, Peter Bishop, Paramsothy Jeyakumar, and Geoff Bates. 2023. "Simulating Gibberellic Acid Effect on Pasture Yield on Naturally Deposited and Fixed Area Urine" Environments 10, no. 7: 112. https://doi.org/10.3390/environments10070112
APA StyleMatse, D. T., Avendano, F., Bishop, P., Jeyakumar, P., & Bates, G. (2023). Simulating Gibberellic Acid Effect on Pasture Yield on Naturally Deposited and Fixed Area Urine. Environments, 10(7), 112. https://doi.org/10.3390/environments10070112







