Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy
Abstract
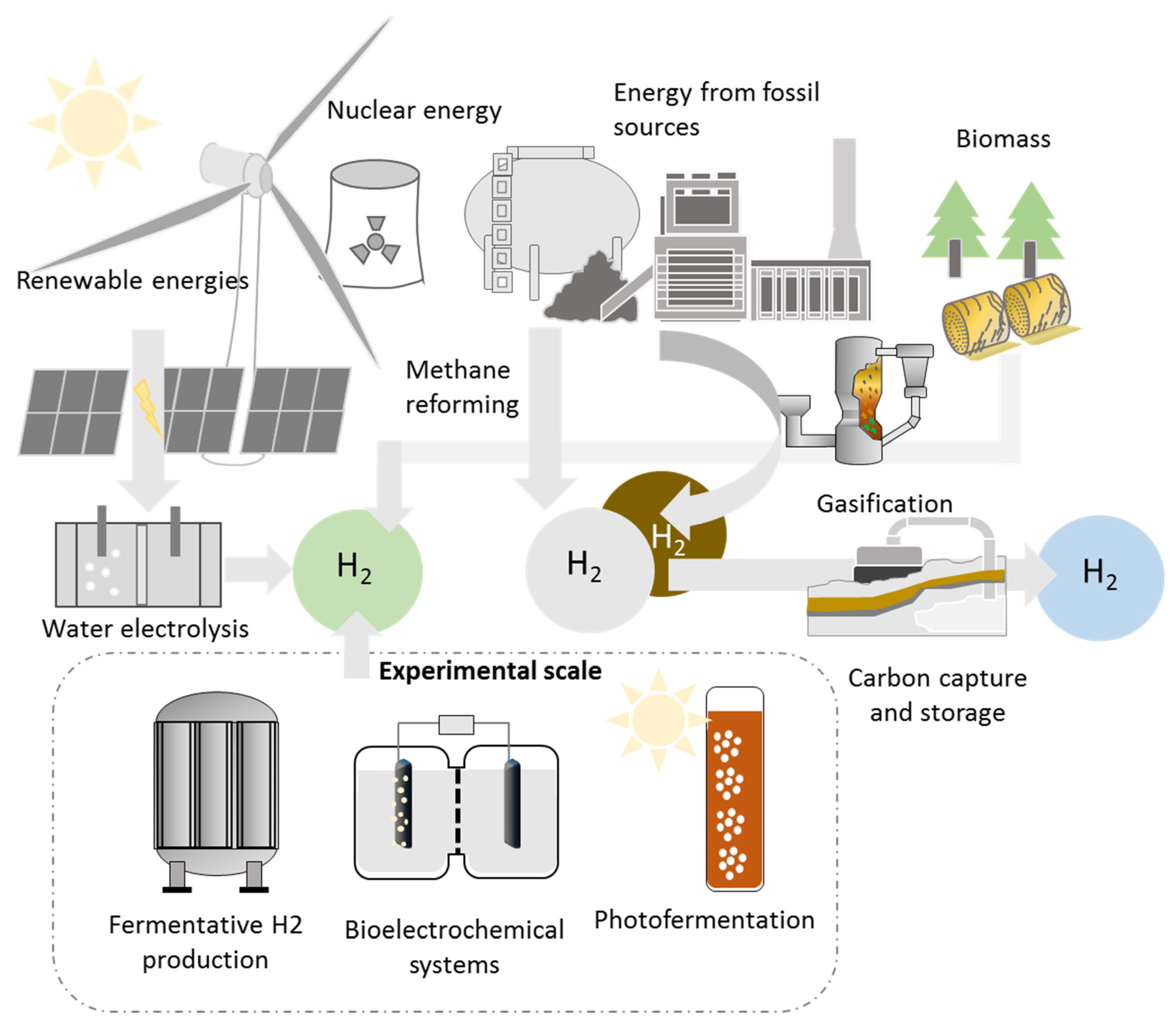
1. Introduction
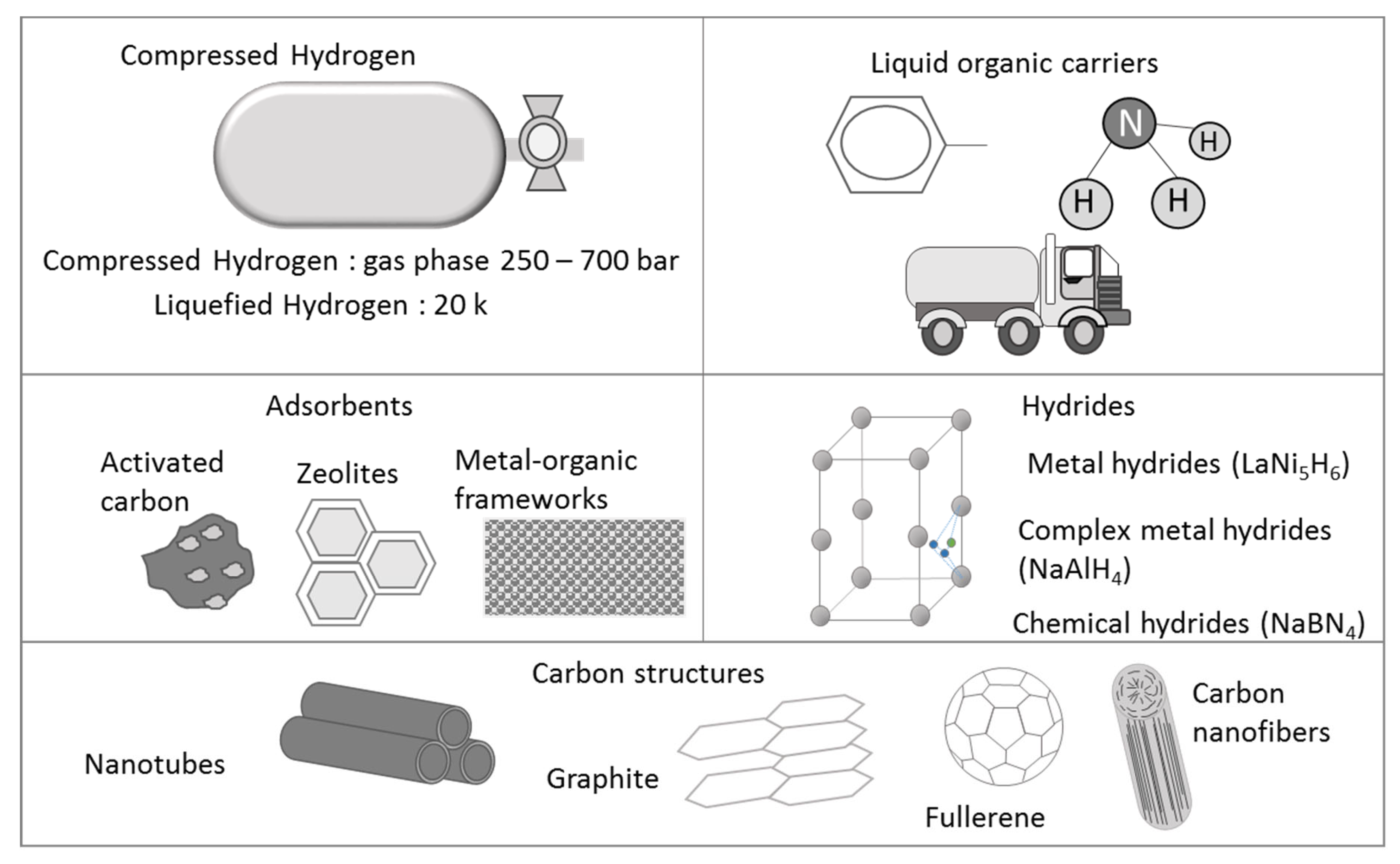
2. Hydrogen Storage
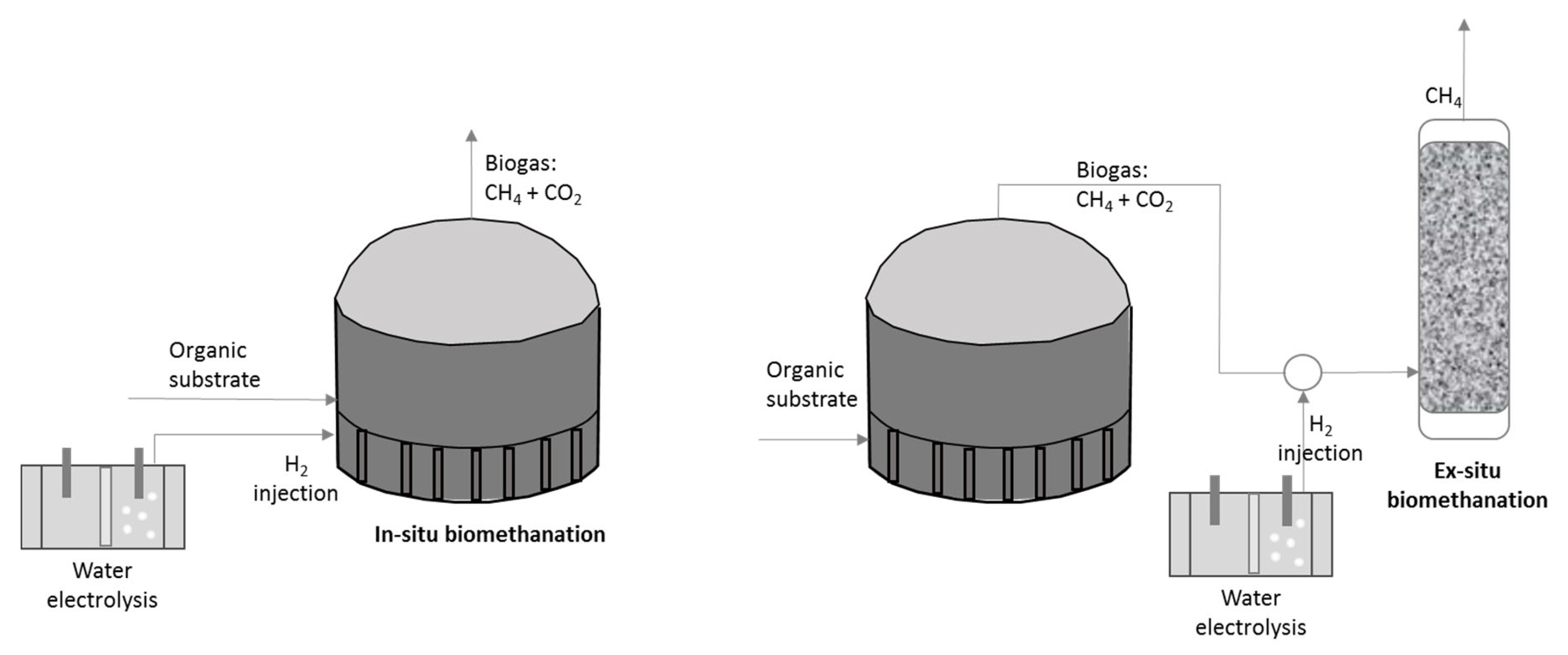
3. Biological Conversion of Hydrogen to Methane in Reactors with Mixed Substrates
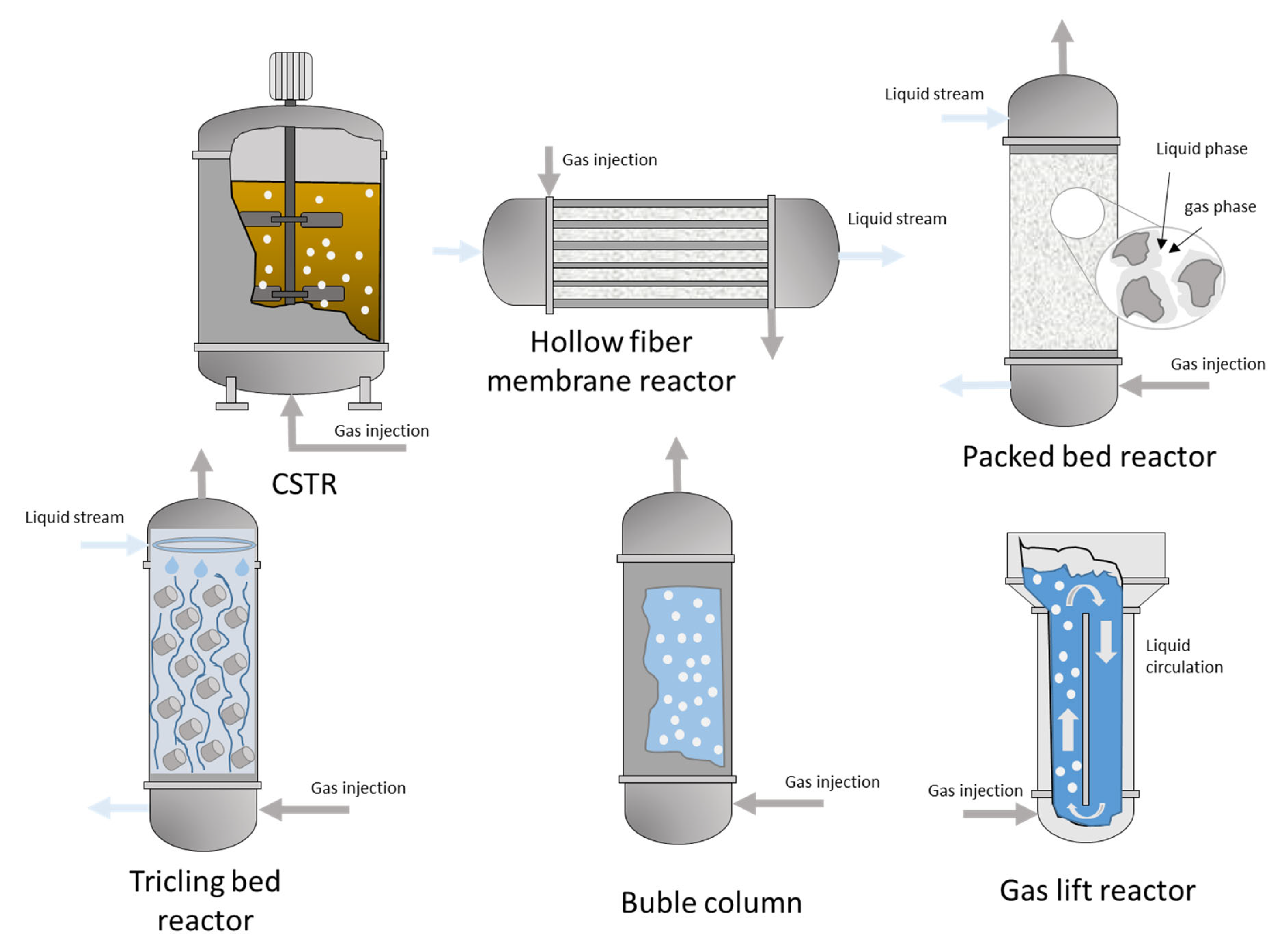
3.1. Process Configuration
3.2. Mass Transfer Limitations
3.3. Effect of Temperature
4. Biological Conversion of Hydrogen to Methane with CO2 as Substrate in Specialized Reactors
Underground Reservoirs as Large-Scale Bioreactors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARES | Advanced Rail Energy Storage |
| CHP | Combined heat and power |
| CSTR | Continuously stirred tank reactor |
| Li-ion | Lithium-ion |
| LHV | Lower heating value |
| LNG | Liquefied natural gas |
| LOHCs | Liquid organic hydrogen carriers |
| MCH | Methylcyclohexane |
| MOFs | Metal–organic frameworks |
| MPR | Methane production rate |
| PAC | Pyrolysis aqueous condensate |
| PtG | Power-to-Gas |
| PtM | Power-to-Methane |
| PUF | Polyurethane foam |
| STP | Standard temperature and pressure conditions |
| USC | Underground Sun Conversion |
| VFA | Volatile fatty acids |
| VSS | Volatiles suspended solids |
| vvm | Volume of gas injected per volume of reactor and minute |
| WWTP | Wastewater treatment plant |
References
- Installed Electricity Capacity Worldwide in 2021, by Source. 2021. Available online: https://www.statista.com/statistics/267358/world-installed-power-capacity/ (accessed on 4 January 2023).
- Electricity Generation Worldwide from 1990 to 2021. 2021. Available online: https://www.statista.com/statistics/270281/electricity-generation-worldwide/ (accessed on 4 January 2023).
- Goldthau, A. Rethinking the governance of energy infrastructure: Scale, decentralization and polycentrism. Energy Res. Soc. Sci. 2014, 1, 134–140. [Google Scholar] [CrossRef]
- Samuel, O.; Almogren, A.; Javaid, A.; Zuair, M.; Ullah, I.; Javaid, N. Leveraging blockchain technology for secure energy trading and least-cost evaluation of decentralized contributions to electrification in Sub-Saharan Africa. Entropy 2020, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Amuzu-Sefordzi, B.; Martinus, K.; Tschakert, P.; Wills, R. Disruptive innovations and decentralized renewable energy systems in Africa: A socio-technical review. Energy Res. Soc. Sci. 2018, 46, 140–154. [Google Scholar] [CrossRef]
- Bakhtiari, H.; Naghizadeh, R.A. Multi-criteria optimal sizing of hybrid renewable energy systems including wind, photovoltaic, battery, and hydrogen storage with ɛ-constraint method. IET Renew. Power Gener. 2018, 12, 883–892. [Google Scholar] [CrossRef]
- Battery Cell Comparison. Available online: https://www.epectec.com/batteries/cell-comparison.html (accessed on 4 January 2023).
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design Strategies for High-Energy-Density Aqueous Zinc Batteries. Angew. Chemie 2022, 61, e202200598. [Google Scholar] [CrossRef]
- Nadeem, F.; Hussain, S.M.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative Review of Energy Storage Systems, Their Roles, and Impacts on Future Power Systems. IEEE Access 2019, 7, 4555–4585. [Google Scholar] [CrossRef]
- Rehman, S.; Al-Hadhrami, L.M.; Alam, M.M. Pumped hydro energy storage system: A technological review. Renew. Sustain. Energy Rev. 2015, 44, 586–598. [Google Scholar] [CrossRef]
- Menéndez, J.; Ordóñez, A.; Álvarez, R.; Loredo, J. Energy from closed mines: Underground energy storage and geothermal applications. Renew. Sustain. Energy Rev. 2019, 108, 498–512. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Cava, F.; Kelly, J.; Peitzke, W.; Brown, M.; Sullivan, S. Advanced rail energy storage: Green energy storage for green energy. In Storing Energy with Special Reference to Renewable Energy Sources; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–86. [Google Scholar] [CrossRef]
- GravityLine. Available online: https://aresnorthamerica.com/gravityline/ (accessed on 4 January 2023).
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Yap, J.; McLellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11. [Google Scholar] [CrossRef]
- Almutairi, K.; Mostafaeipour, A.; Jahanshahi, E.; Jooyandeh, E.; Himri, Y.; Jahangiri, M.; Issakhov, A.; Chowdhury, S.; Dehshiri, S.J.H.; Dehshiri, S.S.H.; et al. Ranking locations for hydrogen production using hybrid wind-solar: A case study. Sustainability 2021, 13, 4524. [Google Scholar] [CrossRef]
- Leonzio, G. Power to Gas Systems Integrated with Anaerobic Digesters and Gasification Systems. Waste Biomass Valorization 2021, 12, 29–64. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.H.; Lim, H.; Won, W. Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Appl. Sci. 2021, 11, 6021. [Google Scholar] [CrossRef]
- Blue-Hydrogen. Available online: https://pmt.honeywell.com/us/en/solutions/sustainability/hydrogen-solutions/blue-hydrogen (accessed on 4 January 2023).
- Carbon Capture. Available online: https://www.lindehydrogen.com/technology/carbon-capture (accessed on 4 January 2023).
- Post Combustion Capture (PCC). Available online: https://www.linde-engineering.com/en/process-plants/co2-plants/carbon-capture/post-combustion-capture/index.html (accessed on 4 January 2023).
- Hydrogen Production: Steam Reforming, Autothermal Reforming and Water Electrolysis. Available online: https://www.thyssenkrupp-uhde.com/en/products-and-technologies/hydrogen-and-gas-technologies/hydrogen (accessed on 4 January 2023).
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main hydrogen production processes: An overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Das, S.R.; Basak, N. Optimization of process parameters for enhanced biohydrogen production using potato waste as substrate by combined dark and photo fermentation. Biomass Convers. Biorefinery 2022, 1–21. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, T.; Lu, S.; Zhu, S.; Li, Y.; Zhang, Q.; Zhang, Z. Study on Comparisons of Bio-Hydrogen Yield Potential and Energy Conversion Efficiency between Stem and Leaf of Sweet Potato by Photo-Fermentation. Fermentation 2022, 8, 165. [Google Scholar] [CrossRef]
- De Gioannis, G.; Dell’Era, A.; Muntoni, A.; Pasquali, M.; Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T. Bio-electrochemical production of hydrogen and electricity from organic waste: Preliminary assessment. Clean Technol. Environ. Policy 2023, 25, 269–280. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Cotta, M.A.; Angenent, L.T. Aerated Shewanella oneidensis in continuously fed bioelectrochemical systems for power and hydrogen production. Biotechnol. Bioeng. 2010, 105, 880–888. [Google Scholar] [CrossRef]
- Jing, Y.; Li, F.; Li, Y.; Jin, P.; Zhu, S.; He, C.; Zhao, J.; Zhang, Z.; Zhang, Q. Statistical optimization of simultaneous saccharification fermentative hydrogen production from corn stover. Bioengineered 2020, 11, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chui, C.; Zhang, S.; Liu, Q.; Li, B.; Shi, J.; Liu, L. Hydrogen Production by the Thermophilic Dry Anaerobic Co-Fermentation of Food Waste Utilizing Garden Waste or Kitchen Waste as Co-Substrate. Sustainability 2022, 14, 7367. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 4 January 2023).
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Du, J.; Sun, X.; Jiang, G.; Zhang, C. Hydrogen capability of bimetallic boron cycles: A DFT and ab initio MD study. Int. J. Hydrog. Energy 2019, 44, 6763–6772. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Zhang, H.; Xia, G.; Sun, D.; Yu, X. Heterostructures Built in Metal Hydrides for Advanced Hydrogen Storage Reversibility. Adv. Mater. 2020, 32, 2002647. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wang, H.; Xue, X.; Lin, Q. Metal-Organic Frameworks Promoted Hydrogen Storage Properties of Magnesium Hydride for In-Situ Resource Utilization (ISRU) on Mars. Front. Mater. 2021, 8, 766288. [Google Scholar] [CrossRef]
- Aboud, M.F.A.; ALOthman, Z.A.; Bagabas, A.A. Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons. Appl. Sci. 2021, 11, 6604. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, H.; Oh, J.-E.; Park, B.Y. Recent Advances in Homogeneous/Heterogeneous Catalytic Hydrogenation and Dehydrogenation for Potential Liquid Organic Hydrogen Carrier (LOHC) Systems. Catalysts 2021, 11, 1497. [Google Scholar] [CrossRef]
- Available online: https://netzerotc-newsroom.prgloo.com/news/project-launched-to-create-hydrogen-highway-from-scotland-to-rotterdam (accessed on 5 January 2023).
- Liquid Organic Hydrogen Carriers. Available online: https://sherlohck.eu/ (accessed on 8 January 2023).
- Together We Are Advancing the Energy Transition Using Hydrogen. Available online: https://www.get-h2.de/wp-content/uploads/geth2_infobroschuere_4seiter_en_200402.pdf (accessed on 5 January 2023).
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Kim, M.; Kim, H.-J.; Suh, J.; Kang, N. Role of Hydrogen and Temperature in Hydrogen Embrittlement of Equimolar CoCrFeMnNi High-entropy Alloy. Met. Mater. Int. 2021, 27, 166–174. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Met. Mater. Trans. B 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrogen Energy 2017, 42, 13329–13338. [Google Scholar] [CrossRef]
- Hydrogen Delivery Liquefaction & Compression. Available online: https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/liquefaction_comp_pres_praxair.pdf (accessed on 19 April 2023).
- Al Ghafri, S.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.M.; Leachman, J.; Span, R.; Kamija, S.; Pearce, G.; et al. Hydrogen liquefaction: A review of the fundamental physics, engineering practice and future opportunities. Energy Environ. Sci. 2022, 15, 2690–2731. [Google Scholar] [CrossRef]
- Crotogino, F. Large-scale hydrogen storage. In Storing Energy, with Special Reference to Renewable Energy Sources, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 613–632. ISBN 9780128245101. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Lanni, D.; Galloni, E.; Fontana, G.; D’Antuono, G. Assessment of the Operation of an SI Engine Fueled with Ammonia. Energies 2022, 15, 8583. [Google Scholar] [CrossRef]
- Seo, Y.; Han, S. Economic Evaluation of an Ammonia-Fueled Ammonia Carrier Depending on Methods of Ammonia Fuel Storage. Energies 2021, 14, 8326. [Google Scholar] [CrossRef]
- Otto, M.; Vesely, L.; Kapat, J.; Stoia, M.; Applegate, N.D.; Natsui, G. Ammonia as an Aircraft Fuel: Thermal Assessment from Airport to Wake, In Proceedings of Turbo Expo: Power for Land, Sea, and Air. Rotterdam, The Netherlands, 13–17 June 2022; Volume 85987, p. V002T03A023. [Google Scholar] [CrossRef]
- Boretti, A.; Castelletto, S. NH3 Prospects in Combustion Engines and Fuel Cells for Commercial Aviation by 2030. ACS Energy Lett. 2022, 7, 2557–2564. [Google Scholar] [CrossRef]
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453. [Google Scholar] [CrossRef]
- Zhang, Z.; Liguori, S.; Fuerst, T.F.; Way, J.D.; Wolden, C.A. Efficient Ammonia Decomposition in a Catalytic Membrane Reactor To Enable Hydrogen Storage and Utilization. ACS Sustain. Chem. Eng. 2019, 7, 5975–5985. [Google Scholar] [CrossRef]
- Khan, W.U.; Alasiri, H.S.; Ali, S.A.; Hossain, M.M. Recent Advances in Bimetallic Catalysts for Hydrogen Production from Ammonia. Chem. Rec. 2022, 22, e202200030. [Google Scholar] [CrossRef] [PubMed]
- Çelık Kazici, H.; Yilmaz, Ş.; Şahan, T.; Yildiz, F.; Er, Ö.F.; Kivrak, H. A comprehensive study of hydrogen production from ammonia borane via PdCoAg/AC nanoparticles and anodic current in alkaline medium: Experimental design with response surface methodology. Front. Energy 2020, 14, 578–589. [Google Scholar] [CrossRef]
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Ag@Pd Core–Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost benefits of optimizing hydrogen storage and methanation capacities for Power-to-Gas plants in dynamic operation. Appl. Energy 2020, 257, 113967. [Google Scholar] [CrossRef]
- Wai, S.; Ota, Y.; Sugiyama, M.; Nishioka, K. Evaluation of a Sabatier Reaction Utilizing Hydrogen Produced by Concentrator Photovoltaic Modules under Outdoor Conditions. Appl. Sci. 2020, 10, 3144. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. Assessment and optimization of an integrated wind power system for hydrogen and methane production. Energy Convers. Manag. 2018, 177, 693–703. [Google Scholar] [CrossRef]
- Audi E-Gas Project, Germany. Available online: https://www.power-technology.com/marketdata/audi-e-gas-project-germany/ (accessed on 10 January 2023).
- Power-to-Gas. Available online: https://www.hz-inova.com/de/renewable-gas/etogas/ (accessed on 10 January 2023).
- Abdel-Mageed, A.M.; Wohlrab, S. Review of CO2 Reduction on Supported Metals (Alloys) and Single-Atom Catalysts (SACs) for the Use of Green Hydrogen in Power-to-Gas Concepts. Catalysts 2022, 12, 16. [Google Scholar] [CrossRef]
- De Roeck, F.G.; Buchmayr, A.; Gripekoven, J.; Mertens, J.; Dewulf, J. Comparative life cycle assessment of power-to-methane pathways: Process simulation of biological and catalytic biogas methanation. J. Clean. Prod. 2022, 380, 135033. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar] [CrossRef]
- González, R.; González, J.; Rosas, J.G.; Smith, R.; Gómez, X. Biochar and Energy Production: Valorizing Swine Manure through Coupling Co-Digestion and Pyrolysis. C 2020, 6, 43. [Google Scholar] [CrossRef]
- González, R.; Ellacuriaga, M.; Aguilar-Pesantes, A.; Carrillo-Peña, D.; García-Cascallana, J.; Smith, R.; Gómez, X. Feasibility of Coupling Anaerobic Digestion and Hydrothermal Carbonization: Analyzing Thermal Demand. Appl. Sci. 2021, 11, 11660. [Google Scholar] [CrossRef]
- Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495. [Google Scholar] [CrossRef]
- Ipiales, R.P.; Mohedano, A.F.; Diaz, E.; de la Rubia, M.A. Energy recovery from garden and park waste by hydrothermal carbonisation and anaerobic digestion. Waste Manag. 2022, 140, 100–109. [Google Scholar] [CrossRef]
- Liquid-Blank-Ammonia. Available online: https://www.aqua-calc.com/page/density-table/substance/liquid-blank-ammonia (accessed on 19 April 2023).
- Liu, Y.T.; Liu, S.; Li, G.R.; Gao, X.P. Strategy of enhancing the volumetric energy density for lithium–sulfur batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2021, 12, 5259–5282. [Google Scholar] [CrossRef]
- Tauber, J.; Ramsbacher, A.; Svardal, K.; Krampe, J. Energetic Potential for Biological Methanation in Anaerobic Sewage Sludge Digesters in Austria. Energies 2021, 14, 6618. [Google Scholar] [CrossRef]
- El Sistema Eléctrico Español. Available online: https://www.ree.es/sites/default/files/publication/2022/03/downloadable/Avance_ISE_2021.pdf (accessed on 8 January 2023).
- Valle-Falcones, L.M.; Grima-Olmedo, C.; Mazadiego-Martínez, L.F.; Hurtado-Bezos, A.; Eguilior-Díaz, S.; Rodríguez-Pons, R. Green Hydrogen Storage in an Underground Cavern: A Case Study in Salt Diapir of Spain. Appl. Sci. 2022, 12, 6081. [Google Scholar] [CrossRef]
- Bekkering, J.; Zwart, K.; Martinus, G.; Langerak, J.; Tideman, J.; van der Meij, T.; Alberts, K.; van Steenis, M.; Nap, J.-P. Farm-scale bio-power-to-methane: Comparative analyses of economic and environmental feasibility. Int. J. Energy Res. 2020, 44, 2264–2277. [Google Scholar] [CrossRef]
- Gavala, H.N.; Angelidaki, I.; Ahring, B.K. Kinetics and modeling of anaerobic digestion process. In Advances in Biochemical Engineering/Biotechnology; Biomethanation, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 81, pp. 57–93. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Arenas, C.B.; Meredith, W.; Snape, C.E.; Gómez, X.; González, J.F.; Martinez, E.J. Effect of char addition on anaerobic digestion of animal by-products: Evaluating biogas production and process performance. Environ. Sci. Pollut. Res. 2020, 27, 24387–24399. [Google Scholar] [CrossRef] [PubMed]
- Arenas Sevillano, C.B.; Chiappero, M.; Gomez, X.; Fiore, S.; Martínez, E.J. Improving the Anaerobic Digestion of Wine-Industry Liquid Wastes: Treatment by Electro-Oxidation and Use of Biochar as an Additive. Energies 2020, 13, 5971. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Salvador, A.F.; Martins, G.; Oliveira, C.C.; Liu, Y.; Martins, V.R.; Castro, A.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Pereira, L.; et al. Multi-Walled Carbon Nanotubes Enhance Methanogenesis from Diverse Organic Compounds in Anaerobic Sludge and River Sediments. Appl. Sci. 2020, 10, 8184. [Google Scholar] [CrossRef]
- Ciezkowska, M.; Bajda, T.; Decewicz, P.; Dziewit, L.; Drewniak, L. Effect of Clinoptilolite and Halloysite Addition on Biogas Production and Microbial Community Structure during Anaerobic Digestion. Materials 2020, 13, 4127. [Google Scholar] [CrossRef]
- Chorukova, E.; Hubenov, V.; Gocheva, Y.; Simeonov, I. Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Appl. Sci. 2022, 12, 6274. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Application of Bioelectrochemical System and Magnetite Nanoparticles on the Anaerobic Digestion of Sewage Sludge: Effect of Electrode Configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Rani, V.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Dhull, N.; et al. Biostimulation of Anaerobic Digestion Using Iron Oxide Nanoparticles (IONPs) for Increasing Biogas Production from Cattle Manure. Nanomaterials 2022, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Ellacuriaga, M.; Cascallana, J.G.; González, R.; Gómez, X. High-solid anaerobic digestion: Reviewing strategies for increasing reactor performance. Environments 2021, 8, 80. [Google Scholar] [CrossRef]
- Diekert, G.; Wohlfarth, G. Metabolism of homoacetogens. Antonie Van Leeuwenhoek 1994, 66, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Karekar, S.; Stefanini, R.; Ahring, B. Homo-Acetogens: Their Metabolism and Competitive Relationship with Hydrogenotrophic Methanogens. Microorganisms 2022, 10, 397. [Google Scholar] [CrossRef]
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, X.; Söderlind, U.; Göransson, K.; Zhang, W.; Yu, C. Identification of the biomethanation pathways during biological CO2 fixation with exogenous H2 addition. Fuel Process. Technol. 2022, 238, 107478. [Google Scholar] [CrossRef]
- Rittmann, S.; Seifert, A.; Herwig, C. Essential prerequisites for successful bioprocess development of biological CH4 production from CO2 and H2. Crit. Rev. Biotechnol. 2015, 35, 141–151. [Google Scholar] [CrossRef]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological hydrogen methanation systems—An overview of design and efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef]
- Barik, S.; Vega, J.L.; Clausen, E.C.; Gaddy, J.L. Biological conversion of coal gas to methane. Appl. Biochem. Biotechnol. 1988, 18, 379–392. [Google Scholar] [CrossRef]
- Sur, R.; Sun, K.; Jeffries, J.B.; Hanson, R.K. Multi-species laser absorption sensors for in situ monitoring of syngas composition. Appl. Phys. B 2014, 115, 9–24. [Google Scholar] [CrossRef]
- Hussain, M.; Zabiri, H.; Uddin, F.; Yusup, S.; Tufa, L.D. Pilot-scale biomass gasification system for hydrogen production from palm kernel shell (part A): Steady-state simulation. Biomass Convers. Biorefinery 2021, 13, 3849–3862. [Google Scholar] [CrossRef]
- Wise, D.L.; Cooney, C.L.; Augenstein, D.C. Biomethanation: Anaerobic fermentation of CO2, H2 and CO to methane. Biotechnol. Bioeng. 1978, 20, 1153–1172. [Google Scholar] [CrossRef]
- Klasson, K.T.; Cowger, J.P.; Ko, C.W.; Vega, J.L.; Clausen, E.C.; Gaddy, J.L. Methane production from synthesis gas using a mixed culture ofR. rubrum M. barkeri, and M. formicicum. Appl. Biochem. Biotechnol. 1990, 24, 317–328. [Google Scholar] [CrossRef]
- Kimmel, D.E.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Performance of trickle-bed bioreactors for converting synthesis gas to methane. Appl. Biochem. Biotechnol. 1991, 28, 457–469. [Google Scholar] [CrossRef]
- Chandolias, K.; Pekgenc, E.; Taherzadeh, M.J. Floating Membrane Bioreactors with High Gas Hold-Up for Syngas-to-Biomethane Conversion. Energies 2019, 12, 1046. [Google Scholar] [CrossRef]
- Zipperle, A.; Reischl, B.; Schmider, T.; Stadlbauer, M.; Kushkevych, I.; Pruckner, C.; Vítězová, M.; Rittmann, S.K.-M.R. Biomethanation of Carbon Monoxide by Hyperthermophilic Artificial Archaeal Co-Cultures. Fermentation 2021, 7, 276. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, D.; Hu, X.; Söderlind, U.; Paladino, G.; Gamage, S.; Hedenström, E.; Zhang, W.; Arrigoni, J.; Lundgren, A.; et al. Low-Grade Syngas Biomethanation in Continuous Reactors with Respect to Gas-Liquid Mass Transfer and Reactor Start-Up Strategy. Fermentation 2023, 9, 38. [Google Scholar] [CrossRef]
- Steelanol Open Door. Available online: http://www.steelanol.eu/en/news/steelanol-opendoor (accessed on 10 January 2023).
- ArcelorMittal Inaugurates Flagship Carbon Capture and Utilisation Project at Its Steel Plant in Ghent, Belgium. Available online: https://corporate.arcelormittal.com/media/press-releases/arcelormittal-inaugurates-flagship-carbon-capture-and-utilisation-project-at-its-steel-plant-in-ghent-belgium (accessed on 10 January 2023).
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Andreides, D.; Pokorna, D.; Zabranska, J. Assessing the syngas biomethanation in anaerobic sludge digestion under different syngas loading rates and homogenisation. Fuel 2022, 320, 123929. [Google Scholar] [CrossRef]
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8. [Google Scholar] [CrossRef]
- Robazza, A.; Welter, C.; Kubisch, C.; Baleeiro, F.C.; Ochsenreither, K.; Neumann, A. Co-Fermenting Pyrolysis Aqueous Condensate and Pyrolysis Syngas with Anaerobic Microbial Communities Enables L-Malate Production in a Secondary Fermentative Stage. Fermentation 2022, 8, 512. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Ul Hai, I.; Neubauer, Y.; Schröder, P.; Oldenburg, H.; Seilkopf, A.; Kölling, A. Gas cleaning strategies for biomass gasification product gas. Int. J. Low-Carbon Technol. 2012, 7, 69–74. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Straub, M.; Dürre, P. Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 2013, 34, 1639–1651. [Google Scholar] [CrossRef]
- On the Mend: Why INEOS Bio Isn’t Producing Ethanol in Florida. 2014. Available online: http://www.biofuelsdigest.com/bdigest/2014/09/05/on-the-mend-why-ineos-bio-isnt-reporting-much-ethanol-production/ (accessed on 10 January 2023).
- Riley, D.M.; Tian, J.; Güngör-Demirci, G.; Phelan, P.; Villalobos, J.R.; Milcarek, R.J. Techno-economic assessment of chp systems in wastewater treatment plants. Environments 2020, 7, 74. [Google Scholar] [CrossRef]
- Gregorie, E.F.J.; Lamb, J.J.; Lien, K.M.; Pollet, B.G.; Burheim, O.S. Hydrogen and biogas. In Micro-Optics and Energy; Springer: Cham, Switzerland, 2020; pp. 131–155. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Biogas Upgrading Technologies—Developments and Innovations. 2009. Available online: https://www.researchgate.net/profile/Anneli-Petersson/publication/228890974_Biogas_Upgrading_Technologies_-_Developments_and_Innovations/links/53db86ab0cf216e4210bf3bc/Biogas-Upgrading-Technologies-Developments-and-Innovations.pdf (accessed on 10 January 2023).
- Sposob, M.; Wahid, R.; Fischer, K. Ex-situ biological CO2 methanation using trickle bed reactor: Review and recent advances. Rev. Environ. Sci. Bio/Technology 2021, 20, 1087–1102. [Google Scholar] [CrossRef]
- Lee, M.J.; Zinder, S.H. Hydrogen Partial Pressures in a Thermophilic Acetate-Oxidizing Methanogenic Coculture. Appl. Environ. Microbiol. 1988, 54, 1457–1461. [Google Scholar] [CrossRef]
- Bajpai, P. Basics of Anaerobic Digestion Process. In Anaerobic Technology in Pulp and Paper Industry; Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Illi, L.; Lecker, B.; Lemmer, A.; Müller, J.; Oechsner, H. Biological methanation of injected hydrogen in a two-stage anaerobic digestion process. Bioresour. Technol. 2021, 333, 125126. [Google Scholar] [CrossRef]
- Martínez, E.J.; Sotres, A.; Arenas, C.B.; Blanco, D.; Martínez, O.; Gómez, X. Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance. Energies 2019, 12, 1228. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Ross, A.; Camargo-Valero, M.A. Enhancing bioenergy production from food waste by in situ biomethanation: Effect of the hydrogen injection point. Food Energy Secur. 2021, 10, e288. [Google Scholar] [CrossRef]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Solubilities-Gases. Available online: https://www.wiredchemist.com/chemistry/data/solubilities-gases (accessed on 14 January 2023).
- Sieblist, C.; Hägeholz, O.; Aehle, M.; Jenzsch, M.; Pohlscheidt, M.; Lübbert, A. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO2 stripping. Biotechnol. J. 2011, 6, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jia, Y.; Wang, L.; Cao, Y. Drag coefficient fluctuation prediction of a single bubble rising in water. Chem. Eng. J. 2017, 316, 553–562. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wen, X.; Shimizu, K.; Lei, Z.; Kobayashi, M.; Zhang, Z.; Sumi, I.; Yao, Y.; Mogi, Y. Enhanced bioconversion of hydrogen and carbon dioxide to methane using a micro-nano sparger system: Mass balance and energy consumption. RSC Adv. 2018, 8, 26488–26496. [Google Scholar] [CrossRef] [PubMed]
- Herkowiak, M.; Łaska-Zieja, B.; Myczko, A.; Wrzesińska-Jędrusiak, E. Problems of Hydrogen Doping in the Methane Fermentation Process and of Energetic Use of the Gas Mixture. Appl. Sci. 2021, 11, 6374. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, J.; Liu, Z.; Huang, Z.; Zhao, M.; Shi, W.; Ruan, W. Optimal the ex-situ biogas biological upgrading to biomethane and its combined application with the anaerobic digestion stage. Energy Sources Part A Recover. Util. Environ. Eff. 2021, 43, 2147–2159. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and Inoculum Origin Influence the Performance of Ex-Situ Biological Hydrogen Methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef]
- Logroño, W.; Kluge, P.; Kleinsteuber, S.; Harms, H.; Nikolausz, M. Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen. Bioengineering 2022, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, A.; Ullrich, T. Effect of Different Operating Temperatures on the Biological Hydrogen Methanation in Trickle Bed Reactors. Energies 2018, 11, 1344. [Google Scholar] [CrossRef]
- Pokorna, D.; Varga, Z.; Andreides, D.; Zabranska, J. Adaptation of anaerobic culture to bioconversion of carbon dioxide with hydrogen to biomethane. Renew. Energy 2019, 142, 167–172. [Google Scholar] [CrossRef]
- Kozak, M.; Köroğlu, E.O.; Cirik, K.; Zaimoğlu, Z. Evaluation of ex-situ hydrogen biomethanation at mesophilic and thermophilic temperatures. Int. J. Hydrogen Energy 2022, 47, 15434–15441. [Google Scholar] [CrossRef]
- Sarker, S.; Wijnsma, S.N.; Lien, K.M. Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion. Energies 2020, 13, 3542. [Google Scholar] [CrossRef]
- Mulat, D.G.; Mosbæk, F.; Ward, A.J.; Polag, D.; Greule, M.; Keppler, F.; Nielsen, J.L.; Feilberg, A. Exogenous addition of H2 for an in situ biogas upgrading through biological reduction of carbon dioxide into methane. Waste Manag. 2017, 68, 146–156. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Kleinsteuber, S.; Sträuber, H.; Harms, H.; Nikolausz, M. Microbial Resource Management for Ex Situ Biomethanation of Hydrogen at Alkaline pH. Microorganisms 2020, 8, 614. [Google Scholar] [CrossRef]
- Daglioglu, S.T.; Ogut, T.C.; Ozdemir, G.; Azbar, N. Comparative Evaluation of Two Packing Materials (Glass Pipe and Ceramic Ball) for Hydrogenothrophic Biomethanation (BHM) of CO2. Waste Biomass Valorization 2021, 12, 3717–3726. [Google Scholar] [CrossRef]
- Kodama, T.; Goto, E.; Minoda, Y. Determination of Dissolved Hydrogen Concentration and in Submerged Culture Vessels. Biosci. Biotechnol. Biochem. 1976, 40, 2373–2377. [Google Scholar] [CrossRef]
- Jee, H.S.; Yano, T.; Nishio, N.; Nagai, S. Biomethanation of H2 and CO2 by Methanobacterium thermoautotrophicum in membrane and ceramic bioreactors. J. Ferment. Technol. 1987, 65, 413–418. [Google Scholar] [CrossRef]
- Daglioglu, S.T.; Karabey, B.; Ozdemir, G.; Azbar, N. CO2 utilization via a novel anaerobic bioprocess configuration with simulated gas mixture and real stack gas samples. Environ. Technol. 2019, 40, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Karabey, B.; Daglioglu, S.T.; Azbar, N.; Ozdemir, G. Bacterial and archeal dynamics of a labscale HYBRID gas fermentation bioreactor fed with CO2 and H2. J. Environ. Sci. Health Part A 2019, 54, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Dupnock, T.L.; Deshusses, M.A. High-Performance Biogas Upgrading Using a Biotrickling Filter and Hydrogenotrophic Methanogens. Appl. Biochem. Biotechnol. 2017, 183, 488–502. [Google Scholar] [CrossRef]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic methanation of hydrogen and carbon dioxide in an anaerobic three-phase system. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Dağlıoğlu, T.; Öğüt, T.C.; Ozdemir, G.; Azbar, N. Comparative analysis of the effect of cell immobilization on the hydrogenothrophic biomethanation of CO2. Greenh. Gases Sci. Technol. 2021, 11, 493–505. [Google Scholar] [CrossRef]
- Ullrich, T.; Lemmer, A. Performance enhancement of biological methanation with trickle bed reactors by liquid flow modulation. GCB Bioenergy 2019, 11, 63–71. [Google Scholar] [CrossRef]
- Banchero, M.; Manna, L.; Sicardi, S.; Ferri, A. Experimental investigation of fast-mode liquid modulation in a trickle-bed reactor. Chem. Eng. Sci. 2004, 59, 4149–4154. [Google Scholar] [CrossRef]
- Ju, D.-H.; Shin, J.-H.; Lee, H.-K.; Kong, S.-H.; Kim, J.-I.; Sang, B.-I. Effects of pH conditions on the biological conversion of carbon dioxide to methane in a hollow-fiber membrane biofilm reactor (Hf–MBfR). Desalination 2008, 234, 409–415. [Google Scholar] [CrossRef]
- Jønson, B.D.; Sieborg, M.U.; Ashraf, M.T.; Yde, L.; Shin, J.; Shin, S.G.; Triolo, J.M. Direct inoculation of a biotrickling filter for hydrogenotrophic methanogenesis. Bioresour. Technol. 2020, 318, 124098. [Google Scholar] [CrossRef] [PubMed]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-situ biogas upgrading and enhancement in different reactor systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A Single-Culture Bioprocess of Methanothermobacter thermautotrophicus to Upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, 157529. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. H2 addition through a submerged membrane for in-situ biogas upgrading in the anaerobic digestion of sewage sludge. Bioresour. Technol. 2019, 280, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-M.; Sung, S.; Kang, S.; Kim, M.-S.; Kim, D.-H. Enrichment of hydrogenotrophic methanogens by means of gas recycle and its application in biogas upgrading. Energy 2017, 135, 294–302. [Google Scholar] [CrossRef]
- Ullrich, T.; Lindner, J.; Bär, K.; Mörs, F.; Graf, F.; Lemmer, A. Influence of operating pressure on the biological hydrogen methanation in trickle-bed reactors. Bioresour. Technol. 2018, 247, 7–13. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic methanation of hydrogen and carbon dioxide in a fixed bed bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef]
- Abe, F.; Horikoshi, K. The biotechnological potential of piezophiles. Trends Biotechnol. 2001, 19, 102–108. [Google Scholar] [CrossRef]
- Oger, P.M.; Jebbar, M. The many ways of coping with pressure. Res. Microbiol. 2010, 161, 799–809. [Google Scholar] [CrossRef]
- Takai, K.; Nakamura, K.; Toki, T.; Tsunogai, U.; Miyazaki, M.; Miyazaki, J.; Hirayama, H.; Nakagawa, S.; Nunoura, T.; Horikoshi, K. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc. Natl. Acad. Sci. USA 2008, 105, 10949–10954. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 2013, 4, 1998. [Google Scholar] [CrossRef] [PubMed]
- Mauerhofer, L.-M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.-S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, R.T.; Pol, L.W.H.; Lettinga, G. Biological sulphate reduction using gas-lift reactors fed with hydrogen and carbon dioxide as energy and carbon source. Biotechnol. Bioeng. 1994, 44, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, R.T.; Yun, S.Y.; Lettinga, G. Thermophilic sulphate and sulphite reduction in lab-scale gas-lift reactors using H2 and CO2 as energy and carbon source. Biotechnol. Bioeng. 1997, 55, 807–814. [Google Scholar] [CrossRef]
- Esposito, G.; Weijma, J.; Pirozzi, F.; Lens, P.N.L. Effect of the sludge retention time on H2 utilization in a sulphate reducing gas-lift reactor. Process Biochem. 2003, 39, 491–498. [Google Scholar] [CrossRef]
- Weijma, J.; Gubbels, F.; Hulshoff Pol, L.W.; Stams, A.J.M.; Lens, P.; Lettinga, G. Competition for H2 between sulfate reducers, methanogens and homoacetogens in a gas-lift reactor. Water Sci. Technol. 2002, 45, 75–80. [Google Scholar] [CrossRef]
- Tyne, R.L.; Barry, P.H.; Lawson, M.; Byrne, D.J.; Warr, O.; Xie, H.; Hillegonds, D.J.; Formolo, M.; Summers, Z.M.; Skinner, B.; et al. Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs. Nature 2021, 600, 670–674. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Underground-Sun-Conversion. Available online: https://www.underground-sun-conversion.at/en/ (accessed on 15 January 2023).
- Almacenamiento Subterraneo Hidrogeno. Available online: http://www.hychico.com.ar/esp/almacenamiento-subterraneo-hidrogeno.html (accessed on 15 January 2023).
- Panfilov, M. Underground Storage of Hydrogen: In Situ Self-Organisation and Methane Generation. Transp. Porous Media 2010, 85, 841–865. [Google Scholar] [CrossRef]
- Eddaoui, N.; Panfilov, M.; Ganzer, L.; Hagemann, B. Impact of Pore Clogging by Bacteria on Underground Hydrogen Storage. Transp. Porous Media. 2021, 139, 89–108. [Google Scholar] [CrossRef]
- Ugarte, E.R.; Salehi, S. A Review on Well Integrity Issues for Underground Hydrogen Storage. J. Energy Resour. Technol. 2021, 144, 042001. [Google Scholar] [CrossRef]
- Hemme, C.; Van Berk, W. Hydrogeochemical Modeling to Identify Potential Risks of Underground Hydrogen Storage in Depleted Gas Fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Simon, K.-M.R.R.; Seifert, A.H.; Bernacchi, S. Kinetics, multivariate statistical modelling, and physiology of CO2-based biological methane production. Appl. Energy 2018, 216, 751–760. [Google Scholar] [CrossRef]
| Compound | Energy Density (MJ/L) | References |
|---|---|---|
| H2 (STP) | 1.08 × 10−4 | |
| CH4 (STP) | 3.58 × 10−4 | |
| LNG | 21–24 | |
| Gasoline | 32 | |
| Compressed H2 at 350 bar | 3.1 | [33] |
| Compressed H2 at 700 bar | 5.0 | [33] |
| Liquefied H2 | 8.5 | [44] |
| Ammonia at −33 °C | 12.7 | [76] |
| MCH when used as hydrogen transport 1 | 2.8 | [49] |
| Li-ion batteries | 0.97–2.7 | [77] |
| Operating Conditions | Regimen | Main Results | References |
|---|---|---|---|
| Fed-Batch system. Substrate: Cattle manure H2 injection into the head space | Mesophilic (39 °C) Working volume: 5 L | 31% increase in CH4 production with 400 mL H2 injected. No significant increase in CH4 concentration No effect reported regarding H2:CO2 molar ratio | [144] |
| H2 injection at 4:1 H2:CO2 molar ratio H2 loading rate: 2 L/Lreactor d | Mesophilic CSTR working volume: 3.6 L Thermophilic CSTR working volume: 4.5 L | Better thermophilic performance but needing longer adaptation time to achieved maximum efficiency. 84% of CO2 conversion efficiency with a methane production 1 of 0.084 L CH4/Lreactor d | [142] |
| Batch 2 Substrate: glucose, and diluted manure to provide nutrients. Intermittent H2 injection | Mesophilic (37 °C) Working volume: 0.5 L 4:1 (H2:CO2) molar ratio considered as optimum | Increase in CH4 content from 67 to 94%. High residual H2 levels caused VFA accumulation. pH increase observed with the increase in CO2 conversion Methanobacterium increased in abundance | [130] |
| Batch Substrate: Maize leaf Hydrogen injection of 507 mL/Lreactor d | Thermophilic (52 °C) Working volume: 20 mL | Excess H2 addition caused VFA accumulation and CO2 depletion, along with stimuli for homoacetogens to convert CO2 and H2 into acetate. pH control needed to avoid excessive increase. Enrichment of Methanobacterium | [145] |
| Acclimation of mixed cultures. Testing shear rate and media composition | Mesophilic (37–38 °C) Working volume: 50 mL and 500 mL | Enrichment attained using a mixed culture. 97% CH4 content obtained. Predominating methanogens were Methanobacterium and Methanoculleus genus | [146] |
| Testing different H2:CO2 ratios 24 h gas residence time | Mesophilic (37 °C) Thermophilic (55 °C) Working volume: 50 mL | The maximum CH4 concentration was obtained at thermophilic condition (81%) with a 4:1 H2:CO2 molar ratio Average H2 utilization efficiency of 92%, with a conversion yield of 0.23 L CH4/L H2 | [143] |
| H2 injection 4:1 H2:CO2 molar ratio Gas loading rate: 6 L/Lreactor d (4.8 L H2/Lreactor d) | Mesophilic (37 °C) Working volume: 4.5 L Packed bed reactor testing glass pipe tubes and ceramic balls as filling material | Average methane production rate between 4 and 5 L CH4/Lreactor d, with glass pipe filling showing better performance 3. | [147] |
| Reactor Configuration | Operating Conditions and Working Volume | Methane Production Rate (MPR) (L CH4/Lreactor d)—Main Results | References |
|---|---|---|---|
| Biotrickling filter Packing material: PUF (Temperature: 52 °C) | H2 injection rate: 11 L/Lreactor d Reactor volume: 0.291 L | MPR: 3.03 (Methane concentration: 98%) Reactor inoculated from a mixed anaerobic culture extracted from a biogas plant digester. Methanobacterium and Methanothermobacter represented 62.3 ± 1.5% and 31.1 ± 1.0% of the total archaea respectively | [160] |
| Bubble column (Temperature: 54 °C) | Gas injection rate: 3 L/Lreactor d (gas composition: CH4:H2:CO2 of 23:62:15 (%)) Reactor volume: 1.2 L | 84% conversion of H2 injected Accumulation of acetate at the end of the experiment Methane content output gas: 97–98% Reactor inoculation with thermophilic digestate. Methanothermobacter thermautotrophicus was the most abundant methanogen. | [161] |
| Pressurized CSTR (Pressure: 122 KPa Temperature: 60 °C) | 0.5 L/min Reactor volume: 3 L Gas injection rate: 240 L/Lreactor d H2:CO2 ratio: 4:1 | MPR: 65.6, for pressurized system 92% increase when compared with atmospheric control system Pure culture of Methanothermobacter thermautotrophicus | [162] |
| CSTR reactor with H2 supplemented using submerged membranes Temperature: 35 °C | H2 injection rate: 0.87 L/Lreactor d Gas recirculation rate: 200 L/Lreactor d Reactor volume: 20 L | MPR: 0.54 Value obtained from combined digestion of sewage sludge and H2 addition. (42% increase in MPR when compared with sewage sludge digestion). Methanoculleus sp., Methanospirillum sp., Methanolinea sp., and Methanobacterium sp. were the hydrogenotrophic archaea dominating in the reactor | [163] |
| Thermophilic anaerobic sludge blanket reactor Temperature: 55 °C | H2 injection rate: 16 L H2/Lreactor d Gas composition: CH4:H2:CO2 of 23:62:15 (%) Reactor volume: 6.3 L | MPR: 3.9 96% H2 conversion. Optimum gas recycle rate: 200 L/Lreactor d Testing mesophilic and thermophilic system founding better performance at higher temperature Methanothermococcus thermolithotrophicus was the dominant species under thermophilic conditions | [164] |
| Anaerobic trickling bed at high pressure Mesophilic: 40–41 °C | H2 injection rate: 10.87–10.98 L H2/Lreactor d Reactor trickling bed volume: 13 L + fixed bed volume: 1.5 L | MPR 1: 4.09 at 1.5 bar, 4.29 at 5.0 bar. Increasing pressure to 9 bar did not lead to significant increase in MPR. Methane concentration in biogas increased with pressure, from 64.13% to 86.51% with pressure increasing from 1.5 to 9 bar | [165] |
| Two-phase fixed bed reactor Temperature: 53–55 °C | H2 injection rate: 25.2 L H2/Lreactor d Effective volume of the two-reactor (serial) system: 4.0 L | MPR: 6.35 (maximum value) Average H2 conversion rate: 97.1% Average MPR at optimal stage: 1.73 | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. https://doi.org/10.3390/environments10050082
González R, Cabeza IO, Casallas-Ojeda M, Gómez X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments. 2023; 10(5):82. https://doi.org/10.3390/environments10050082
Chicago/Turabian StyleGonzález, Rubén, Iván Orlando Cabeza, Miguel Casallas-Ojeda, and Xiomar Gómez. 2023. "Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy" Environments 10, no. 5: 82. https://doi.org/10.3390/environments10050082
APA StyleGonzález, R., Cabeza, I. O., Casallas-Ojeda, M., & Gómez, X. (2023). Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments, 10(5), 82. https://doi.org/10.3390/environments10050082







