Abstract
Human and animal waste, including waste products originating from human or animal digestive systems, such as urine, feces, and animal manure, have constituted a nuisance to the environment. Inappropriate disposal and poor sanitation of human and animal waste often cause negative impacts on human health through contamination of the terrestrial environment, soil, and water bodies. Therefore, it is necessary to convert these wastes into useful resources to mitigate their adverse environmental effect. The present study provides an overview and research progress of different thermochemical and biological conversion pathways for the transformation of human- and animal-derived waste into valuable resources. The physicochemical properties of human and animal waste are meticulously discussed, as well as nutrient recovery strategies. In addition, a bibliometric analysis is provided to identify the trends in research and knowledge gaps. The results reveal that the USA, China, and England are the dominant countries in the research areas related to resource recovery from human or animal waste. In addition, researchers from the University of Illinois, the University of California Davis, the Chinese Academy of Sciences, and Zhejiang University are front runners in research related to these areas. Future research could be extended to the development of technologies for on-site recovery of resources, exploring integrated resource recovery pathways, and exploring different safe waste processing methods.
1. Introduction
Human and animal waste management is a growing concern globally. Human and animal waste, in this context, refers to waste products originating from human or animal digestive systems, including urine and feces. In contrast, animal manure comprises feces, urine, and other excrement produced by animals, as well as animal manure derived from animal feces. The lack of sewerage in densely populated areas, especially in underdeveloped countries, often leads to the indiscriminate and unhealthy disposal of solid waste, including feces and urine [1]. The World Health Organization (WHO) projected that more than 20% of 7.8 billion individuals in the world do not have access to well-managed sanitation and are often practicing open defecation [2]. Approximately three-fifths of globally generated fecal wastes are not subjected to any treatment process and are often disposed of in small or large water bodies [3]. As a result, the water bodies become unsafe for drinking and pose severe contamination risks to the aquatic ecosystem. Approximately 500,000 deaths of children under 5 years old are recorded yearly from diarrhea, due to drinking water contaminated by human and animal wastes [4]. There is no doubt that human and animal waste constitute serious environmental nuisances.
Poor sanitation and fecal sludge management can affect human health and contaminate soil and water bodies. Moreover, human feces and animal waste contain an array of pathogens that can cause waterborne diseases if released into the environment without adequate treatment. Additionally, the progressing production rate of human- and animal-derived waste is another issue that requires attention. The increasing world population, as well as industrialization and urbanization, have contributed to the increasing demand for dairy and animal products, thereby increasing the amount of animal waste produced annually. For example, China’s livestock industry generated approximately 4 billion tons of manure, which is six times that of the past 40 years, followed by the United States, with approximately 1.4 billion tons [2]. Animal manure has always been used as a soil conditioner and as a nutrient to enhance crop growth [5]. However, when manure decomposes, it releases methane gas (CH4), which is a contributor to global warming and a major greenhouse gas (GHG).
Improved sanitation could help reduce the adverse effect of human waste and provide a decent barrier between humans and harmful pathogens [6]. However, factors such as sustainability concerns related to the emissions of GHG from domestic wastewater treatment facilities and the rapidly increasing population of individuals without sanitation have hindered the development of sanitation technologies. Therefore, it is imperative to consider alternative waste valorization routes to complement the development of advanced sanitation facilities worldwide.
Human and animal waste can be converted into valuable resources through several process steps and conversion pathways. The two key conversion pathways are thermochemical and biological processes [7]. Thermochemical processes include gasification, pyrolysis, and liquefaction. Such processes proceed with the aid of thermal and chemical energy at high temperatures. In contrast, biological processes, such as anaerobic digestion (AD) and fermentation, employ microorganisms for the degradation of organic waste [8]. Thermochemical or biological valorization of human and animal waste could help promote sustainable development goals (SDGs) 6 (clean water and sanitation) and 7 (affordable and clean energy).
Human and animal waste are complex and heterogeneous, and most of the waste also contains high moisture contents. Compositing human and animal waste has been an effective traditional method of eliminating pathogenic bacteria and balancing the carbon-to-nitrogen ratio [9]. AD is also another effective method for human and animal waste valorization [9]. Several studies have reported that human or animal waste could be an effective source of energy or nutrients [10,11,12,13]. Hunter and Deshusses [3] developed nitrification and denitrification filters to post-treat human waste-derived digestate, so that they can efficiently recover nutrients that may be used as fertilizer [3]. Oa [14] studied the recovery of resources from animal waste. They proposed an integrated system for the recovery of nutrients. The integrated system comprises incineration processes, anaerobic digestion or microbial fuel cells, mechanical vapor compression distillation for recovering nitrogen, and struvite precipitation for recovering phosphorus [14].
Some authors have also published excellent reviews related to the valorization of human and animal waste, as summarized in Table 1. However, the available information on resource recovery from human or animal waste is scattered in the literature considering the environmental relevance of the topic. Moreover, most of the available studies either focus on one type of waste (human or animal waste) or one resource recovery pathway (biological or thermochemical routes). Although, there have been some pioneering studies on the use of human urine as fertilizer [15]. To the best of the authors’ knowledge, comprehensive studies that consider both human and animal waste, including animal manure, as well as all possible resource recovery methods, are scarce in the literature. Furthermore, a bibliometric analysis of research studies related to the valorization of human and animal waste has not been documented in the literature. Thus, this study comprehensively reviewed different pathways for the recovery of resources from human and animal waste. It also discusses and compares the physical and chemical properties of human and livestock urine and feces. Human and livestock urine and feces, as a source of fertilizer, were also discussed in this paper. An overview of the key contents of this study is summarized in Table 1.

Table 1.
Summary of review articles related to resource recovery from human and animal waste.
2. Physical and Chemical Properties of Human and Livestock Urine and Feces
Figure 1 shows the different classifications of human and animal waste considered in this study. Human waste, such as feces and urine, are meticulously reviewed. The combination of human feces and urine is known as excreta. In contrast, animal manure from livestock, domestic animals, poultry, and horses are also discussed.

Figure 1.
Classification of human and animal waste considered in this study. Note that, while there are several waste classifications, the one presented herein is based on the authors’ experience and the scope of the present study.
Manures are valuable organic matter derived from solid animal waste, including cow dung, and biogas plant slurries. They can be used as a nutrient source to enhance crop growth and as a soil conditioner. Animal waste is a combination of waste feeds, feces, urine, and bedding materials. It consists of macronutrients such as nitrogen (N), phosphorus (P), and potassium (K) and micronutrients such as iron (Fe), sodium (Na), cobalt (Co), manganese (Mn), sulphur (S), and magnesium (Mg). The physicochemical properties of manure are one of the most important factors in evaluating the most promising valorization pathways [16,18]. Animal manure can also be classified based on its moisture content into liquid manure (up to 95% moisture content), semisolid and slurry manure (between 75% and 95% moisture content), and solid manure (less than 75% moisture content) [19]. The average composition and nutrient content of human excreta (urine and feces) and animal manure are comprehensively compared and summarized in Table 2.

Table 2.
Composition and nutrient content of animal manure and human excreta [20,21,22].
The data in Table 2 show that the composition of animal manure varies among animals. However, they are all rich in N, P, and K contents, making them valuable fertilizer resources. They also contain high moisture content between 70 and 92%. Compared to animal manure, human excreta have a higher biological oxygen demand (BOD). BOD is an indication of the amount of oxygen that is required by microorganisms to break down organic matter in water. Given that organic matter in the water can have a detrimental effect on aquatic life, it is a crucial indicator of the quality of the water. High BOD levels can be a sign of excess nutrients, such as nitrogen and phosphorus, which can cause eutrophication or excessive growth of plants and algae. Aquatic life may be harmed or killed if the substance’s oxygen levels fall as a result. High BOD levels may also be a sign of the existence of pathogenic (disease-causing) organisms that could be dangerous to people.
The proximate and ultimate analysis of human feces and animal manure is presented in Table 3. The manures are rich in carbon content and volatile matter. Chicken manure has a relatively high volatile matter content (65.6 wt.%), compared to human feces (50.2 wt.%). In contrast, the carbon content of human feces is higher (43.5 wt.%).

Table 3.
Proximate and ultimate analysis of human and livestock wastes, expressed on a dry basis.
The fixed carbon content provides an overview of the amount of char formation during the thermochemical conversion process. It is the solid combustible residue that remains after the volatile matter is driven off. A relatively high fixed carbon indicates an improved char production during the thermochemical conversion process. However, the fixed carbon content of animal manure is less than 26%. Lower ash content is also favorable for thermochemical processes. Therefore, the low fixed carbon of human and animal waste makes them promising thermochemical process precursors. However, severe agglomeration and erosion problems could occur, as the ash content significantly exceeded the 6% threshold.
Both human and animal waste are also characterized by low S content and high O content (Table 3). The energy content of human and animal waste is defined by the higher heating value (HHV). The HHV of animal manure ranges between 13.2 and 22.5 MJ/kg. This value is within the range of the HHV value of a typical lignocellulosic biomass (15–21 MJ/kg) [21]. The higher HHV and lower S content confirm that human and animal waste are promising fuel sources. The H/C and O/C ratios of animal manures are higher than those of coal and biochar, as indicated in the van Krevelen diagram in Figure 2 [22]. The position of the animal manures in the van Krevelen plot is similar to the position of plant biomass. Pig manure had a significantly lower O/C ratio, while beef manure had a low H/C ratio. Low O/C and H/C ratios may indicate that a compound is more reduced, meaning that it contains more hydrogen and less oxygen, relative to carbon. Compounds with low O/C and H/C ratios may be more stable and have lower reactivity, compared to compounds with high O/C and H/C ratios.

Figure 2.
Van Krevelen diagram representing the heating values of the different types of animal manures, compared with solid fuels. Adapted from Shen et al. 2 [22] with permission from Elsevier.
The physicochemical characterization of different animal manures for energy production has been the focus of some research studies. Shen et al. [22] revealed that different types of animal manure presented a wide variation in their composition. Their study on the composition analysis of different Chinese animal manures showed a significant difference in composition among the various types of animal manures.
The physicochemical properties of human excreta depend on gender, age, protein, the quantity of fiber, calories taken in, geographical location, diet, and sociocultural factors. Human excreta is mostly composed of water. Feces water content is a factor of health status, the quantity of water taken in, and the duration the feces spends in the intestine before it is excreted [20,29]. Fresh human feces contain 75–80% moisture content, while the rest are solids (ash, undigested fats, food remnants, and proteins). The pH of feces is approximately 5–7 [30], and urine contains approximately 90% water, with the rest being inorganic salts, organic compounds, organic ammonium salts, and urea [31].
An individual excretes approximately 50 kg of feces and 500 kg of urine each year with the following composition: 1.2 kg K, 0.6 kg P, and 5.7 kg N, but only 60–65% of total P, 50–80% of total K, and 90% of total N are excreted in the urine [32]. The average urine per capita per year is 500 L. North America and Europe produce feces of 130–520 g person−1 d−1 and 100–200 person−1 d−1, respectively. Urine has lower K/N and P/N ratios than synthetic fertilizers [32].
Generally, animal manure also contains heavy metals, such as Pb, Cu, Zn, Fe, and Sr, in large quantities. A recent study showed that lactating dromedary manure samples contain 2189 mg kg−1 Fe, 183 mg kg−1 Mn, 293 mg kg−1 Sr, and 87 mg kg−1 Zn heavy metals [32]. The presence of heavy metals in animal manure could constitute environmental challenges.
3. Thermochemical Conversion of Human and Animal Waste
The thermochemical conversion processes discussed in this section include pyrolysis, gasification, and liquefaction. These processes are used in the conversion of human and animal waste into various valuable chemicals and biofuels.
3.1. Pyrolysis
Pyrolysis refers to the thermal decomposition of biogenic waste in an oxygen-free environment to produce bio-oil, gases, and biochar, depending on the reaction conditions. Operating conditions such as the pressure, temperature, physicochemical properties of the feed materials, residence time, and heating rate influence the product yields and composition during pyrolysis.
Pyrolysis can be classified into slow pyrolysis, flash pyrolysis, and fast pyrolysis, based on the operating conditions utilized in the process [8]. The operating conditions and key products of different types of pyrolysis are summarized in Figure 3. Slow pyrolysis requires a lower temperature and longer residence time to complete the process with biochar as the major product. Slow pyrolysis yields higher biochar yields (15–40 wt.%) and lower bio-oil (25–35 wt.%) and gas yields (10–25 wt.%). In contrast, fast pyrolysis occurs under a very high temperature, high heating rate, and relatively short residence time. This results in bio-oil formation as the key product during slow and flash pyrolysis. Flash pyrolysis is an extremely rapid thermal decomposition pyrolysis that occurs at a relatively high heating rate and short residence time and temperatures between 800 and 1000 °C. The rapid heating and cooling rates in flash pyrolysis allow for the production of high-quality pyrolysis products with minimal degradation or secondary reactions. The major products are gases and bio-oil with minimal biochar formation. Generally, flash pyrolysis produces approximately 60–75 wt.% bio-oil, 10–25 wt.% biochar, and 10–30 wt.% gases [33].

Figure 3.
Overview of different types of pyrolysis. Data were obtained from Okolie et al. [8].
Several studies have reported the pyrolysis of human and animal waste for biochar and bio-oil production [34,35,36,37]. Most of the studies either focus on process optimization or evaluation of the influence of reaction conditions on biochar production [38], a study of the reaction mechanism, or the development of new catalysts [39]. Cantrell et al. [38] reported the effect of pyrolysis temperature on the physicochemical properties of biochar derived from five different animal manure precursors (poultry litter, swine-separated solids, turkey litter, dairy manure, and paved feedlot manure). The study revealed that the biochar obtained from dairy manure had the highest energy density, carbon, and volatile matter, coupled with the lowest contents of N, S, and ash. Moreover, the biochar produced from poultry litter had the highest electrical conductivity. Their results showed that the properties of animal manure precursors and reaction temperatures play a significant role in the physicochemical properties of the produced biochar. Recently, some researchers have explored the application of pyrolysis technology for the intermediate conversion of partially wet sanitary fecal sludge generated on train toilets [36]. The novel twin auger pyrolysis reactor produced about 50% bio-oil yield, 40% syngas yield, and 10% biochar yield at 500 °C.
Krounbi et al. [1] performed a comparative study to determine the soil amendment quality by torrefaction, composting, and pyrolysis of human wastes (feces and urine). The physicochemical properties of pyrolysis-treated human waste were largely influenced by temperature. Additionally, pyrolysis-treated human waste at 600 °C had five times the available potassium (7400 mg K/kg) and four times phosphorus (3120 mg P/kg), when compared to thermophilic composting. Yacob et al. [24] performed human feces pyrolysis at low, intermediate, and high temperatures, with a heating rate of 10 °C/min. They reported that slow pyrolysis (at low temperatures) gave a 20.9% yield of non-condensable gases. Carbon dioxide (CO2) and methane (CH4) were dominant during mild pyrolysis (at intermediate temperature). Moreover, fast pyrolysis (>600 °C) gave a 45% yield of hydrogen. Mong et al. [40] developed a microwave pyrolysis technology for the conversion of horse manure into biofuels and biochemicals. The reactor produced gaseous products with 67% syngas and 37% biochar yield.
Few studies have also explored the soil amendment potential of human and animal waste-derived biochar. Liu et al. [41] showed that pyrolysis-derived biochar from dry human waste could be used as a promising additive for enhancing soil fertility. Zhou et al. [42] reported a comparative study on the characteristics of biochar from the slow pyrolysis of chicken manure, pig manure, and cattle manure. Regarding carbon content, chicken manure had the highest carbon content (41 wt. %), followed by cattle manure (35–38 wt. %) and then pig manure. Pig manure had the highest ash content, followed by cattle manure (46–47 wt. %) and chicken manure (21 wt. %). The produced biochar is perceived as a promising soil additive.
Catalysts have been employed during the pyrolysis of human and animal waste for several reasons. First, the use of catalysts ensures that the reaction proceeds at lower temperatures, thereby significantly lowering the energy demand. Additionally, catalysts help improve the quality of the product and yield, especially for fast and flash pyrolysis [43]. Catalyst deactivation, due to coke or char formation, is still a major challenge. Overall, pyrolysis catalysts could be homogeneous or heterogeneous. Homogeneous catalysts exist in a single phase (usually a liquid solution), while heterogeneous catalysts are solid and have the advantages of ease of regeneration and reuse. Commonly used heterogeneous catalysts for waste pyrolysis include metals supported on carbon and basic oxides, zeolites, and solid acid catalysts. Studies related to catalytic and noncatalytic human and animal waste pyrolysis are summarized in Table 4.

Table 4.
Summary of past studies on the pyrolysis of human and livestock wastes.
In addition to catalytic studies, some authors have also explored the mechanism of animal manure pyrolysis. He et al. [51] developed a detailed mechanistic reaction pathway for the pyrolysis of cattle manure (Figure 4). The pathway indicates that major products, such as aldehydes, ketones, acids, hydrocarbons, phenolic compounds, and nitrogenous compounds, in bio-oil are formed in the temperature range of 180–350 °C. Alkenes are formed at higher temperatures above 500 °C. Moreover, the cracking of cellulose and CC chains led to the formation of acetaldehyde at 180 °C. Increasing temperature inhibits acetaldehyde formation, while promoting the generation of ketones and acids.

Figure 4.
Mechanistic reaction pathways for the pyrolysis of animal manure to bio-oils. Adapted from He et al. [51].
3.2. Gasification
Gasification is another thermochemical conversion pathway that can convert human and animal waste into solid, liquid, and gaseous products (syngas). Gasification is often referred to as a biomass-to-gas conversion process because its target is hydrogen-rich gas comprising mostly H2 and CO. Substantial quantities of hydrocarbons, such as CH4, as well as CO2 and H2O, and often N2, are also present in the gaseous product from gasification [7]. Synthetic gas (syngas) is defined as a gas with H2 and CO as the main components, and it is a product of gasification. Syngas has myriad applications as a precursor for the production of higher alcohols, green fuels, and chemicals via syngas fermentation of Fischer–Tropsch (FT) synthesis.
Gasification can be grouped as conventional or hydrothermal gasification, based on the temperature, pressure, and gasifying agents. It should be mentioned that both gasification processes have identical products, but their composition and yield are often different. Figure 5 outlines the differences between conventional and hydrothermal gasification processes.

Figure 5.
An overview of the difference between conventional and hydrothermal gasification.
Studies on the gasification of human and animal waste are related to the parametric evaluation of the impact of process conditions on gaseous yield, process optimization [52], energy and exergy analysis [53], kinetics studies [54], and techno-economic and life cycle assessment [55,56]. Some researchers have also focused on developing innovative heterogeneous catalysts for improving product yield and selectivity or co-gasification with other waste materials [57].
Liu et al. [57] studied the synergistic effect of a gasifying mixture of petroleum coke and chicken manure. They reported that chicken manure addition during the gasification of petroleum coke helped increase the hydrogen content in the produced syngas. The authors also explored the catalytic effect of chicken manure ash on gasification efficiency. Chicken manure ash increased the gasification efficiency by five times. They suggested that this must have been due to the ash’s high potassium and calcium content. Onabanjo et al. [53] explored the possibility of producing hydrogen from human feces through conventional gasification. Aspen Plus thermodynamic equilibrium modelling was used to estimate the quantity of energy that could be produced from human feces. They reported that a 3–6 wt.% ash content in fresh human feces makes it a viable raw material for gasification. The product gas obtained from human feces at an optimal equivalence ratio (ER) of 0.31 was characterized by 24 MJ/kg and 17 MJ/kg lower heating and exergy values, respectively [53].
Hussein et al. [23] studied the effects of gasifying agents (air, steam, carbon dioxide, and nitrogen) and temperatures ranging from 600 to 1000 °C on the pyrolysis and gasification of chicken manure. It was reported that a higher temperature will produce a higher syngas yield. Regarding the gasifying media, in terms of increasing order, the air medium produced the lowest yield, followed by nitrogen, steam, and carbon dioxide. They revealed that the reaction time is inversely proportional to the energy yield. The feasibility of a two-step gasification route for producing hydrogen gas from cattle manure was examined by studying the temperature impact on not only biochar characteristics, but also product distribution. The biochar from the joint pyrolysis-carbonization was found to have a low volatile percentage composition and high carbon composition at 500 °C. This result suggests that hydrogen can be produced from the two-step gasification process [23].
Hydrothermal gasification is a suitable valorization pathway for animal manure, due to the high moisture content of the materials [58,59]. Nanda et al. [59] explored the feasibility of hydrogen production from horse manure via alkali catalyst-enhanced hydrothermal gasification. The impact of temperature (400–600 °C), two biomass-to-water ratios (BTW) of 1:5 and 1:10, and reaction time (15–45 min) at a pressure range of 23–25 MPa on hydrogen yield was comprehensively explored. The maximum hydrogen yield was obtained at a high temperature of 600 °C and a 1:10 biomass-to-water ratio for 45 min with a 2 wt.% Na2CO3 catalyst. Another study showed that cattle manure could be used as a promising feedstock for hydrothermal gasification with Ni heterogeneous catalysts supported by hydrochar prepared from cattle feed [60]. Liu et al. [61] proposed a mechanism for the transformation of nitrogenous compounds during the hydrothermal gasification of chicken manure (Figure 6). Two main mechanisms were inherent: ionic and free radical degradation, with both mechanisms dependent on temperature. The ionic mechanism occurs at low temperature subcritical or near critical conditions, while the free radical degradation is inherent at high temperatures >400 °C. Some intermediate products, such as amino acids, are also produced via the Maillard reaction. Three main reactions are involved in free radical degradation: steam reforming, pyrolysis, and Maillard reaction. An elevation in temperature led to the migration of nitrogenous compounds in the raw materials to the aqueous liquid, while the Maillard reaction conditions are created by the hydrolysis of proteins and amino acids in the initial phases. As the temperature increases beyond 400 °C, some nitrogen-containing compounds are converted into condensable gases via stream reforming and pyrolysis (Figure 5).

Figure 6.
An overview of the reaction mechanism for nitrogen recovery during the hydrothermal gasification of chicken manure. Adapted from Liu et al. [61].
A summary of the studies related to heterogeneous catalyzed gasification of human and animal waste, kinetics studies, and process integration studies is presented in Table 5. Detailed information on the gasification of different biogenic waste can also be found elsewhere [7,62].
Hydrothermal gasification is still a challenging technology, due to the high temperature and pressure requirement. The mechanism of hydrothermal gasification of human and animal waste is still unclear. Although some researchers have documented the reaction mechanism of liquid feedstock decomposition, such as glycerol in supercritical water [63]. Problems such as corrosion issues, reactor material durability, waste heat utilization, safety, and risk are associated with extreme reaction conditions. Catalysts are often used to reduce the reaction temperature during hydrothermal gasification. However, the harsh environment facilitates the decline in the surface area of metallic catalysts and some structural changes causing significant deactivation, a phenomenon known as catalyst sintering [7].

Table 5.
Summary of the studies related to heterogeneous catalyzed gasification of human and animal waste, kinetics studies, and process integration studies.
Table 5.
Summary of the studies related to heterogeneous catalyzed gasification of human and animal waste, kinetics studies, and process integration studies.
| Type of Gasification and Key Products | Key Findings | References |
|---|---|---|
| Gasification type: Conventional fluidized bed gasification Feedstock: Poultry litter Study focus: Parametric studies and process optimization To investigate the effect of adding limestone (CaCO3), different gasifying agents and temperatures on product gas yield, and cold gas efficiency during gasification |
| Pandey et al. [64] |
| Gasification type: Conventional gasification Feedstock: Chicken manure Study focus: Co-gasification and catalytic studies. Study the synergistic effect of gasifying petroleum coke and chicken manure, while the chicken manure is a catalyst |
| Liu et al. [61] |
| Gasification type: Conventional gasification Feedstock: Human faces Study focus: Thermodynamic and energy analysis with Aspen Plus simulation. Explored the viability of human feces as a raw material for gasification. Estimation of the quantity of energy that could be produced from human feces |
| Onabanjo et al. [53] |
| Gasification type: Conventional gasification Feedstock: Chicken manure Study focus: Parametric studies The effect of gasifying media (air, steam, carbon dioxide, and nitrogen) and temperatures ranging from 600 °C to 1000 °C on the pyrolysis and gasification of chicken manure |
| Hussein et al. [23] |
| Gasification type: Conventional gasification Feedstock: Cattle manure Study focus: Parametric studies The viability of a two-step gasification route for producing hydrogen gas was examined by studying the temperature impact on biochar characteristics and product distribution |
| Xin et al. [58] |
| Gasification type: Hydrothermal gasification Feedstock: Horse manure Catalyst: Homogeneous alkali catalyst including NaOH, Na2CO3, and K2CO3 Study focus: Parametric studies Explored the effect of reaction temperature (400–600 °C), biomass-to-water ratio (1:5 and 1:10), and reaction time (15–45 min) at a pressure range of 23–25 MPa on product yield during horse manure gasification in supercritical water |
| Nanda et al. [59] |
| Gasification type: Conventional gasification Feedstock: Pig manure compost Heterogeneous catalyst: Ni/Al2O3, Ni-loaded brown coal char Study focus: Catalytic effect of supported Ni catalyst during gasification and parametric studies. |
| Xiao et al. [65] |
| Gasification type: Hydrothermal gasification Feedstock: Chicken manure Catalyst: K2CO3 Study focus: Parametric studies, kinetics, and reaction mechanism evaluation |
| Liu et al. [57] |
3.3. Liquefaction
Liquefaction is a biomass-to-liquid (BTL) conversion pathway, similar to pyrolysis, but different in terms of operating temperature (200–450 °C), pressure (5–25 MPa), and the presence of a solvent, usually water or alcohols. It is mostly used for the conversion of wet biomass (wastes) into crude oil, also referred to as biocrude oil [66]. The yield and quality of biocrude oil are dependent on the raw material composition, residence time, catalyst, temperature, pressure, and solvent [67].
Hydrothermal liquefaction (HTL) is different from other hydrothermal processes, such as supercritical water gasification and hydrothermal carbonization (HTC), in terms of the desired products and operating conditions. Figure 7 describes different phase diagrams for hydrothermal processes, as well as the operating conditions ranges. Hydrothermal gasification is favorable at high-temperature supercritical conditions (>400 °C) and the desired product is high-quality syngas [67]. In contrast, HTC is focused on the formation of solid fuels with improved physicochemical properties for subsequent energy and environmental applications.

Figure 7.
Hydrothermal processing condition in water phase diagram. Adapted from [67].
The biocrude oil obtained from liquefaction has a lower oxygen content, is more viscous, and has an elevated HHV, compared to pyrolysis oils [47]. Hydrothermal liquefaction (HTL) offers the advantages of feedstock flexibility and does not require precursor drying. Studies on the liquefaction of human waste focus on developing pathways for nitrogen transformation [68], process optimization, or the development of novel heterogeneous catalysts for improving oil yield and quality [68]. Lu et al. [69] investigated the effects of temperature (260 °C, 300 °C, 340 °C), retention time (10 min, 30 min, 50 min), and total solid content (5%, 15%, 25%) on biocrude yield and nutrient recovery during the HTL of human feces. They revealed that approximately half of the carbon content in human feces was converted to biocrude oil, while 72% of nitrogen was found in the aqueous phase. They also reported that human feces contained metals such as calcium, aluminum, magnesium, zinc, iron, sodium, and potassium. The results showed that human feces are a potential source of energy and nutrient recovery. The authors proposed possible reaction pathways for the conversion of human feces via HTL (Figure 8).

Figure 8.
Proposed reaction pathways involved in the hydrothermal conversion of waste materials, such as human feces to biocrude and value-added chemicals. Adapted from Lu et al. [69]. ((a) hydrolysis; (b) dehydration; (c) decarboxylation; (d) deamination; (e) Maillard reaction; (f) cyclization; (g) polymerization; (h) decomposition).
The pathways in Figure 8 include intermediate reactions, such as (a) hydrolysis; (b) dehydration; (c) decarboxylation; (d) deamination; (e) Maillard reaction; (f) cyclization; (g) polymerization; and (h) decomposition [69]. The hydrocarbons found in the biocrude oil during HTL were produced through the decarboxylation of the fatty acids. It should be mentioned that the fatty acids are from the hydrolysis of lipids in human waste. The ketones and aldehyde functional groups were produced from polysaccharides through a series of intermediate reactions, including hydrolysis, dehydration, and decomposition reactions [69]. In contrast, nitrogenous compounds such as pyridine, pyrrolidine indole, and quinolone are derived from monosaccharides and amino acids through the cyclization and Maillard reactions.
Conti et al. [70] explored the possibility of recovering nutrients and energy production from the biodegradation of animal manure, sewage sludge, and fish sludge, with or without K2CO3 (catalyst) under subcritical (350 °C) and supercritical conditions (450 °C). They reported that all the wastes gave a high yield of biocrude, with fish sludge and sewage sludge giving yields of 50% and 45%, respectively. Moreover, animal manure was able to produce a quality biocrude in the presence of the catalyst and under supercritical conditions. Inorganics, such as calcium, phosphorus, and magnesium, were obtained from the solid phase, as approximately 55–80% and 50–60% of carbon were recovered from fish and swine sludge, respectively.
Li et al. [71] compared the HTL of dairy manure, broiler manure, laying hen manure, swine manure, and beef manure. They revealed that swine manure had the highest biocrude oil yield (30.85%) at 340 °C, while the other manure yields ranged from 15% to 25%. The heavy metals from these manures (copper, zinc, lead, cadmium) are heavily distributed in the solid residue after hydrothermal liquefaction. The influence of temperature, solvent filling rate, and solid–liquid rate on the constituents, features, and yield of bio-oil derived from pig manure was examined by some researchers [27]. The highest yield of the bio-oil was found to be 35.56%, while its heating value ranged from 34.39 to 37.03 MJ/kg. The bio-oil was composed of ketones, nitrogen compounds, organic acids, long-chain hydrocarbons, ethanol, and esters. The yield of the bio-oils had an inverse relationship with the solid–liquid rates, while it increased and decreased with solvent filling rates and temperature, respectively [27]. A summary of previous studies related to the liquefaction of human and animal waste is presented in Table 6.

Table 6.
Summary of past studies on the gasification of human and livestock wastes.
3.4. Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is a thermochemical process for the pre-treatment of high moisture content biomass to make it viable for energy production. HTC uses relatively low temperatures and is suitable for any kind of biomass feedstock [75]. HTC can convert human and animal waste into solid hydrochar, which has better physicochemical characteristics than raw feedstocks [76] and produces liquid products that contain organic and inorganic value-added chemicals. The HTC hydrochar exhibits lower O/C and H/C ratios, compared to dry torrefaction, and turns into more lignin or coal-type materials [77]. HTC hydrochar can be used in a wide range of processes, such as soil amendment [78], CO2 capture [78], nanoparticles (for making composites) [79], energy production [80], and water purification [81], due to their unique physicochemical properties [82].
HTC could be adopted for the sustainable and environmentally benign treatment of human and animal waste for several environmental and energy applications. Some researchers have explored the conversion of human feces to hydrochar via HTC [83]. The effect of temperature (180, 210, and 240 °C) and reaction times (30, 60, and 120 min) during the HTC of human excreta reveals that hydrochar yield decreased with elevating HTC temperature [83]. In addition, the calorific value of the produced hydrochar rose from 24.7 to 27.6 MJ/kg with a rise in temperature. Afolabi et al. [84] showed that an hydrochar, with improved HHV ranging from 19.79–25.01 MJ/kg, was produced during the microwave HTC of raw human fecal sludges with increasing temperature ranges of 160–200 °C. Some researchers also assessed the energy efficiency during the HTC of human fecal wastes [85]. The energy balance results obtained during the HTC of fecal waste conducted at 200 °C, with a reaction time of 30 min, reveals that feces with 25% solids requires 63–65% of the overall reactor input energy for efficient heating. On the contrary, feces with lower solids (15% solid) would need lower energy (about 62–64%) [85]. Another study showed that HTC could be used as an effective technology for the mitigation of SARS-CoV-2 in sewage sludge [86].
HTC can also be used to upgrade the properties of livestock manure for subsequent fuel applications. Jang et al. [87] reported that the HTC of livestock manure reduced the amount of C–O and C–H functional groups and elevated the number of aromatic C–H functional groups. Additionally, HTC increased the fixed carbon content and energy density of livestock manure, while the H/C and O/C ratios decreased. HTC could also be used aa nutrient recovery strategy from animal waste. Qaramaleki et al. [88] showed that cow manure is a promising source of phosphorus and nitrogen via HTC. However, the extent of phosphate extraction is dependent on the reaction temperature, acid addition, and acid concentration. Increasing HTC temperature and the addition of either citric acid or HCl led to an improved phosphate recovery in the aqueous phase. HTC could also be used for the valorization of anaerobic digestate to promote the circular economy and optimize material utilization [89].
4. Biological Conversion of Human and Animal Waste
As mentioned earlier, the biological conversion process is a waste-to-energy process that uses microorganisms to convert biogenic materials into energy or other value-added products. Anaerobic digestion (AD) and fermentation are the two main biological processes used for the valorization of human and animal waste. Both processes involve microorganisms breaking down organic matter in the absence of oxygen. Detailed information about the advantages and limitations of each process, as well as the reaction pathways, can be found elsewhere [8]. Most of the studies related to resource recovery from human and animal waste via biological conversion pathways mostly focus on anaerobic digestion. In contrast, fermentation uses microorganisms to convert sugars into carbon dioxide and ethanol.
4.1. Anaerobic Digestion
AD is a biological process that converts wastes into biogas and digestate under anaerobic conditions. These wastes include animal manure, human waste, sewage sludge, food waste, and lignocellulosic materials. Its products, which include digestate and biogas, have proven to be useful. The former can be used as a fertilizer because it contains nutrients such as nitrogen, potassium, and phosphorus. In contrast, the former can be used to generate electricity and heat, thus reducing the overreliance on fossil fuels.
AD of biogenic waste proceeds in four different stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [90]. In hydrolysis, compounds with high molecular mass (polymers) are deconstructed into lower molecular weight compounds (monomers). This stage is of paramount importance because it is where microorganisms in the digestion medium have access to the material’s energy potential [90,91]. Acidogenic bacteria further decompose the remaining compounds into volatile fatty acids (VFAs), carbon dioxide, ammonia, and other byproducts in the acidogenesis stage. The next stage (acetogenesis) involves acetogens digesting the monomers formed in the acidogenesis stage to form acetic acid, hydrogen, and carbon dioxide. Methanogens then convert the previously formed products into methane, carbon dioxide, and water, which constitute biogas.
AD of human and animal waste for biogas production has been the focus of some research studies, as shown in Table 7. Although, AD could also be adopted for the treatment of sludge. A survey of the available literature shows that mono-feedstock digestion is more common. The use of a single feedstock has been reported to be problematic because of several challenges, such as poor biogas yield, limited year-round feedstock availability, and digester instability [92]. To some extent, these challenges can be overcome through different methods, such as pH adjustment [93], manipulation of the digestion time [94], intermittent feeding [95], multistage AD processes [96], the introduction of external microorganisms, and co-digestion [97]. Among these options, co-digestion of more than one feedstock has been reported to be very successful in overcoming the challenges associated with mono-feedstock digestion. Co-digestion results in higher biogas yields, improved digester stability, and, in particular, the combination of multiple feedstocks, with their unique properties providing a more balanced nutrient composition, a diversified microbial community, and a better buffering capacity against the accumulation of VFAs [98]. For example, Ihoeghian et al. [12] investigated the co-digestion of cattle rumen content and food waste. They found that both materials had desirable characteristics necessary for biogas production. Their findings showed that co-digestion enhanced the carbon-to-nitrogen ratio with an attendant-positive impact on biogas yield and pH of the AD medium. A 50:50 ratio of cattle rumen content and food waste was found to be optimum for biogas production, with a maximum cumulative biogas yield of 320.52 mL gVS-1. The profile of pH, total ammonia nitrogen, and volatile fatty acids were improved via co-digestion.
Ma et al. [99] employed the meta-analysis approach to compare methane yield between mono-digestion and co-digestion of animal manure with other feedstocks. A higher methane yield was obtained from the co-digestion (animal manure mixed with other feedstock (249 Lkg−1 was recorded), compared to mono-digestion (171 Lkg−1). The superior methane yield was attributed to the high volatile solid concentration and the carbon-to-nitrogen ratio of the manure. In another study, an equal proportion of rice straw and human feces (50:50) was the optimal ratio that produced the highest biogas yield (61% yield, volume of 6391 mL) during the anaerobic co-digestion of rice straw and human feces. In other studies, Kaur and Kommalapati [100] reported a biogas yield of 262 mlgVS−1 for the 20:80 co-digestion of goat manure and cotton gin trash, while a value of 250 mlgVS−1 was reported by Alfa et al. [101] for the 25:75 co-digestion of cow dung and horse dung. Hazmah et al. [102] showed that co-digestion of pineapple waste with cow dung improved the process stability, in terms of the C/N ratio, total ammonia nitrogen, VS removal, and pH. Arifan and Sumardiono [103] studied the effectiveness of co-digestion of chicken manure, cow manure, and liquid tofu waste for producing biogas. They then revealed that the best proportion that gave the highest biogas yield was 15% chicken manure, 70% cow manure, and 15% liquid tofu waste, totaling 3252 mL of biogas. Moreover, they also reported a huge decrease in BOD and COD at 95% and 98%, respectively.
The AD process is a complex series of biochemical reactions that involve the conversion of various biomolecules by a diverse microbial community [104]. Direct interspecies electron transfer (DIET) between the microorganisms that facilitate the biodegradation of complex biodegradable compounds and methanogens has been reported to be responsible for facilitating the syntrophic conversion process during the methanogenesis stage of AD [105]. The interruption of this syntrophic process is often the cause of digester instability, which results from the lowering of the pH, as a consequence of the accumulation of VFAs [106]. Research efforts have been directed toward mitigating this situation, with a particular focus on the introduction (into the digester) of conductive materials that can also serve as immobilizing media for microbes [107]. Examples of materials that have been assessed in this regard include polyethylene [108], glass [109], polyvinyl alcohol [110], activated carbon [111], oxides of iron [112], and biochar [113]. The porous nature of these materials provides the necessary surface area for microbial immobilization, which has an overall effect of shortening the AD start-up phase and enhancing feedstock digestibility, especially when the feedstock is a recalcitrant lignocellulosic material.

Table 7.
Summary of past studies on anaerobic digestion and fermentation of human and livestock wastes.
Table 7.
Summary of past studies on anaerobic digestion and fermentation of human and livestock wastes.
| Authors | Aim of Study | Key Findings |
|---|---|---|
| Ihoeghian et al. [12] | Conversion pathway: Anaerobic digestion Investigated and established the best co-digestion ratio for cattle rumen content and food waste for synergistic biogas production. | A 50:50 ratio of cattle rumen content and food waste was recommended for biogas production Co-digestion of cattle rumen content and food waste enhanced biogas production |
| Ma et al. [99] | Conversion pathway: Anaerobic digestion Adopted the meta-analysis approach to compare the methane yield between mono-digestion and co-digestion of animal manure with other feedstock. | Higher methane yield was obtained from the co-digestion (animal manure mixed with other feedstock) when compared to mono-digestion. |
| Adjama et al. [114] | Conversion pathway: Anaerobic digestion To investigate the proportions of anaerobic co-digestion of rice straws and human feces that will give the optimal biogas yield. | An equal ratio of rice straws and human feces produced the highest biogas yield (61% percentage yield). |
| Arifan et al. [103] | Conversion pathway: Anaerobic digestion To study the effectiveness of co-digestion of chicken manure, cow manure, and liquid tofu waste for producing biogas. | The best combination of feed materials that produced the optimum yield are as follow: 15% chicken manure, 70% cow manure, and 15% liquid tofu waste. |
| Eduok et al. [115] | Conversion pathway: Anaerobic digestion To compare the effectiveness of water, human urine, and sodium bicarbonate (Na2CO3) as a buffering agent for the codigestion of poultry feces and lignocellulosic biomass for the generation of biogas. | The urine-buffered reactors produced the highest yield up to five times greater than those buffered with sodium bicarbonate and water. |
| Silwadi et al. [116] | Conversion pathway: Anaerobic digestion To compare the biogas yield and composition resulting from mono-digestion (cow, chicken, and camel) and co-digestion (mixtures of cow, chicken, and camel). | The co-digestion gave a higher yield than the mono-digestion. Biogas yield increased 5 (co-digestion with chicken manure), 12 (co-digestion with cow manure), and 28 (co-digestion with camel manure) times when compared to mono-digestion. |
| Pan et al. [117] | Conversion pathway: Anaerobic digestion Investigated the role of wood-based biochar during AD of chicken manure. | 25% reduction in TAN accumulation. 69% increase in biogas production compared to the control. |
| Kizito et al. [118] | Conversion pathway: Anaerobic digestion Investigated the role of biochar on the removal of TAN during AD of piggery waste | 60% reduction in TAN accumulation which enhanced AD stability. |
| Recebli et al. [119] | Conversion pathway: Fermentation To compare the daily biogas production rate from poultry manure and bovine animal manure. | Approximately 0.83 m3 and 6.33 m3 of biogas are produced daily from poultry manure and bovine animal manure, respectively. The lower heating value of the produced methane and biogas is 34,000 KJ/m3 and 21,000 kJ/m3 respectively. |
| Zlateva et al. [120] | Conversion pathway: Fermentation To determine the quantity of biogas and energy produced from the anaerobic fermentation of cow manure, chicken manure, and pig manure. | It was revealed that approximately 556,000 kWh per annum of energy is produced. At the same time, 55,660 methane is released per annum, with pig manure, cow manure, and chicken manure contributing to the release of 7493 Nm3CH4/a, 234,111 Nm3CH4/a, and 24,756 Nm3CH4/a, respectively. |
| Andreev et al. [121] | Conversion pathway: Fermentation To subject human urine to lactic acid fermentation to reduce its odor and enhance its fertilizing ability. | The pH of the treated urine ranges from 3.8–4.7 compared to the untreated which is 6.1. The ammonia composition decreases by 20–30% compared to the untreated, whose ammonia composition increases by 30% owing to hydrolysis. |
| Andreev et al. [122] | Conversion pathway: Fermentation To subject human excreta to lactic acid fermentation to reduce the loss of nutrients and the number of pathogens present in them. | Human excreta is a promising source of nutrients via lactic acid fermentation. The nutrient loss is lowered in the presence of lactic acid with 7–10 days fermentation. |
| Adjama et al. [114] | Conversion pathway: Fermentation To investigate anaerobic fermentation chicken manure and straw mixtures in a batch reactor at a temperature of 37 °C for ten weeks | The straw ratio of 3% gave the highest methane yield of 292.87 mLgVS−1 which is 17% greater than pure chicken manure. |
| Dong-Jun Lee et al. [123] | Conversion pathway: Fermentation Evaluate the impact of two different pretreatment methods (NaOH and H2SO4) on the bioethanol yield during horse manure fermentation. | Alkaline/enzyme-hydrolysates showed higher bioethanol productivity (0.075 g L−1h−1) than those of acid/enzyme-hydrolysates (0.050 g L−1h−1). Fermentation of hydrolysates produced less inhibitory compounds due to the alkaline pretreatment. |
Anaerobic co-digestion of lignocellulosic materials with animal manure is also a promising strategy to maintain its stability and keep the pH between 6.8 and 7.2 [115]. Human urine has been shown to be a promising waste material with a similar composition to buffer materials used during anaerobic digestion [115]. Eduok et al. [115] compared the performance of water, human urine, and sodium bicarbonate (Na2CO3) as buffering agents for the co-digestion of poultry feces and lignocellulosic biomass for the generation of biogas. The urine-buffered reactors were found to have a mean volume of 37 ± 8 mL gVS−1 to 101 ± 18 mL gVS−1. These values are 1 to 5 times greater than those of reactors buffered with sodium bicarbonate and water. The positive effect of urine as a buffering agent for biogas production is reflected in its volatile fatty acid concentration, which was reported to fall between 396 and 1400 mg L−1, in contrast to that of water (386 and 3109 mg L−1). Another study showed that human urine could be used as a co-feedstock to improve the gas yield during anaerobic co-digestion with cassava liquid waste [124,125]. Twizerimana et al. [126] further confirmed that source-separated human urine could help stabilize the anaerobic digestion of cotton yarn waste and improve the gas yield. Significant fluctuations in pH during AD are not desirable, as they can negatively impact microbial activity. This is important, as reduced fluctuations will encourage a better performance from the microbes, as their performance is highly pH-dependent [127].
Some studies have explored the role of biochar in process stability during AD. Biochar’s ability to confer stability to an AD system has been linked to its buffering ability, which is a consequence of its alkaline nature. Pan et al. [117] reported that wood-based biochar reduced the TAN concentration by 25%, in comparison with the control, which resulted in enhanced biogas production, while Kizito et al. [118] reported a 60% TAN reduction for the digestion of biochar-amended piggery waste, with a consequent increase in biogas production.
4.2. Fermentation
Fermentation is a biological process that deals with the decomposition of wastes (manures) into biogas and digestate at temperatures appropriate for mesophilic or thermophilic bacteria [128]. The fermentation process could either be dry or wet fermentation based on the moisture content of the feed material. For the former, the feed material usually has over 85% water content, while for the latter, the feed material usually has approximately 60% water content. The fermentation process is influenced by the pH of the medium, temperature, raw materials composition, sludge stirring, and humidity [128,129].
During the fermentation of manure, the feed is collected and transferred to a fermentation tank. The collected manure will then be blended with the reactor’s agitator, so that they become homogenized for the microorganisms to decompose the manure into methane (CH4), carbon dioxide (CO2), and organic fertilizer in an anaerobic environment. The fermentation process normally lasts up to 23 days, either for thermophilic-type (33–34 °C) or mesophilic-type (53–55 °C) conditions [120].
Several studies have documented the fermentation of human and animal waste for biofuel production, as shown in Table 7. Recebli et al. [119] conducted a comparative study to determine the daily biogas production rate from poultry and bovine animal manure. They separately filled 375 kg of poultry manure blend and 350 kg of bovine animal manure into 0.5 m3 of the fermentation reactor. Their results revealed that approximately 0.83 m3 and 6.33 m3 of biogas were produced daily from poultry manure and bovine animal manure, respectively. Zhang et al. [130] showed that the co-fermentation of waste-activated sludge and animal manure promotes glucocorticoid degradation. Their study revealed that chicken manure is a better co-fermentation precursor than dairy manure.
Fertilizers made from human excreta can cause eutrophication in aquatic bodies if there is runoff. Andreev et al. [121] explored the lactic acid fermentation of human urine to reduce its odor and enhance its fertilizing ability. To do this, they fermented the urine with bacterial inoculum in a closed jar for approximately 36 days. The pH of the treated urine ranged from 3.8–4.7 to the untreated 6.1, and the ammonia composition decreased by 20–30%, compared to the untreated, whose ammonia composition increased by 30%, owing to hydrolysis. The results showed that the treated urine has a reduced odor intensity and can be a good fertilizer because it lowers the volatilization of ammonia.
Bioethanol is an environmentally benign fuel that provides comparable efficiency at a lower cost, when compared with gasoline. AD intermediates, including the fermentable sugars obtained from hydrolysis and acetogenesis, can be used as useful feedstocks for bioethanol production via fermentation. The process is characterized by faster reaction kinetics and lower CO2 production. Some researchers have demonstrated that lignocellulosic feedstock, including animal manure, can be converted to bioethanol and green chemicals via enzyme hydrolysis and fermentation or through simultaneous saccharification and fermentation processes [123,131]. Figure 9 shows the sequential steps involved in the simultaneous saccharification and fermentation process. Doreswamy et al. [132] adopted the conventional anaerobic fermentation method for the production of bioethanol from piggery excreta. The authors reported approximately 89.59% high-purity bioethanol production and a theoretical yield of 0.765–1.02 gm/200 mL. Yan et al. [133] reported that the use of Saccharomyces cerevisiae for simultaneous saccharification and fermentation could produce bioethanol from pretreated and anaerobically digested cow dung, with yields of 0.19 and 0.13 g/g-raw biomass.

Figure 9.
An overview of the sequential steps involved in the simultaneous saccharification with the fermentation process.
5. Nutrient and Fertilizer Recovery from Human and Animal Waste
Fertilizer is known for its beneficial impact on agricultural practices, with the common impact being enhancing soil fertility. Hence, its use has increased tremendously over the past years. This is reflected in its global demand, which rose from 186.6 Mt in 2016 to 194.4 Mt in 2018, and this value is projected to increase every year [134]. The fertilizer could be synthetic (chemical fertilizer) or organic fertilizer (animal manure manure-based fertilizers and human waste-based fertilizers).
Chemical fertilizers are inorganic fertilizers that enhance plant growth when added to the soil. They are also known as synthetic fertilizers. They usually have an equal distribution of the three important nutrients (nitrogen, potassium, and phosphorus) needed for plant growth [135]. They include urea, ammonium chloride, ammonium nitrate, and ammonium sulphate. However, this fertilizer is known for its unstable and soaring price and environmental degradation (polluting water bodies, acidifying soil, and global warming contributor), a result of its increased and continuous usage [136]. Hence, there is a need to switch to a cost-effective fertilizer type that is also environmentally friendly.
Organic fertilizers, on the other hand, which are bio-based, are the perfect alternative to chemical fertilizers because they also increase the productivity and yield of crops, improve the quality of soil, and enhance sustainability, while being friendly to the environment. They are rich in carbon and essential nutrients needed for plant growth. They cause minimal or no degradation, compared to synthetic fertilizers [137]. They are usually animal manure or human wastes (urine and feces).
Over the years, human feces have demonstrated excellent fertilization prospects by releasing organic matter and plant nutrients that help improve the structure of the soil and prevent erosion. Unprocessed human feces could be harmful to the soil, as they contain pathogens that reduce soil fertility [138]. Composting can be employed to eliminate all pathogens in human feces, so that they can be used to produce fertilizers. Human feces contain nutrients (potassium, nitrogen, sulphur phosphorus, magnesium, and calcium) for plant growth. Comparatively, human feces are richer in potassium, phosphorus, and organic matter than human urine [19]. Moya et al. [139] conducted a comparative study on the effect of artificial fertilizers and human-based fertilizers on the growth of French beans in Nairobi. They then reported that human feces-based fertilizers gave a 30% yield increase, compared to artificial fertilizers.
Human urine also contains potassium, phosphorus, sulphur, and nitrogen. Plants easily absorb these nutrients because they are in ionic forms [140]. Pathy et al. [141] reported that NPK biofertilizers can be obtained from human urine. Akpan-idiok et al. [142] compared the effect of human urine-based fertilizers and inorganic fertilizers on the Abelmoschus esculentus crop. It was reported that human urine-based fertilizers gave a higher yield of the crop than inorganic fertilizers.
The characteristics of urine are affected by the quantity of water taken in, the organism’s body size and feeding habits, the source of the urine, and the well-being of the microorganisms that excrete the urine. Urine is made up of organic compounds (primarily creatine, uric acid, and creatinine) and inorganic compounds (primarily nitrogen, potassium, and phosphorus) [29]. The percentage concentration of the critical elements in urine mainly determines the various components of urine. Ions such as Na+, Mg2+, Cl−, and Cu2+ are also constituents of urine. They enhance the growth of plants. The pH of stored urine is different from that of fresh urine because of the former hydrolyses ammonia and bicarbonate in a germ-free environment. The products (ammonia and bicarbonate) are released into the environment, while the residual bicarbonate increases the pH of the stored urine. Urine is reported to typically contain 1 g and 9.1 g of phosphorus and nitrogen per litre, respectively. The high composition of nutrients in unadulterated urine enables the development of more efficient energy recovery techniques and practical nutrient recovery concepts [29]. The developed nutrient recovery concept will help mitigate the environmental degradation that nutrients (potassium, phosphorus, and nitrogen) are likely to cause.
5.1. Overview of Nutrient Recovery Technologies
Nutrient recovery technologies are adopted for the recovery of nutrients from human and animal waste. Primary macronutrients, such as phosphorous, nitrogen, and potassium, as well as secondary macronutrients, including sulphur, magnesium, and calcium, are relevant nutrients that are in demand in several industries, such as the food, pharmaceutical, and fertilizer industries. Some of these technologies, including struvite precipitation, ammonia stripping, evaporation, and selective adsorption of nutrients, are briefly discussed as follows. For detailed information and a technical background of each recovery method, the readers are referred to the excellent review by Veneeckhaute et al. [143]. Patel et al. [144] also provided a comparative review of different nutrient recovery technologies from human urine.
5.1.1. Selective Adsorption
This technique employs an adsorbent to obtain nutrients from urine that are suitable for soil conditioning and amendment. It is mainly used to recover nitrogen with a zeolite adsorbent, which is composed of alumina and silica [145]. It can also be used to recover phosphorus if combined with struvite precipitation. A magnesium oxide (MgO) concentration of 0.5 mg/L and a supernatant phosphorus concentration of 10 gm−3 are reported to give the highest recovery of nitrogen. In contrast, 0.015 mg/L of zeolite with 1000 gNm−3 of supernatant nitrogen concentration gave the highest recovery of nitrogen. The nitrogen obtained from this technique can be used as a soil conditioner because it has proven helpful in enhancing the water retention and nutrients of soils with low nutrients [69].
5.1.2. Struvite Precipitation
This technology can be used for the simultaneous recovery of phosphorus and nitrogen. It is also employed in the treatment of wastewater. Struvite is an equimolar ratio of the anion (phosphate) and cations (ammonium ions and magnesium). Struvite precipitation for nutrient recovery can be made more effective by adding magnesium to urine. The precipitate produced can then be washed and filtered after it has been dried. Ahmed et al. [146] revealed that 12.5% of phosphorus and 5.7% of nitrogen constitute dry and pure struvite. The higher phosphorus concentration could be traced to the drying and precipitation processes. With this method, it is possible to recover nitrogen in the form of ammonium and phosphorus.
5.1.3. Ammonia Stripping
Ammonia stripping is used to recover ammonia from urine. During this process, ammonia is transferred between the liquid and gaseous phases. The recovery of ammonia is triggered by the pH, which can be increased by adding sodium hydroxide (NaOH) and calcium oxide (CaO) [146]. Papurello et al. [147] reported a 97% ammonia recovery from their study, where ammonia was stripped in a batch reactor with air, followed by its absorption in sulfuric acid. The product of this reaction, ammonia sulfate solution, can be used as a fertilizer. Stripping stored urine at 0.4 bar and 40 °C, followed by adsorbing the resulting gas at 5 bar and 20 °C, resulted in 10% ammonia recovery.
5.1.4. Evaporation
This technique is a volume reduction method because it helps reduce the water content in urine, thereby increasing the concentration of other nutrients. This technology is usually faced with problems such as energy and ammonia loss. The former can be prevented by recovering the energy, while the latter can be addressed by employing an acidification process [146]. Table 8 provides a summary of different nutrient recovery technologies and their efficiencies.

Table 8.
Summary of nutrient recovery technologies from human and livestock urine.
6. Bibliometric Research Trends on Resource Recovery from Human and Animal Waste
A bibliometric analysis is presented in this article to investigate the development of resource recovery from human and animal waste and identify the trends in publishing, dominant contributing authors, institutions, countries, potential publishing sources, and the most cited publications in this research area. Through bibliometric analysis, gaps in research were identified and presented in the next section. A summary of the methodology used in developing the bibliometric analysis is presented in Figure 10. The Web of Science (WoS) Core Collection database was used to compile the data used in the bibliometric analysis. The database was selected because of its large spectrum of data, compared to other databases. All research articles and proceeding papers related to resource recovery from human and animal waste for the last fifteen years were selected, with keywords such as animal manure, human feces, and urine (Figure 10). All relevant data were imported into the VOS viewer to create network maps for co-authorships, countries, and institutions. In addition, maps were developed for journal participation and keywords co-occurrence.

Figure 10.
Flow chart of the bibliometric analysis methodology.
Figure 11 shows the network visualization maps of journals with a minimum of five citations per source between 2007 and 2022. It should be mentioned that the size of the circle on a map was determined by the number of citations. Therefore, journals with relatively large circles are highly cited. The journals were grouped into six distinct clusters, based on the circle colors. The journals with the highest number of citations in each cluster include scientific reports journal under nature portfolio, PLoS ONE, applied and environmental microbiology, nutrients, science of the total environment, and xenobiotica. The strong participation in journals such as scientific reports, science of total environments, and PLoS ONE is expected, since most of them are focused on resource recovery from hazardous waste materials, including human and animal waste. Some of them also explore different environmental remediation techniques.
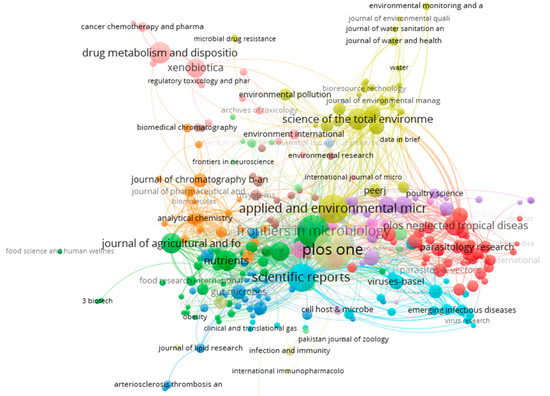
Figure 11.
Network visualization map of journals with a minimum of 5 publications related to resource recovery from human and animal waste (2007–2022).
Figure 12 presents the co-authorship map of countries collaborating in resource recovery from human and animal waste. The map outlines how countries relate to each other in the research area. The size of the circle represents the collaboration intensity of each country. In this case, the USA, China, and England are the leading countries, in terms of collaboration intensity. This could be attributed to the significant amount of investment in new technologies from these countries. Moreover, the line thickness indicates the link strength between two items, while the distance between two countries indicates the similarity between them.

Figure 12.
Co-authorship map of countries collaborating in resource recovery from human and animal waste.
The line thickness and distance analysis in Figure 12 shows that there is a significant research collaboration between the USA and other closer countries in the cluster. The institution participation map presented in Figure 13 also confirms that most of the institutions reported to be actively involved in resource recovery from human and animal waste research are found in China and the USA. The University of Illinois, University of California Davis, the Chinese Academy of Sciences, Zhejiang University, and the University of Reading had the highest number of publications, compared to the rest of the map.

Figure 13.
Network visualization map showing organizations’ contributions.
7. Conclusions and Future Research Directions
Over the years, human and animal wastes have caused pollution (groundwater, soil, air, and land pollution), which has resulted in environmental degradation. This has resulted in researchers looking for a means to reuse, recycle, or recover byproducts from waste. Biological processes (fermentation and anaerobic digestion) and thermochemical methods (pyrolysis, gasification, and liquefaction) are promising valorization pathways. The present study provides an overview and the research progress in the valorization of human and animal waste via thermochemical and biological conversion pathways. While both pathways are promising routes for the conversion of waste into biofuels and value-added materials, they face several limitations. Thermochemical processes are expensive and require extremely high temperatures. In contrast, biological processes often produce low biofuel yields and require extended processing times. Therefore, an integrated biorefinery combining the two processes is suggested for the effective valorization of human and animal waste.
A bibliometric analysis was also presented in this study. The analysis confirmed that the USA, China, and England are the most productive countries in this research area. The University of Illinois, University of California Davis, the Chinese Academy of Sciences, Zhejiang University, and the University of Reading had the highest number of publications. Based on the results presented herein, the following future research gaps are observed.
- Developing effective and safe methods for processing human and animal waste is needed. There is a need for safe and effective methods for processing hazardous waste to optimize nutrients and resource recovery.
- It is imperative to examine the most effective ways to use the recovered resources. Once resources have been recovered from human or animal waste, there is a need to determine the most effective ways to use them, such as for agricultural purposes or as a source of energy, while considering the environmental impacts of different utilization methods.
- Understanding the potential impacts of using recovered resources: It is important to understand any potential negative impacts of using recovered resources on the environment on a large scale. Usually, a cradle-to-grave lifecycle assessment should be performed.
- Developing technologies for the on-site recovery of resources: There is a need for technologies that can be used to recover resources from feces on-site, such as at a wastewater treatment plant or in a portable system. Offsite or district waste processing facilities with improved heat optimization and materials recovery could also be a viable alternative.
Author Contributions
Conceptualization, T.J, A.N.A. and J.A.O.; methodology, C.C.O., T.J., A.N.A., J.A.O., P.U.O., P.P.I., F.G., O.A, A.A.A. and J.A.O.; investigation, C.C.O., T.J., A.N.A., J.A.O., P.U.O., P.P.I., F.G., O.A., A.A.A. and J.A.O.; software and data processing; J.A.O. and P.U.O.; writing original draft, C.C.O., T.J., A.N.A., J.A.O., P.U.O., P.P.I., F.G., O.A, A.A.A. and J.A.O.; writing review draft, C.C.O., P.U.O., F.G. and J.A.O. project administration, J.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krounbi, L.; Enders, A.; van Es, H.; Woolf, D.; von Herzen, B.; Lehmann, J. Biological and thermochemical conversion of human solid waste to soil amendments. Waste Manag. 2019, 89, 366–378. [Google Scholar] [CrossRef] [PubMed]
- WHO. Population Practising Open Defecation (%). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4823 (accessed on 20 January 2023).
- Hunter, B.; Deshusses, M.A. Resources recovery from high-strength human waste anaerobic digestate using simple nitrification and denitrification filters. Sci. Total. Environ. 2019, 712, 135509. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.A.; Umar, L.W. Diarrhoeal Disease. Trop. Dr. 2000, 30, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Szogi, A.A.; Vanotti, M.; Ro, K. Methods for Treatment of Animal Manures to Reduce Nutrient Pollution Prior to Soil Application. Curr. Pollut. Rep. 2015, 1, 47–56. [Google Scholar] [CrossRef]
- Orner, K.D.; Mihelcic, J.R. A review of sanitation technologies to achieve multiple sustainable development goals that promote resource recovery. Environ. Sci. Water Res. Technol. 2018, 4, 16–32. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. Process. Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Zhu, Z.; Zhang, Y.; Zhao, Y.; Li, R.; Watson, J.; Li, B.; Liu, Z. Simultaneous production of biocrude oil and recovery of nutrients and metals from human feces via hydrothermal liquefaction. Energy Convers. Manag. 2017, 134, 340–346. [Google Scholar] [CrossRef]
- Min, K.J.; Park, K.Y. Economic feasibility of phosphorus recovery through struvite from liquid anaerobic digestate of animal waste. Environ. Sci. Pollut. Res. 2021, 28, 40703–40714. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Igbokwe, V.C.; Ezugworie, F.N. Decentralized Anaerobic Digestion Technology for Improved Management of Human Excreta in Nigeria. In Anaerobic Biodigesters for Human Waste Treatment; Springer: Cham, Switzerland, 2022; pp. 137–163. [Google Scholar] [CrossRef]
- Ihoeghian, N.A.; Amenaghawon, A.N.; Ajieh, M.U.; Oshoma, C.E.; Ogofure, A.; Erhunmwunse, N.O.; Edosa, V.I.; Tongo, I.; Obuekwe, I.S.; Isagba, E.S.; et al. Anaerobic co-digestion of cattle rumen content and food waste for biogas production: Establishment of co-digestion ratios and kinetic studies. Bioresour. Technol. Rep. 2022, 18, 101033. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhang, Z.; Nghiem, L.D.; Wang, Q. Urine pretreatment significantly promotes methane production in anaerobic waste activated sludge digestion. Sci. Total. Environ. 2022, 853, 158684. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oa, S.-W. Resource-recovery processes from animal waste as best available technology. J. Mater. Cycles Waste Manag. 2015, 18, 201–207. [Google Scholar] [CrossRef]
- Kirchmann, H.; Pettersson, S. Human urine—Chemical composition and fertilizer use efficiency. Nutr. Cycl. Agroecosyst. 1994, 40, 149–154. [Google Scholar] [CrossRef]
- Rout, P.R.; Pandey, D.S.; Haynes-Parry, M.; Briggs, C.; Manuel, H.L.C.; Umapathi, R.; Mukherjee, S.; Panigrahi, S.; Goel, M. Sustainable Valorisation of Animal Manures via Thermochemical Conversion Technologies: An Inclusive Review on Recent Trends. Waste Biomass-Valorization 2022, 14, 553–582. [Google Scholar] [CrossRef]
- Olugasa, T.T.; Odesola, I.; Oyewola, M. Energy production from biogas: A conceptual review for use in Nigeria. Renew. Sustain. Energy Rev. 2014, 32, 770–776. [Google Scholar] [CrossRef]
- Malomo, G.A.; Madugu, A.S.; Bolu, S.A. Sustainable Animal Manure Management Strategies and Practices. In Agricultural Waste and Residues; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Zseni, A. Human Excreta Management: Human Excreta as an Important Base of Sustainable Agriculture. Proceedings of the 4th MAC 2015, Prague, Czech Republic, 20–21 February 2015; MAC Prague consulting Ltd.: Prague, Czech Republic, 2015. [Google Scholar]
- Tsai, W.-T.; Huang, C.-N.; Chen, H.-R.; Cheng, H.-Y. Pyrolytic Conversion of Horse Manure into Biochar and Its Thermochemical and Physical Properties. Waste Biomass Valorization 2015, 6, 975–981. [Google Scholar] [CrossRef]
- Afolabi, I.C.; Epelle, E.I.; Gunes, B.; Güleç, F.; Okolie, J.A. Data-Driven Machine Learning Approach for Predicting the Higher Heating Value of Different Biomass Classes. Clean Technol. 2022, 4, 1227–1241. [Google Scholar] [CrossRef]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional characteristics and energy potential of Chinese animal manure by type and as a whole. Appl. Energy 2015, 160, 108–119. [Google Scholar] [CrossRef]
- Hussein, M.; Burra, K.; Amano, R.; Gupta, A. Temperature and gasifying media effects on chicken manure pyrolysis and gasification. Fuel 2017, 202, 36–45. [Google Scholar] [CrossRef]
- Yacob, T.W.; Fisher, R.; Linden, K.G.; Weimer, A.W. Pyrolysis of human feces: Gas yield analysis and kinetic modeling. Waste Manag. 2018, 79, 214–222. [Google Scholar] [CrossRef]
- Nitsche, M.; Hensgen, F.; Wachendorf, M. Energy Generation from Horse Husbandry Residues by Anaerobic Digestion, Combustion, and an Integrated Approach. Sustainability 2017, 9, 358. [Google Scholar] [CrossRef]
- Chong, C.T.; Mong, G.R.; Ng, J.-H.; Chong, W.W.F.; Ani, F.N.; Lam, S.S.; Ong, H.C. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Zheng, X.; Liu, F.; Wang, A.; Zou, D.; Yuan, J.; Xiao, Z. Thermochemical liquefaction of pig manure: Factors influencing on oil. Fuel 2020, 264, 116884. [Google Scholar] [CrossRef]
- Islam, M.N.; Park, J.-H. A short review on hydrothermal liquefaction of livestock manure and a chance for Korea to advance swine manure to bio-oil technology. J. Mater. Cycles Waste Manag. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Salana, S.; Banerji, T.; Kumar, A.; Singh, E.; Kumar, S. Resource Recovery-Oriented Sanitation and Sustainable Human Excreta Management. In Sustainable Resource Management: Technologies for Recovery and Reuse of Energy and Waste Materials; Wiley VCH: Weinheim, Germany, 2021; pp. 109–136. [Google Scholar] [CrossRef]
- Biswas, J.K.; Rana, S.; Meers, E. Bioregenerative Nutrient Recovery from Human Urine: Closing the Loop in Turning Waste into Wealth. In Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Simha, P.; Ganesapillai, M. Ecological Sanitation and nutrient recovery from human urine: How far have we come? A review. Sustain. Environ. Res. 2017, 27, 107–116. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Rowles, L.S.; Morgan, V.L.; Li, Y.; Zhang, X.; Watabe, S.; Stephen, T.; Lohman, H.A.C.; DeSouza, D.; Hallowell, J.; Cusick, R.D.; et al. Financial Viability and Environmental Sustainability of Fecal Sludge Treatment with Pyrolysis Omni Processors. ACS Environ. AU 2022, 2, 455–466. [Google Scholar] [CrossRef]
- Selim, O.M.; Amano, R.S. Co-Pyrolysis of Chicken and Cow Manure. J. Energy Resour. Technol. 2021, 143, 011301. [Google Scholar] [CrossRef]
- Beik, F.; Williams, L.; Brown, T.; Wagland, S. Development and prototype testing of a novel small-scale pyrolysis system for the treatment of sanitary sludge. Energy Convers. Manag. 2023, 277, 116627. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Li, R.; Zhang, Z. Improvement of the composition and humification of different animal manures by black soldier fly bioconversion. J. Clean. Prod. 2021, 278, 123397. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Lee, D.-J.; Jung, S.; Jeong, K.-H.; Lee, S.-H.; Park, Y.-K.; Kwon, E.E. Catalytic pyrolysis of cow manure over a Ni/SiO2 catalyst using CO2 as a reaction medium. Energy 2020, 195, 117077. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Ng, J.-H.; Chong, W.W.F.; Ong, H.C.; Tran, M.-V. Multivariate optimisation study and life cycle assessment of microwave-induced pyrolysis of horse manure for waste valorisation and management. Energy 2021, 216, 119194. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Zhang, Y.; Feng, R.; Mahmood, I.B. Characterization of human manure-derived biochar and energy-balance analysis of slow pyrolysis process. Waste Manag. 2014, 34, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag. 2019, 88, 85–95. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Bleuler, M.; Gold, M.; Strande, L.; Schönborn, A. Pyrolysis of Dry Toilet Substrate as a Means of Nutrient Recycling in Agricultural Systems: Potential Risks and Benefits. Waste Biomass-Valorization 2021, 12, 4171–4183. [Google Scholar] [CrossRef]
- Koutcheiko, S.; Monreal, C.; Kodama, H.; McCracken, T.; Kotlyar, L. Preparation and characterization of activated carbon derived from the thermo-chemical conversion of chicken manure. Bioresour. Technol. 2007, 98, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Erdogdu, A.E.; Polat, R.; Ozbay, G. Pyrolysis of goat manure to produce bio-oil. Eng. Sci. Technol. Int. J. 2018, 22, 452–457. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Pighinelli, A.L.; Boateng, A.A. Hydrodeoxygenation of fast-pyrolysis bio-oils from various feedstocks using carbon-supported catalysts. Fuel Process. Technol. 2014, 123, 11–18. [Google Scholar] [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability 2019, 11, 2533. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Ng, J.-H.; Chong, W.W.F.; Lam, S.S.; Ong, H.C.; Ani, F.N. Microwave pyrolysis for valorisation of horse manure biowaste. Energy Convers. Manag. 2020, 220, 113074. [Google Scholar] [CrossRef]
- Lee, D.-J.; Jung, S.; Jang, Y.; Jo, G.; Park, S.H.; Jeon, Y.J.; Park, Y.-K.; Kwon, E.E. Offering a new option to valorize hen manure by CO2-assisted catalytic pyrolysis over biochar and metal catalysts. J. CO2 Util. 2020, 42, 101344. [Google Scholar] [CrossRef]
- He, S.; Cao, C.; Wang, J.; Yang, J.; Cheng, Z.; Yan, B.; Pan, Y.; Chen, G. Pyrolysis study on cattle manure: From conventional analytical method to online study of pyrolysis photoionization time-of-flight mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 151, 104916. [Google Scholar] [CrossRef]
- Ren, C.; Guo, S.; Wang, Y.; Liu, S.; Du, M.; Chen, Y.; Guo, L. Thermodynamic analysis and optimization of auto-thermal supercritical water gasification polygeneration system of pig manure. Chem. Eng. J. 2022, 427, 131938. [Google Scholar] [CrossRef]
- Onabanjo, T.; Patchigolla, K.; Wagland, S.; Fidalgo, B.; Kolios, A.; McAdam, E.; Parker, A.; Williams, L.; Tyrrel, S.; Cartmell, E. Energy recovery from human faeces via gasification: A thermodynamic equilibrium modelling approach. Energy Convers. Manag. 2016, 118, 364–376. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Fernandez, A.; Al-Ansari, T.; Mackey, H.R.; Rodriguez, R.; McKay, G. Thermal degradation characteristics and gasification kinetics of camel manure using thermogravimetric analysis. J. Environ. Manag. 2021, 287, 112345. [Google Scholar] [CrossRef]
- Sobamowo, G.M.; Ojolo, S.J. Techno-Economic Analysis of Biomass Energy Utilization through Gasification Technology for Sustainable Energy Production and Economic Development in Nigeria. J. Energy 2018, 2018, 4860252. [Google Scholar] [CrossRef]
- Jia, J.; Shu, L.; Zang, G.; Xu, L.; Abudula, A.; Ge, K. Energy analysis and techno-economic assessment of a co-gasification of woody biomass and animal manure, solid oxide fuel cells and micro gas turbine hybrid system. Energy 2018, 149, 750–761. [Google Scholar] [CrossRef]
- Liu, M.; Li, F.; Liu, H.; Wang, C.-H. Synergistic effect on co-gasification of chicken manure and petroleum coke: An investigation of sustainable waste management. Chem. Eng. J. 2020, 417, 128008. [Google Scholar] [CrossRef]
- Xin, Y.; Cao, H.; Yuan, Q.; Wang, D. Two-step gasification of cattle manure for hydrogen-rich gas production: Effect of biochar preparation temperature and gasification temperature. Waste Manag. 2017, 68, 618–625. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of horse manure through catalytic supercritical water gasification. Waste Manag. 2016, 52, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, A.; Aslan, M.; Salimi, M.; Balou, S.; Pirbazari, S.; Hashemi, H.; Kohansal, K. Influence of the blend nickel/porous hydrothermal carbon and cattle manure hydrochar catalyst on the hydrothermal gasification of cattle manure for H2 production. Energy Convers. Manag. 2018, 173, 15–28. [Google Scholar] [CrossRef]
- Liu, S.; Cao, W.; Wang, Y.; Wei, W.; Li, L.; Jin, H.; Guo, L. Characteristics and mechanisms of nitrogen transformation during chicken manure gasification in supercritical water. Waste Manag. 2022, 153, 240–248. [Google Scholar] [CrossRef]
- De Blasio, C. Notions of Biomass Gasification. In Fundamentals of Biofuels Engineering and Technology; Springer: Cham, Switzerland, 2019; pp. 307–334. [Google Scholar] [CrossRef]
- Salierno, G.; Marinelli, F.; Likozar, B.; Ghavami, N.; De Blasio, C. Supercritical Water Gasification of glycerol: Continuous reactor kinetics and transport phenomena modeling. Int. J. Heat Mass Transf. 2022, 183, 122200. [Google Scholar] [CrossRef]
- Pandey, D.S.; Kwapinska, M.; Gómez-Barea, A.; Horvat, A.; Fryda, L.E.; Rabou, L.P.L.M.; Leahy, J.J.; Kwapinski, W. Poultry Litter Gasification in a Fluidized Bed Reactor: Effects of Gasifying Agent and Limestone Addition. Energy Fuels 2016, 30, 3085–3096. [Google Scholar] [CrossRef]
- Xiao, X.; Cao, J.; Meng, X.; Le, D.D.; Li, L.; Ogawa, Y.; Sato, K.; Takarada, T. Synthesis gas production from catalytic gasification of waste biomass using nickel-loaded brown coal char. Fuel 2013, 103, 135–140. [Google Scholar] [CrossRef]
- Perera, S.M.; Wickramasinghe, C.; Samarasiri, B.; Narayana, M. Modeling of thermochemical conversion of waste biomass—A comprehensive review. Biofuel Res. J. 2021, 8, 1481–1528. [Google Scholar] [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 121166. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Y.; Liu, Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain. Chem. Eng. 2018, 6, 13570–13578. [Google Scholar] [CrossRef]
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Seehar, T.H.; Nielsen, A.H.; Rosendahl, L.A. Valorization of animal and human wastes through hydrothermal liquefaction for biocrude production and simultaneous recovery of nutrients. Energy Convers. Manag. 2020, 216, 112925. [Google Scholar] [CrossRef]
- Li, H.; Lu, J.; Zhang, Y.; Liu, Z. Hydrothermal liquefaction of typical livestock manures in China: Biocrude oil production and migration of heavy metals. J. Anal. Appl. Pyrolysis 2018, 135, 133–140. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Yin, Z.; Kong, S.; Han, W.; Zhang, J. Catalytic liquefaction of human feces over Ni-Tm/TiO2 catalyst and the influence of operating conditions on products. Energy Convers. Manag. 2018, 157, 239–245. [Google Scholar] [CrossRef]
- Alherbawi, M.; Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Potential of drop-in biofuel production from camel manure by hydrothermal liquefaction and biocrude upgrading: A Qatar case study. Energy 2021, 232, 121027. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Cheng, Z.; Dong, H.; Yan, B.; Chen, G. Synergetic effect and primary reaction network of corn cob and cattle manure in single and mixed hydrothermal liquefaction. J. Anal. Appl. Pyrolysis 2021, 155, 105076. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Sabio, E.; Ledesma, B.; Román, S.; González-García, C. Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy 2016, 94, 600–608. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Guangzhi, Y.; Jinyu, Y.; Yuhua, Y.; Zhihong, T.; DengGuang, Y.; Junhe, Y. Preparation and CO2 adsorption properties of porous carbon from camphor leaves by hydrothermal carbonization and sequential potassium hydroxide activation. RSC Adv. 2017, 7, 4152–4160. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Hoekman, S.K.; Liu, T.; Gai, C.; Peng, N. Homogeneously Dispersed Zerovalent Iron Nanoparticles Sup-ported on Hydrochar-Derived Porous Carbon: Simple, in situ Synthesis and Use for Dechlorination of PCBs. ACS Sustain. Chem. Eng. 2016, 4, 3261–3267. [Google Scholar] [CrossRef]
- Fan, F.; Xing, X.; Shi, S.; Zhang, X.; Zhang, X.; Li, Y.; Xing, Y. Combustion Characteristic and Kinetics Analysis of Hydrochars. Trans. Chin. Soc. Agric. Eng. 2016, 32, 219–224. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons for Water Treatment Prepared by Phosphoric Acid Ac-tivation of Hydrothermally Treated Beer Waste. Ind. Eng. Chem. Res. 2014, 53, 15389–15397. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Spitzer, R.Y.; Mau, V.; Gross, A. Using hydrothermal carbonization for sustainable treatment and reuse of human excreta. J. Clean. Prod. 2018, 205, 955–963. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Thomas, C.P.L. Microwave Hydrothermal Carbonization of Human Biowastes. Waste Biomass-Valorization 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.; Martin, S.; Shama, G.; Wheatley, A. Process energetics for the hydrothermal carbonisation of human faecal wastes. Energy Convers. Manag. 2015, 105, 1115–1124. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Jang, E.-S.; Ryu, D.-Y.; Kim, D. Hydrothermal carbonization improves the quality of biochar derived from livestock manure by removing inorganic matter. Chemosphere 2022, 305, 135391. [Google Scholar] [CrossRef] [PubMed]
- Qaramaleki, S.V.; Villamil, J.A.; Mohedano, A.F.; Coronella, C.J. Factors Affecting Solubilization of Phosphorus and Nitrogen through Hydrothermal Carbonization of Animal Manure. ACS Sustain. Chem. Eng. 2020, 8, 12462–12470. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Chianese, S.; Musmarra, D.; De Blasio, C. Process simulation of hydrothermal carbonization of digestate from energetic perspectives in Aspen Plus. Energy Convers. Manag. 2022, 270, 116215. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R. BMC Energy Waste to Bioenergy: A Review on the Recent Conversion Technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Dalke, R.; Demro, D.; Khalid, Y.; Wu, H.; Urgun-Demirtas, M. Current status of anaerobic digestion of food waste in the United States. Renew. Sustain. Energy Rev. 2021, 151, 111554. [Google Scholar] [CrossRef]
- Ilo, O.; Simatele, M.; Nkomo, S.; Mkhize, N.; Prabhu, N. Methodological Approaches to Optimising Anaerobic Digestion of Water Hyacinth for Energy Efficiency in South Africa. Sustainability 2021, 13, 6746. [Google Scholar] [CrossRef]
- Ripoll, V.; Agabo-García, C.; Solera, R.; Perez, M. Anaerobic digestion of slaughterhouse waste in batch and anaerobic sequential batch reactors. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Rajendran, K.; Mahapatra, D.; Venkatraman, A.V.; Muthuswamy, S.; Pugazhendhi, A. Advancing anaerobic digestion through two-stage processes: Current developments and future trends. Renew. Sustain. Energy Rev. 2020, 123, 109746. [Google Scholar] [CrossRef]
- Donkor, K.O.; Gottumukkala, L.D.; Lin, R.; Murphy, J.D. A perspective on the combination of alkali pre-treatment with bioaugmentation to improve biogas production from lignocellulose biomass. Bioresour. Technol. 2022, 351, 126950. [Google Scholar] [CrossRef]
- Awosusi, A.; Sethunya, V.; Matambo, T. Synergistic effect of anaerobic co-digestion of South African food waste with cow manure: Role of low density-polyethylene in process modulation. Mater. Today Proc. 2021, 38, 793–803. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total. Environ. 2020, 728, 138224. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kommalapati, R.R. Optimizing anaerobic co-digestion of goat manure and cotton gin trash using biochemical methane potential (BMP) test and mathematical modeling. SN Appl. Sci. 2021, 3, 724. [Google Scholar] [CrossRef]
- Alfa, M.I.; Owamah, H.I.; Onokwai, A.O.; Gopikumar, S.; Oyebisi, S.O.; Kumar, S.S.; Bajar, S.; Samuel, O.D.; Ilabor, S.C. Evaluation of biogas yield and kinetics from the anaerobic co-digestion of cow dung and horse dung: A strategy for sustainable management of livestock manure. Energy, Ecol. Environ. 2020, 6, 425–434. [Google Scholar] [CrossRef]
- Hamzah, A.F.A.; Hamzah, M.H.; Man, H.C.; Jamali, N.S.; Siajam, S.I.; Show, P.L. Biogas Production Through Mono- and Co-digestion of Pineapple Waste and Cow Dung at Different Substrate Ratios. BioEnergy Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Arifan, F.; Abdullah, A.; Sumardiono, S. Effectiveness Analysis of Anaerobic Digestion Method in Making Biogas from Animal Manure and Tofu Liquid Waste. J. Ilmu Teknol. Has. Ternak 2021, 16, 84–94. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Xu, H.; Chang, J.; Wang, H.; Liu, Y.; Zhang, X.; Liang, P.; Huang, X. Enhancing direct interspecies electron transfer in syntrophic-methanogenic associations with (semi)conductive iron oxides: Effects and mechanisms. Sci. Total. Environ. 2019, 695, 133876. [Google Scholar] [CrossRef]
- Lv, N.; Zhao, L.; Wang, R.; Ning, J.; Pan, X.; Li, C.; Cai, G.; Zhu, G. Novel strategy for relieving acid accumulation by enriching syntrophic associations of syntrophic fatty acid-oxidation bacteria and H2/formate-scavenging methanogens in anaerobic digestion. Bioresour. Technol. 2020, 313, 123702. [Google Scholar] [CrossRef]
- Wang, P.; Peng, H.; Adhikari, S.; Higgins, B.; Roy, P.; Dai, W.; Shi, X. Enhancement of biogas production from wastewater sludge via anaerobic digestion assisted with biochar amendment. Bioresour. Technol. 2020, 309, 123368. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.-H.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Effects of plastics on reactor performance and microbial communities during acidogenic fermentation of food waste for production of volatile fatty acids. Bioresour. Technol. 2021, 337, 125481. [Google Scholar] [CrossRef]
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Sitthi, S.; Hatamoto, M.; Watari, T.; Yamaguchi, T. Enhancing anaerobic syntrophic propionate degradation using modified polyvinyl alcohol gel beads. Heliyon 2020, 6, e05665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Euverink, G.J.W. Effect of bioaugmentation combined with activated charcoal on the mitigation of volatile fatty acids inhibition during anaerobic digestion. Chem. Eng. J. 2022, 428, 131015. [Google Scholar] [CrossRef]
- Lu, X.; Wang, H.; Ma, F.; Zhao, G.; Wang, S. Improved process performance of the acidification phase in a two-stage anaerobic digestion of complex organic waste: Effects of an iron oxide-zeolite additive. Bioresour. Technol. 2018, 262, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Y.; Zhang, T.; Hu, Q.; Tong, Y.W.; He, Y.; Dai, Y.; Wang, C.-H.; Peng, Y. Food waste treating by biochar-assisted high-solid anaerobic digestion coupled with steam gasification: Enhanced bioenergy generation and porous biochar production. Bioresour. Technol. 2021, 331, 125051. [Google Scholar] [CrossRef]
- Adjama, I.; Derkyi, N.S.A.; Uba, F.; Akolgo, G.A.; Opuko, R. Anaerobic Co-Digestion of Human Feces with Rice Straw for Biogas Production: A Case Study in Sunyani. Model. Simul. Eng. 2022, 2022, 608045. [Google Scholar] [CrossRef]
- Eduok, S.; John, O.; Ita, B.; Inyang, E.; Coulon, F. Enhanced Biogas Production From Anaerobic Co-digestion of Lignocellulosic Biomass and Poultry Feces Using Source Separated Human Urine as Buffering Agent. Front. Environ. Sci. 2018, 6, 67. [Google Scholar] [CrossRef]
- Silwadi, M.; Mousa, H.; Al-Hajji, B.Y.; Al-Wahaibi, S.S.; Al-Harrasi, Z.Z. Enhancing biogas production by anaerobic digestion of animal manure. Int. J. Green Energy 2022, 20, 1–8. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total. Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Recebli, Z.; Selimli, S.; Ozkaymak, M.; Gonc, O. Biogas Production from Animal Manure. J. Eng. Sci. Technol. 2015, 10, 722–729. [Google Scholar]
- Zlateva, P.; Terziev, A.; Yordanov, K. Study of regime parameters of the fermenter in the production of biogas from animal liquid waste materials. E3S Web Conf. 2021, 286, 02010. [Google Scholar] [CrossRef]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Wernli, M.; Zubcov, E.; Bagrin, N.; Borodin, N.; Lens, P. Lactic acid fermentation of human urine to improve its fertilizing value and reduce odour emissions. J. Environ. Manag. 2017, 198, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Andreev, N.; Ronteltap, M.; Boincean, B.; Lens, P.N.L. Lactic acid fermentation of human excreta for agricultural application. J. Environ. Manag. 2018, 206, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Yim, J.H.; Jung, S.; Jang, M.-S.; Jeong, G.-T.; Jeong, K.-H.; Kim, J.K.; Tsang, Y.F.; Jeon, Y.J.; Kwon, E.E. Valorization of animal manure: A case study of bioethanol production from horse manure. Chem. Eng. J. 2021, 403, 126345. [Google Scholar] [CrossRef]
- Jimenez, J.; Bott, C.; Love, N.; Bratby, J. Source Separation of Urine as an Alternative Solution to Nutrient Management in Biological Nutrient Removal Treatment Plants. Water Environ. Res. 2015, 87, 2120–2129. [Google Scholar] [CrossRef]
- Koffi Konan, F.; Kouame, M.K.; Author, C.; Koffi Félix, K.; Nazo Edith, K.-K.; Théophile, G.; Kotchi Yves, B.; Kouamé Martin, K.; Yao Francis, K.; Kablan, T. Improving Anaerobic Biodigestion of Manioc Wastewater with Human Urine as Co-Substrate. Int. J. Innov. Appl. Stud. 2013, 2, 335–343. [Google Scholar]
- Twizerimana, M.; Marimi, M.; Bura, X.; Nganyi, E.O. Biogas Production from Co-digestion of Cotton Yarn Waste and Human Urine. J. Energy Res. Rev. 2020, 6, 20–29. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 2019, 7, 103067. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, J.J.; Nie, J.M.; Wu, N.; Yang, F.; Yang, R.J. Biogas production of Chicken Manure by Two-stage fermentation process. E3S Web Conf. 2018, 38, 01048. [Google Scholar] [CrossRef]
- Zhang, A.; He, J.; Shen, Y.; Xu, X.; Liu, Y.; Li, Y.; Wu, S.; Xue, G.; Li, X.; Makinia, J. Enhanced degradation of glucocorticoids, a potential COVID-19 remedy, by co-fermentation of waste activated sludge and animal manure: The role of manure type and degradation mechanism. Environ. Res. 2021, 201, 111488. [Google Scholar] [CrossRef]
- Svetlitchnyi, A.V.; Kensch, O.; A Falkenhan, D.; Korseska, S.G.; Lippert, N.; Prinz, M.; Sassi, J.; Schickor, A.; Curvers, S. Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol. Biofuels 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Doreswamy, R.; Deb, R.; De, S. Potential use of piggery excreta as a viable source of bioethanol production. J. Clean. Prod. 2021, 316, 128246. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow manure as a lignocellulosic substrate for fungal cellulase expression and bioethanol production. AMB Express 2018, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Dadrasnia, A.; Muñoz, I.D.B.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable nutrient recovery from animal manure: A review of current best practice technology and the potential for freeze concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Ruiz, D.; Miguel, G.S.; Corona, B.; Gaitero, A.; Domínguez, A. Environmental and economic analysis of power generation in a thermophilic biogas plant. Sci. Total. Environ. 2018, 633, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.F.; Queiroz, I.D.S.; Mikhael, J.E.R.; Oliveira, R.C.; Borges, E.N. Enriched animal manure as a source of phosphorus in sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 2019, 8, 203–210. [Google Scholar] [CrossRef]
- Martin, T.M.P.; Esculier, F.; Levavasseur, F.; Houot, S. Human urine-based fertilizers: A review. Crit. Rev. Environ. Sci. Technol. 2020, 52, 890–936. [Google Scholar] [CrossRef]
- Sugihara, R. Reuse of Human Excreta in Developing Countries: Agricultural Fertilization Optimization. Consilience 2020, 22, 58–64. [Google Scholar] [CrossRef]
- Moya, B.; Parker, A.; Sakrabani, R. Challenges to the use of fertilisers derived from human excreta: The case of vegetable exports from Kenya to Europe and influence of certification systems. Food Policy 2019, 85, 72–78. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Ogunbiyi, O.D.; Omotola, E.O.; Adeyemi, W.J.; Agboola, O.O.; Onwudiwe, D.C. Urine: Useless or useful “waste”? Results Eng. 2022, 16, 100522. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Challenges and opportunities of nutrient recovery from human urine using biochar for fertilizer applications. J. Clean. Prod. 2021, 304, 127019. [Google Scholar] [CrossRef]
- Akpan-Idiok, A.U.; Udo, I.A.; Braide, E.I. The use of human urine as an organic fertilizer in the production of okra (Abelmoschus esculentus) in South Eastern Nigeria. Resour. Conserv. Recycl. 2012, 62, 14–20. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Patel, A.; Mungray, A.A.; Mungray, A.K. Technologies for the recovery of nutrients, water and energy from human urine: A review. Chemosphere 2020, 259, 127372. [Google Scholar] [CrossRef]
- Harder, R.; Wielemaker, R.; Larsen, T.A.; Zeeman, G.; Öberg, G. Recycling nutrients contained in human excreta to agriculture: Pathways, processes, and products. Crit. Rev. Environ. Sci. Technol. 2019, 49, 695–743. [Google Scholar] [CrossRef]
- Ahmed, N.; Shim, S.; Won, S.; Ra, C. Struvite recovered from various types of wastewaters: Characteristics, soil leaching behaviour, and plant growth. Land Degrad. Dev. 2018, 29, 2864–2879. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Wei, S.P.; van Rossum, F.; van de Pol, G.J.; Winkler, M.-K.H. Recovery of phosphorus and nitrogen from human urine by struvite precipitation, air stripping and acid scrubbing: A pilot study. Chemosphere 2018, 212, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Masrura, S.U.; Dissanayake, P.; Sun, Y.; Ok, Y.S.; Tsang, D.C.W.; Khan, E. Sustainable use of biochar for resource recovery and pharmaceutical removal from human urine: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 3016–3048. [Google Scholar] [CrossRef]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining Nutrients (N, K, P) from Urban Source-Separated Urine by Forward Osmosis Dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Azuara, M.; Kersten, S.R.; Kootstra, A.M.J. Recycling phosphorus by fast pyrolysis of pig manure: Concentration and extraction of phosphorus combined with formation of value-added pyrolysis products. Biomass-Bioenergy 2012, 49, 171–180. [Google Scholar] [CrossRef]
- Zhang, T.; Bowers, K.E.; Harrison, J.H.; Chen, S. Releasing Phosphorus from Calcium for Struvite Fertilizer Production from Anaerobically Digested Dairy Effluent. Water Environ. Res. 2010, 82, 34–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).