A Comprehensive Review of Risk Assessments of Organic Effluents in Car Workshops
Abstract
:1. Introduction
2. Services Carried Out in Automobile Workshops and Sources of Organic Pollution
2.1. Tire Changing and Shear Stresses with the Floor
2.2. Braking System Servicing
2.3. Fuel and Oil Leaks
2.4. Maintenance of Coolant Circuit
2.5. Windshield Washer Filling
2.6. Detergents
3. Organic Compounds with a High Risk of Leakage to Be Monitored as a Priority in Washing Water
3.1. N-alkanes
3.2. Polycyclic Aromatic Hydrocarbons (PAHs)
3.3. Glycol Ethers
3.4. Alkylbenzenes
3.5. Monitoring the Organic Pollution of Car Workshop Floor Wash Water
4. The Criticality Matrix: An Environmental Management Tool
4.1. Goals
4.2. Presentation of the Criticality Matrix
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lamprea, K.; Bressy, A.; Mirande-Bret, C.; Caupos, E.; Gromaire, M.-C. Alkylphenol and Bisphenol A Contamination of Urban Runoff: An Evaluation of the Emission Potentials of Various Construction Materials and Automotive Supplies. Environ. Sci. Pollut. Res. 2018, 25, 21887–21900. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, A.; Björklund, K.; Eriksson, E.; Kalmykova, Y.; Strömvall, A.-M.; Siopi, A. Emissions of Organic Pollutants from Traffic and Roads: Priority Pollutants Selection and Substance Flow Analysis. Sci. Total Environ. 2017, 580, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Tamis, J.E.; Koelmans, A.A.; Dröge, R.; Kaag, N.H.B.M.; Keur, M.C.; Tromp, P.C.; Jongbloed, R.H. Environmental Risks of Car Tire Microplastic Particles and Other Road Runoff Pollutants. Microplast. Nanoplast. 2021, 1, 10. [Google Scholar] [CrossRef]
- Mallick, S.K.; Chakraborty, S. Bioremediation of Wastewater from Automobile Service Station in Anoxic-Aerobic Sequential Reactors and Microbial Analysis. Chem. Eng. J. 2019, 361, 982–989. [Google Scholar] [CrossRef]
- Sarmadi, M.; Foroughi, M.; Najafi Saleh, H.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient Technologies for Carwash Wastewater Treatment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Policy in the Field of Water. In Official Journal No L 327 of 22 December 2000. pp. 1–73. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0060 (accessed on 19 August 2022).
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public. Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire Wear Particles in the Aquatic Environment—A Review on Generation, Analysis, Occurrence, Fate and Effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Panko, J.M.; Chu, J.; Kreider, M.L.; Unice, K.M. Measurement of Airborne Concentrations of Tire and Road Wear Particles in Urban and Rural Areas of France, Japan, and the United States. Atmos. Environ. 2013, 72, 192–199. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of Fine Organic Aerosol. 3. Road Dust, Tire Debris, and Organometallic Brake Lining Dust: Roads as Sources and Sinks. Environ. Sci. Technol. 1993, 27, 1892–1904. [Google Scholar] [CrossRef]
- European Commission. Directive 2005/69/EC of the European Parliament and of the Council of 26 November 2005 Amending for the 27th Time Council Directive 76/769/EEC on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Limitation of Marketing and Use of Certain Dangerous Substances and Preparations (Polycyclic Aromatic Hydrocarbons Contained in Extender Oils and Tyres). In Official Journal No L 323 of 9 December 2005. pp. 1–4. Available online: https://eur-lex.europa.eu/legal-content/FR/TXT/?uri=CELEX%3A32005L0069 (accessed on 9 August 2022).
- Wahlström, J.; Olander, L.; Olofsson, U. Size, Shape, and Elemental Composition of Airborne Wear Particles from Disc Brake Materials. Tribol. Lett. 2010, 38, 15–24. [Google Scholar] [CrossRef]
- Garg, B.D.; Cadle, S.H.; Mulawa, P.A.; Groblicki, P.J.; Laroo, C.; Parr, G.A. Brake Wear Particulate Matter Emissions. Environ. Sci. Technol. 2000, 34, 4463–4469. [Google Scholar] [CrossRef]
- Alves, C.; Evtyugina, M.; Vicente, A.; Conca, E.; Amato, F. Organic Profiles of Brake Wear Particles. Atmos. Res. 2021, 255, 105557. [Google Scholar] [CrossRef]
- Caban, J.; Vrábel, J.; Šarkan, B.; Kuranc, A.; Słowik, T. Operational Tests of Brake Fluid in Passenger Cars. Period. Polytech. Transp. Eng. 2020, 49, 126–131. [Google Scholar] [CrossRef]
- Colombano, S.; Saada, A.; Victoire, E.; Guerin, V.; Zornig, C.; Amalric, L.; Blessing, M.; Widory, D.; Hube, D.; Blanc, C.; et al. Nature of petroleum products and origin of aging: Attempt to identify the source by taking into account the impacts and analyzing the approximate age of the spills. Final BRGM Rep. 2014, 1–166. Available online: https://ssp-infoterre.brgm.fr/fr/rapport/nature-produits-petroliers (accessed on 19 July 2022).
- Solano-Serena, F.; Marchal, R.; Vandecasteele, J.P. Biodégradabilité de l’essence dans l’environnement: De l’évaluation globale au cas des hydrocarbures récalcitrants. Oil Gas Sci. Technol. 2001, 56, 479–498. [Google Scholar] [CrossRef]
- Marchal, R.; Penet, S.; Solano-Serena, F.; Vandecasteele, J.P. Gasoline and Diesel Oil Biodegradation. Oil Gas Sci. Technol. 2003, 58, 441–448. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Ecological Implications of Motor Oil Pollution: Earthworm Survival and Soil Health. Soil Biol. Biochem. 2015, 85, 72–81. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Ori-jesu, M. Biodegradation of Mineral Oils—A Review. Afr. J. Biotechnol. 2009, 8, 6. [Google Scholar]
- Vazquez-Duhalt, R. Environmental Impact of Used Motor Oil. Sci. Total Environ. 1989, 79, 1–23. [Google Scholar] [CrossRef]
- Starostin, M.; Tamir, S. New Engine Coolant for Corrosion Protection of Magnesium Alloys. Mater. Corros. 2006, 5, 345–349. [Google Scholar] [CrossRef]
- Directorate-General for Internal Marlet, Industry, Entrepreneuship and SMEs. New Restriction on Methanol in Car Windscreen Washing Fluids. European Commission Press Release. Available online: https://single-market-economy.ec.europa.eu/news/new-restriction-methanol-car-windscreen-washing-fluids-2018-04-18_en (accessed on 18 August 2022).
- Sablayrolles, C.; Breton, A.; Vialle, C.; Vignoles, C.; Montréjaud-Vignoles, M. Priority Organic Pollutants in the Urban Water Cycle (Toulouse, France). Water Sci. Technol. 2011, 64, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Khodadoost, F. Effects of Detergents on Natural Ecosystems and Wastewater Treatment Processes: A Review. Environ. Sci. Pollut. Res. 2019, 26, 26439–26448. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A Comprehensive Review of Aliphatic Hydrocarbon Biodegradation by Bacteria. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef] [PubMed]
- Stroud, J.L.; Paton, G.I.; Semple, K.T. Microbe-Aliphatic Hydrocarbon Interactions in Soil: Implications for Biodegradation and Bioremediation. J. Appl. Microbiol. 2007, 102, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Kanzari, F.; Syakti, A.D.; Asia, L.; Malleret, L.; Piram, A.; Mille, G.; Doumenq, P. Distributions and Sources of Persistent Organic Pollutants (Aliphatic Hydrocarbons, PAHs, PCBs and Pesticides) in Surface Sediments of an Industrialized Urban River (Huveaune), France. Sci. Total Environ. 2014, 478, 141–151. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Hu, X.; Zhang, H.; He, S.; Lv, S. Distributions and Sources of Petroleum, Aliphatic Hydrocarbons and Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Sediments from Bohai Bay and Its Adjacent River, China. Mar. Pollut. Bull. 2015, 90, 88–94. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Environmental Cancer Prevention Department Leon Berard Cancer Center. Polycyclic Aromatic Hydrocarbons. Available online: https://www.cancer-environnement.fr/fiches/expositions-environnementales/hydrocarbures-aromatiques-polycycliques-hap/ (accessed on 29 August 2022).
- Brignon, J.; Soleille, S. Données technico-économiques sur les substances chimiques en France—Hydrocarbures Aromatiques Polycycliques. INERIS 2006, 1–50. Available online: https://substances.ineris.fr/fr/page/14 (accessed on 26 August 2022).
- Ministry of Ecological Transition and Territorial Cohesion. Technical note of 20 September 2020 relating to the national objectives for reducing emissions, discharges and losses of hazardous substances in surface waters and their implementation in the SDAGEs 2022–2027. Fr. Republic. 2020, 1–20. Available online: https://www.bulletin-officiel.developpement-durable.gouv.fr/notice?id=Bulletinofficiel-0031593&reqId=4ef35dfc-a41e-40b1-9c27-8e626220c821&pos=5 (accessed on 16 February 2022).
- De Ketttenis, P. The Historic and Current Use of Glycol Ethers: A Picture of Change. Toxicol. Lett. 2005, 156, 5–11. [Google Scholar] [CrossRef]
- Kelsey, J.R. Ethylene Oxide Derived Glycol Ethers: A Review of the Alkyl Glycol Ethers Potential to Cause Endocrine Disruption. Regul. Toxicol. Pharmacol. 2022, 129, 105113. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Boatman, R.J.; Cano, M.L. Ethylene Glycol Ethers: An Environmental Risk Assessment. Chemosphere 1998, 36, 1585–1613. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Davis, J.W. An Examination of the Physical Properties, Fate, Ecotoxicity and Potential Environmental Risks for a Series of Propylene Glycol Ethers. Chemosphere 2002, 49, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Multigner, L.; Catala, M.; Cordier, S.; Delaforge, M.; Fenaux, P.; Garnier, R.; Rico-Lattes, I.; Vasseur, P. The INSERM Expert Review on Glycol Ethers: Findings and Recommendations. Toxicol. Lett. 2005, 156, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lenoble, C.; Gouzy, A.; Brignon, J. Données technico-économiques sur les substances chimiques en France—Ethers de glycol. INERIS 2015, 1–43. Available online: https://substances.ineris.fr/fr/page/14 (accessed on 26 August 2022).
- European Commission. Regulation n°1272/2008 of the European Parliament and of the Council of 16 December 2008 Relating to the Classification, Labeling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and Amending Regulation (EC) No 1907/2006. In Official Journal No L 353 of 31 December 2008. pp. 1–1355. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 3 November 2022).
- Kocal, J.A.; Vora, B.V.; Imai, T. Production of Linear Alkylbenzenes. Appl. Catal. Gen. 2001, 221, 295–301. [Google Scholar] [CrossRef]
- Fernández, C.; Alonso, C.; García, P.; Tarazona, J.V.; Carbonell, G. Toxicity of Linear Alkyl Benzenes (LABs) to the Aquatic Crustacean Daphnia Magna through Waterborne and Food Chain Exposures. Bull. Environ. Contam. Toxicol. 2002, 68, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-Serrano, M.; Bastolla, C.L.V.; Mattos, J.J.; Lima, D.; Freire, T.B.; Nogueira, D.J.; De-la-Torre, G.E.; Righetti, B.P.H.; Zacchi, F.L.; Gomes, C.H.A.M.; et al. Microplastics and Linear Alkylbenzene Levels in Oysters Crassostrea Gigas Driven by Sewage Contamination at an Important Aquaculture Area of Brazil. Chemosphere 2022, 307, 136039. [Google Scholar] [CrossRef]
- Ni, H.-G.; Lu, F.-H.; Wang, J.-Z.; Guan, Y.-F.; Luo, X.-L.; Zeng, E.Y. Linear Alkylbenzenes in Riverine Runoff of the Pearl River Delta (China) and Their Application as Anthropogenic Molecular Markers in Coastal Environments. Environ. Pollut. 2008, 154, 348–355. [Google Scholar] [CrossRef]
- Gledhill, W.E.; Saeger, V.W.; Trehy, M.L. An Aquatic Environmental Safety Assessment of Linear Alkylbenzene. Environ. Toxicol. Chem. 1991, 10, 169–178. [Google Scholar] [CrossRef]
- Belanger, S.E.; Brill, J.L.; Rawlings, J.M.; Price, B.B. Development of Acute Toxicity Quantitative Structure Activity Relationships (QSAR) and Their Use in Linear Alkylbenzene Sulfonate Species Sensitivity Distributions. Chemosphere 2016, 155, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Mungray, A.K.; Kumar, P. Fate of Linear Alkylbenzene Sulfonates in the Environment: A Review. Int. Biodeterior. Biodegrad. 2009, 63, 981–987. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Yu, D.; Pang, Y.; Cai, H.; Liu, Y. Toxicity of Linear Alkylbenzene Sulfonate to Aquatic Plant Potamogeton perfoliatus L. Environ. Sci. Pollut. Res. 2018, 25, 32303–32311. [Google Scholar] [CrossRef] [PubMed]
- Verge, C.; Moreno, A. Infuence of Water Hardness on the Bioavailability and Toxicity of Linear Alkylbenzene Sulphonate (LAS). Chemosphere 2001, 44, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Greater Avignon Urban Area Community. Order authorising the discharge of non-domestic wastewater from the Avignon Sud Norauto garage into the Avignon municipal collection system. 24 July 2014; 1–3, (pers. com.). [Google Scholar]
- EN 858-1:2001; Separator Systems for Light Liquids (Oil and Petrol)—Part 1: Principles of Product Design, Performance and Testing, Marking and Quality Control. European Standard AFNOR: Saint-Denis, France, 2001; pp. 1–72.
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Mazumder, D.; Mukherjee, S. Treatment of automobile service station wastewater by coagulation and activated sludge process. Int. J. Environ. Sci. Devel. 2011, 2, 64–69. [Google Scholar] [CrossRef]
- Hauts-de-France Regional Economic, Social and Environmental Council (CESER). Towards a Major Water Policy in Hauts-de-France. In Report Following the CESER Opinion Adopted on 26 April 2022. pp. 1–176. Available online: https://ceser.hautsdefrance.fr/rapports/article/vers-une-grande-politique-de-l-eau-en-hauts-de-france-833 (accessed on 23 November 2022).
- Campos, J.C.; Borges, R.M.H.; Oliveira Filho, A.M.; Nobrega, R.; Sant’anna, G.L., Jr. Oilfield wastewater treatment by combined microfiltration and biological processes. Water Res. 2002, 36, 95–104. [Google Scholar] [CrossRef]
- Sirivedhin, T.; McCue, J.; Dallbauman, L. Reclaiming produced water for beneficial use: Salt removal by electrodialysis. J. Membr. Sci. 2004, 243, 335–343. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced bioreactors treatments of hydrocarbon-containing wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef]
- Carranza-Diaz, O.; Schultze-Nobre, L.; Moeder, M.; Nivala, J.; Kuschk, P.; Koeser, H. Removal of selected organic micropollutants in planted and unplanted pilot-scale horizontal flow constructed wetlands under conditions of high organic load. Ecol. Eng. 2014, 71, 234–245. [Google Scholar] [CrossRef]
- Szarka, A.; Mihová, V.; Horváth, G.; Hrouzková, S. Development of an Advanced Inspection of the Degradation of Volatile Organic Compounds in Electrochemical Water Treatment of Paint-Industrial Water Effluents. Appl. Sci. 2023, 13, 443. [Google Scholar] [CrossRef]
| Pollutant | Sources | Some Physicochemical Properties |
|---|---|---|
| N-alkanes | Changing and shear stresses with the floor | Depend on the length of the carbon chain |
| Braking system servicing | n-octane: Log Kow * 5.34, WS ** 0.632 mg L−1 | |
| Fuel leak from an injector | n-heptadecane: Log Kow 10.92, WS 0.0017 mg L−1 | |
| Oil leak during engine and gearbox maintenance | ||
| Floor cleaning with detergents | ||
| Polycyclic aromatic hydrocarbons (PAHs) | Changing and shear stresses with floor | Depend on number of benzene rings benzo[a]pyrene: Log Kow 6.06, WS 0.0038 mg L−1 |
| Braking system servicing Used oil leak during oil change Improper storage of used oil | ||
| Glycol ethers | Brake fluid replacement | Soluble or miscible (water) |
| Maintenance of coolant circuit | Log Kow −1.46 to 1.9 | |
| Windshield washer filling | ||
| Alkylbenzenes | Vehicle structures and floor cleaning with detergents | Hydrophobic, |
| Log Kow 7 to 10 |
| Organic Pollution Indicators | French Regulations | * Upstream of OWS | * Downstream of OWS |
|---|---|---|---|
| Chemical oxygen demand (COD) | 1500 mg L−1 | 1210–87,300 mg L−1 | 866–1100 mg L−1 |
| Biochemical oxygen demand (BOD) | 600 mg L−1 | 740–10,200 mg L−1 | 256–545 mg L−1 |
| Total hydrocarbons | 5 mg L−1 | 14–1100 mg L−1 | 0.363–1.74 mg L−1 |
| Impacts | Frequency | Target | Phase |
|---|---|---|---|
| Low = 1 | WTR * < 10% | Product used in the vehicle mechanics; its flow no longer possible | Aerosol |
| Medium = 2 | WTR: 10–50% | Product used in the vehicle mechanics; its flow still possible | Paste |
| High = 3 | WTR > 50% | Product used on the vehicle external surface | Liquid |
| Impacts | Prevention Factor |
|---|---|
| Low = 1 | A prevention solution is systematically used by mechanics |
| Medium = 2 | A prevention solution is partially used by mechanics |
| High = 3 | No preventative solution exists |
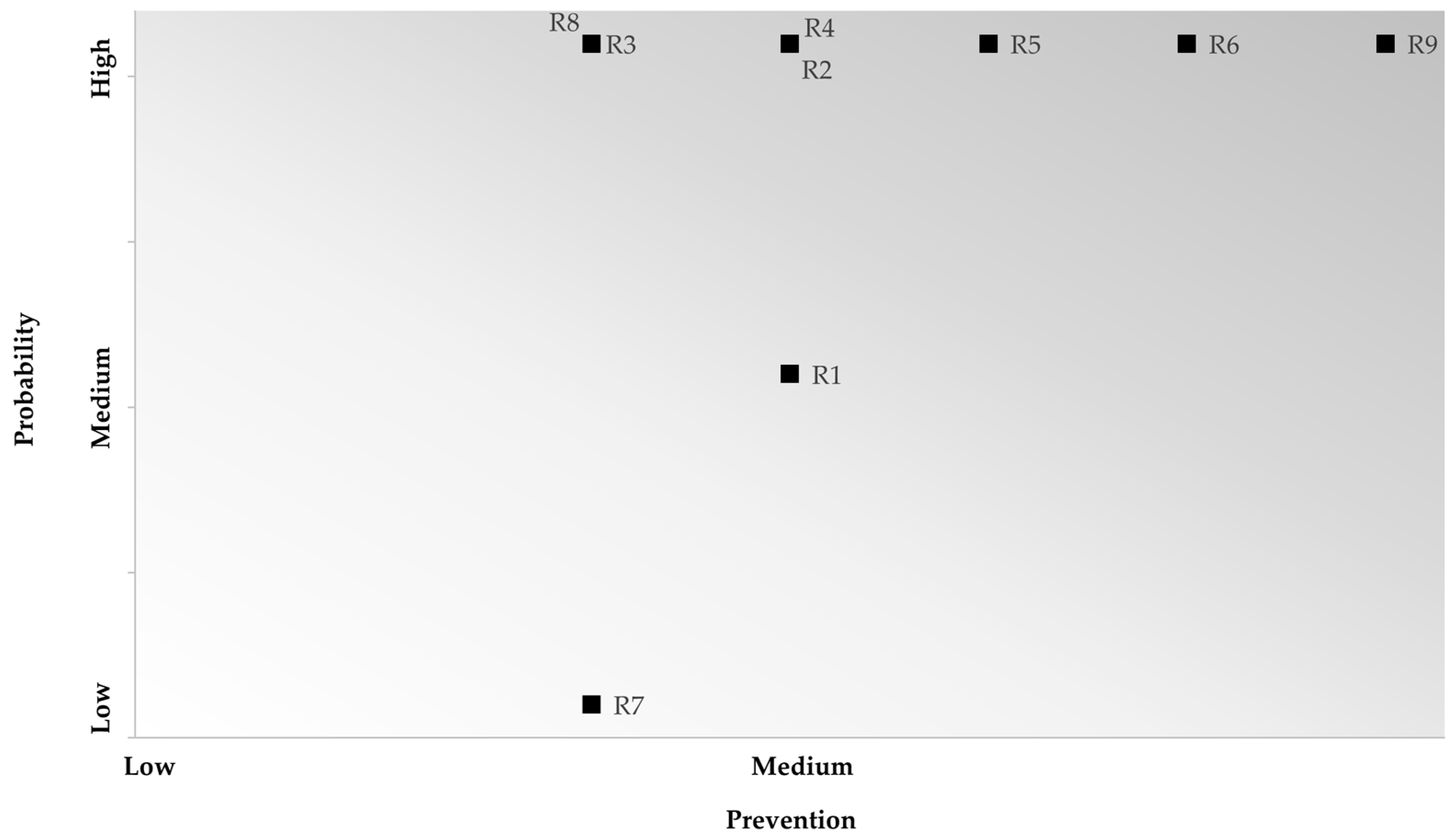
| Criticality Matrix | ||||
|---|---|---|---|---|
| Probability | 3 | 3 | 6 | 9 |
| 2 | 2 | 4 | 6 | |
| 1 | 1 | 2 | 3 | |
| 1 | 2 | 3 | ||
| Prevention | ||||
| Categories | Code | Frequency | Target | Phase | Probability Factor | Prevention Factor | Risk Score |
|---|---|---|---|---|---|---|---|
| Engine oil | R1 | 2 | 1 | 3 | 2 | 2 | 4 |
| Cooling liquid | R2 | 2 | 1 | 3 | 2 | 3 | 6 |
| Transmission oil | R3 | 1 | 1 | 3 | 1.7 | 3 | 5.1 |
| Penetrating oil | R4 | 2 | 3 | 1 | 2 | 3 | 6 |
| Rim cleaner | R5 | 3 | 3 | 1 | 2.3 | 3 | 6.9 |
| Leak detector | R6 | 3 | 3 | 2 | 2.7 | 3 | 8.1 |
| Brake fluid | R7 | 1 | 1 | 3 | 1.7 | 1 | 1.7 |
| Brake cleaner | R8 | 1 | 1 | 3 | 1.7 | 3 | 5.1 |
| Floor detergent | R9 | 3 | 3 | 3 | 3 | 3 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchiat, R.; Veignie, E.; Kaczmarek, F.; Dorchy, J.; Fortunato, A.-D.; Rafin, C. A Comprehensive Review of Risk Assessments of Organic Effluents in Car Workshops. Environments 2023, 10, 220. https://doi.org/10.3390/environments10120220
Bouchiat R, Veignie E, Kaczmarek F, Dorchy J, Fortunato A-D, Rafin C. A Comprehensive Review of Risk Assessments of Organic Effluents in Car Workshops. Environments. 2023; 10(12):220. https://doi.org/10.3390/environments10120220
Chicago/Turabian StyleBouchiat, Rémi, Etienne Veignie, Fabien Kaczmarek, Julien Dorchy, Anne-Danièle Fortunato, and Catherine Rafin. 2023. "A Comprehensive Review of Risk Assessments of Organic Effluents in Car Workshops" Environments 10, no. 12: 220. https://doi.org/10.3390/environments10120220
APA StyleBouchiat, R., Veignie, E., Kaczmarek, F., Dorchy, J., Fortunato, A.-D., & Rafin, C. (2023). A Comprehensive Review of Risk Assessments of Organic Effluents in Car Workshops. Environments, 10(12), 220. https://doi.org/10.3390/environments10120220






