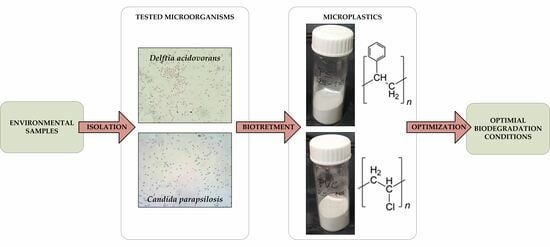
Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization
Abstract
:1. Introduction
| Microorganisms | Enzymes | Plastics | Reference | |
|---|---|---|---|---|
| Fungi | Trametes versicolor, Pleurotus ostreatus, Pleurotus ostreatus, and Trametes pubescen | Laccase | PE | [21] |
| Trichoderma harzianum | Laccase and manganese peroxidase | [30] | ||
| Pleurotus ostreatus | Lignin peroxidase, manganese peroxidase, and laccase | [31] | ||
| Fusarium graminearum | Peroxidase | [32] | ||
| Aspergillus flavus | Laccase and laccase-like multicopper oxidase | [33] | ||
| Bjerkandera adusta | Laccase | [34] | ||
| Phanerocheate chrysosporium | Lignin peroxidase | PVC | [35] | |
| Cochliobolus sp. | Laccase | [36] | ||
| Penicillium citrinum | Polyesterase | PET | [37] | |
| Candida antarctica | Lipase | [38] | ||
| Lentinus tigrinus | Esterase | PS | [39] | |
| Candida antarctica | Lipase | PUR | [40] | |
| Aspergillus flavus | Esterase | [41] | ||
| Aspergillus tubingenesis | Esterase | [42] | ||
| Bacteria | Rhodococcus ruber | Laccase | PE | [22] |
| Pseudomonas sp. | Alkane hydroxylase | [43] | ||
| Pseudomonas aeruginosa | Alkane hydroxylase and rubredoxin reductase | [44] | ||
| Ideonella sakaiensis | PETase | PET | [45] | |
| Streptomyces scabies | Glycosyl hydrolase and esterase | [46] | ||
| Thermobifida fusca | Cutinase-like hydrolase | [47] | ||
| Delftia acidovorans | Esterase | PUR | [48] |
2. Materials and Methods
2.1. Preparation of Microplastics
2.2. Selection of Bacteria and Yeasts Strains
2.3. Biodegradation Experiments
2.4. Modeling of the Optimal Conditions
2.5. Surface Analysis of MP Particles
3. Results and Discussion
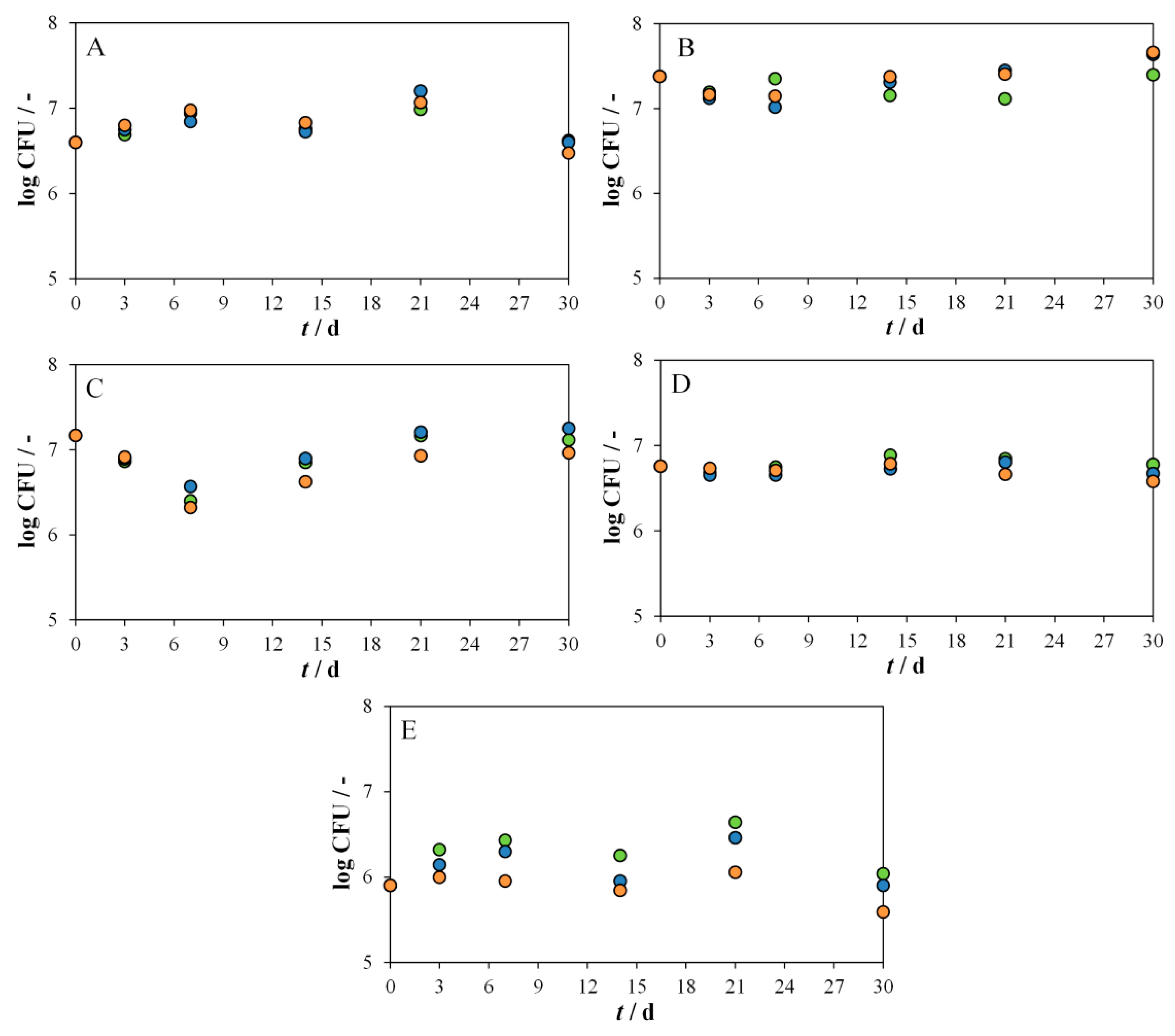
3.1. Screening of the Bacterial and Yeast Strains for Their Potential for PS and PVC Biodegradation
3.2. Determination of Optimal Conditions for the Biodegradation of PS and PVC MPs by Delftia acidovorans and Candida parapsilosis
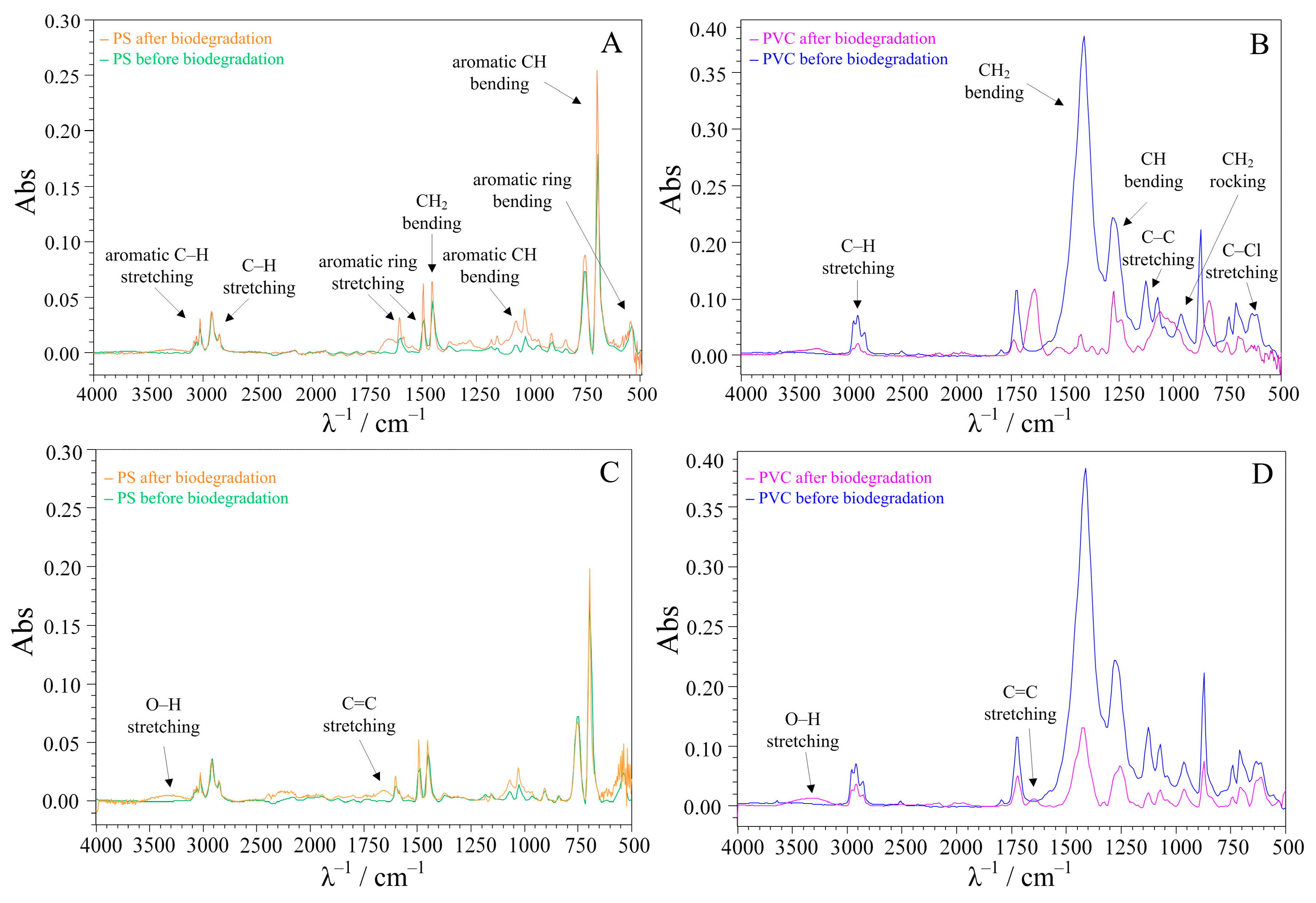
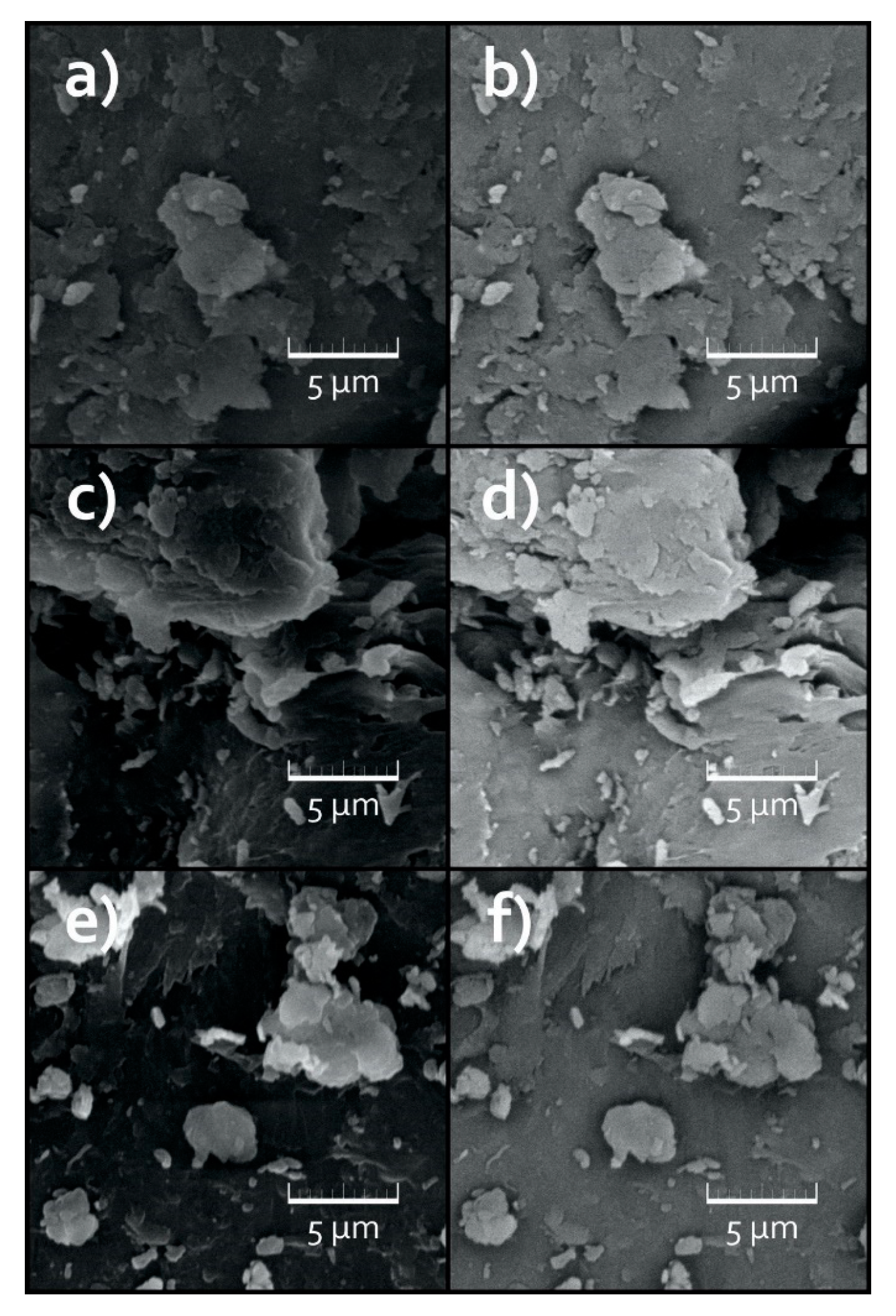
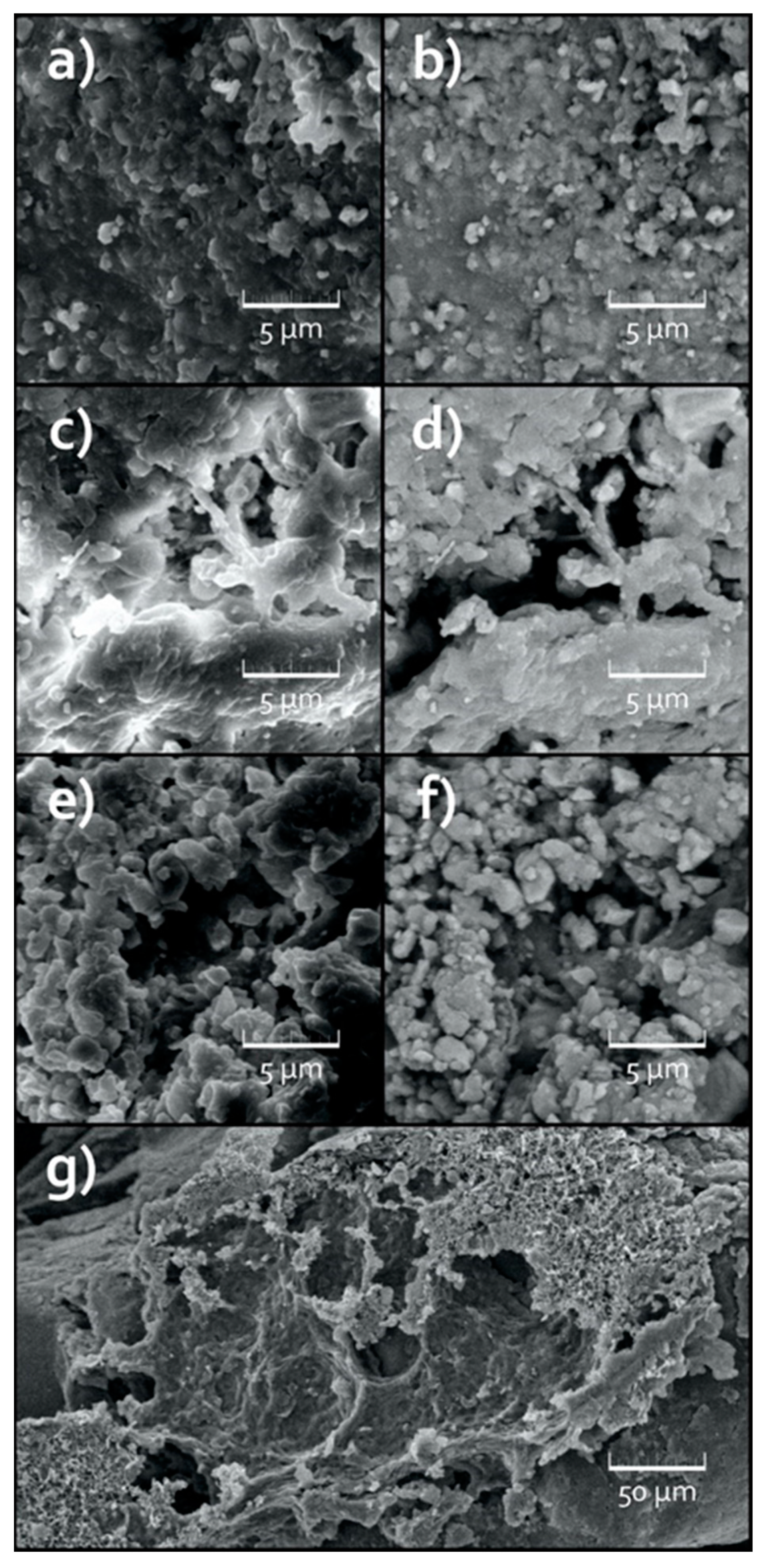
3.3. Surface Analysis of the Treated MPs
3.4. Analysis of the Aqueous Phases in Contact with MPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastics—The Facts. 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 20 March 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 1622–1628. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.-X.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- Erceg, M.; Tutman, P.; Vojanić Varezić, D.; Bobanović, A. Karakterizacija mikroplastike u sedimentu plaže Prapratno. Kem. Ind. 2020, 69, 253–260. [Google Scholar] [CrossRef]
- Westphalen, H.; Abdelrasoul, A. Challenges and Treatment of Microplastics in Water. In Water Challenges of an Urbanizing World; Glavan, M., Ed.; InTech: Rijeka, Croatia, 2018; pp. 71–84. [Google Scholar] [CrossRef]
- Ozturk, R.C.; Altinok, I. Interaction of Plastics with Marine Species. Turk. J. Fish. Aquat. Sci. 2020, 20, 647–658. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Wang, L.; Nabi, G.; Yin, L.; Wang, Y.; Li, S.; Hao, Z.; Li, D. Birds and plastic pollution: Recent advances. Avian Res. 2021, 12, 59. [Google Scholar] [CrossRef]
- Hardesty, B.D.; Good, T.P.; Wilcox, C. Novel methods, new results and science-based solutions to tackle marine debris impacts on wildlife. Ocean Coast. Manag. 2015, 115, 4–9. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.W.S.; Teo, J.Y.Q.; Loh, X.J.; Lim, J.Y.C. Polyolefins and Polystyrene as Chemical Resources for a Sustainable Future: Challenges, Advances, and Prospects. ACS Mater. Lett. 2021, 3, 1660–1676. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Barrick, A.; Champeau, O.; Chatel, A.; Manier, N.; Northcott, G.; Tremblay, L.A. Plastic additives: Challenges in ecotox hazard assessment. PeerJ 2021, 9, e11300. [Google Scholar] [CrossRef]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Obst, M.; Brachmann, A.; Horn, M.A.; Rambold, G. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci. Rep. 2021, 11, 13214. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Singh, J.J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2020, 39, 31–34. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Mohammadreza Davoodi, S.; Pulicharla, R.; Kaur Brar, S.; Mag-douli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Uses of laccases in the food industry. Enzyme. Res. 2010, 2010, 918761. [Google Scholar] [CrossRef] [PubMed]
- Santo, M.; Weitsman, R.; Sivan, A. The Role of the Copper-Binding Enzyme—Laccase—In the Biodegradation of Polyethylene by the Actinomycete Rhodococcus Ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2023, 13, 1001750. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth Rates Made Easy. Mol. Biol. Evol. 2013, 31, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Doelle, H.W.; Fiechter, A.; Schlegel, G.; Sell, D.; Shimizu, S.; Ulber, R.; Yamada, H. Biotechnology, 1. General. Ullmann’s Encycl. Ind. Chem. 2009, 5, 689–712. [Google Scholar] [CrossRef]
- Ali, S.R.M.; Fradi, A.J.; Al-araji, A.M. Effect of some physical factors on growth of five fungal species. Eur. Acad. Res. 2017, 2, 1069–1078. [Google Scholar]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 19, 112. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.-P. Hydrophobins—Unique Fungal Proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environ. Monit. Assess. 2014, 186, 577–6586. [Google Scholar] [CrossRef]
- Gómez-Méndez, L.D.; Moreno-Bayona, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Pedroza-Rodríguez, A.M.; Vargas, A.; Bogoya, J.M. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE 2018, 13, e0203786. [Google Scholar] [CrossRef]
- Ganesh, P.; Dineshrajn, D.; Yoganathan, K. Production and screening of depolymerising enzymes by potential bacteria and fungi isolated from plastic waste dump yard sites. Int. J. Appl. Res. 2017, 3, 693–695. [Google Scholar]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.B.; Song, H.A.; Lee, T.K. Accelerating the Biodegradation of High-Density Polyethylene (HDPE) Using Bjerkandera adusta TBB-03 and Lignocellulose Substrates. Microorganisms 2019, 7, 304. [Google Scholar] [CrossRef]
- Khatoon, N.; Jamal, A.; Ali, M.I. Lignin peroxidase isoenzyme: A novel approach to biodegrade the toxic synthetic polymer waste. Environ. Technol. 2019, 40, 1366–1375. [Google Scholar] [CrossRef]
- Sumathi, T.; Viswanath, B.; Sri Lakshmi, A.; SaiGopal, D.V.R. Production of Laccase by Cochliobolus sp. Isolated from Plastic Dumped Soils and Their Ability to Degrade Low Molecular Weight PVC. Biochem. Res. Int. 2016, 2016, 9519527. [Google Scholar] [CrossRef]
- Liebminger, S.; Eberl, A.; Sousa, F.; Heumann, S.; Fischer-Colbrie, G.; Cavaco-Paulo, A.; Guebitz, G.M. Hydrolysis of PET and bis-(benzoyloxyethyl) terephthalate with a new polyesterase from Penicillium citrinum. Biocatal. Biotransform. 2007, 25, 171–177. [Google Scholar] [CrossRef]
- Carniel, A.; Valoni, É.; Nicomedes, J.; da Conceição Gomes, A.; de Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Tahir, L.; Ali, M.I.; Zia, M.; Atiq, N.; Hasan, F.; Ahmed, S. Production and Characterization of Esterase in Lantinus tigrinus for Degradation of Polystyrene. Pol. J. Microbiol. 2013, 62, 101–108. [Google Scholar] [CrossRef]
- Takamoto, T.; Shirasaka, H.; Uyama, H.; Kobayashi, S. Lipase-catalyzed hydrolytic degradation of polyurethane in organic solvent. Chem. Lett. 2001, 30, 492–493. [Google Scholar] [CrossRef]
- Mathur, G.; Prasad, R. Degradation of Polyurethane by Aspergillus flavus (ITCC 6051) Isolated from Soil. Appl. Biochem. Biotechnol. 2012, 167, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.G.; Jeon, H.J.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J. Bioremed. Biodegrad. 2012, 3, 145. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. Int. Biodeterior. Biodegrad. 2015, 103, 141–146. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Taniguchi, I.; Oda, K. Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. Meth. Enzymol. 2021, 648, 187–205. [Google Scholar] [CrossRef]
- Jabloune, R.; Khalil, M.; Ben Moussa, I.E.; Simao-Beaunoir, A.-M.; Lerat, S.; Brzezinski, R.; Beaulieu, C. Enzymatic Degradation of p-Nitrophenyl Esters, Polyethylene Terephthalate, Cutin, and Suberin by Sub1, a Suberinase Encoded by the Plant Pathogen Streptomyces scabies. Microbes Environ. 2020, 35, ME19086. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.-J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.-D. Enzymatic Degradation of Poly(Ethylene Terephthalate): Rapid Hydrolyse Using a Hydrolase from T. Fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Nakajima-Kambe, T.; Onuma, F.; Kimpara, N.; Nakahara, T. Isolation and Characterization of a Bacterium Which Utilizes Polyester Polyurethane as a Sole Carbon and Nitrogen Source. FEMS Microbiol. Lett. 1995, 129, 39–42. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A Review of the Fungi That Degrade Plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef]
- Lear, G.; Kingsbury, J.; Franchini, S.; Gambarini, V.; Maday, S.; Wallbank, J.; Weaver, L.; Pantos, O. Plastics and the Microbiome: Impacts and Solutions. Environ. Microbiome 2021, 16, 2. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- DSouza, G.C.; Sheriff, R.S.; Ullanat, V.; Shrikrishna, A.; Joshi, A.V.; Hiremath, L.; Entoori, K. Fungal biodegradation of low-density polyethylene using consortium of Aspergillus species under controlled conditions. Heliyon 2021, 7, e07008. [Google Scholar] [CrossRef]
- Kyaw, B.M.; Champakalakshmi, R.; Kishore Sakharkar, M.; Lim, C.S.; Sakharkar, K.R. Biodegradation of low density poly-ethene (LDPE) by Pseudomonas species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.L.; Carreres-Calabuig, J.A.; Gorokhova, E.; Posth, N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. Lett. 2020, 5, 18–36. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, H.R.; Jeon, E.; Yu, H.C.; Lee, S.; Li, J.; Kim, D.-H. Evaluation of the biodegradation efficiency of four various types of plastics by Pseudomonas aeruginosa isolated from the gut extract of superworms. Microorganisms 2020, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Screening for polypropylene degradation potential of bacteria isolated from Mangrove ecosystems in Peninsular Malaysia. Int. J. Biosci. Biochem. Bioinform. 2017, 7, 245–251. [Google Scholar] [CrossRef]
- Sangeetha Devi, R.; Rajesh Kannan, V.; Nivas, D.; Kannan, K.; Chandru, S.; Robert Antony, A. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef]
- Sáenz, M.; Borodulina, T.; Diaz, L.; Banchon, C. Minimal Conditions to Degrade Low Density Polyethylene by Aspergillus terreus and niger. J. Ecol. Eng. 2019, 20, 44–51. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508. [Google Scholar] [CrossRef]
- Kučić Grgić, D.; Miloloža, M.; Ocelić Bulatović, V.; Ukić, Š.; Slouf, M.; Gajdosova, V. Screening the Efficacy of a Microbial Consortium of Bacteria and Fungi Isolated from Different Environmental Samples for the Degradation of LDPE/TPS Films. Separations 2023, 10, 79. [Google Scholar] [CrossRef]
- Black, J.G. Microbiology: Principles and Explorations, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 975. [Google Scholar]
- Gong, Y.; Ding, P.; Xu, M.-J.; Zhang, C.-M.; Xing, K.; Qin, S. Biodegradation of phenol by a halotolerant versatile yeast Candida tropicalis SDP-1 in wastewater and soil under high salinity conditions. J. Environ. Manag. 2021, 289, 112525. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Wolski, E.A.; Murialdo, S.E.; Gonzalez, J.F. Effect of pH and inoculum size on pentachlorophenol degradation by Pseudomonas sp. Water SA 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Aranda, B. Microbial Growth under Limiting Conditions-Future Perspectives. Microorganisms 2023, 11, 1641. [Google Scholar] [CrossRef]
- Peixoto, J.; Silva, L.P.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef]
- Lou, Y.; Li, Y.; Lu, B.; Liu, Q.; Yang, S.-S.; Liu, B.; Ren, N.; Wu, W.-M.; Xiang, D. Response of the yellow mealworm (Tenebrio molitor) gut microbiome to diet shifts during polystyrene and polyethylene biodegradation. J. Hazard. Mater. 2021, 416, 126222. [Google Scholar] [CrossRef]
- Liu, J.; Xu, G.; Dong, W.; Xu, N.; Xian, F.; Ma, J.; Fang, Y.; Zhou, J.; Jiang, M. Biodegradation of diethyl terephthalate (DET) and polyethylene terephthalate (PET) by a novel identified degrader Delftia sp. WL-3 and its proposed metabolic pathway. Lett. Appl. Microbiol. 2018, 67, 254–261. [Google Scholar] [CrossRef]
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and Properties of a Polyester Polyurethane-Degrading Enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Pedersen, J.N.; Eser, B.E.; Guo, Z. Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol. Adv. 2022, 60, 107991. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Piñeros, M.A.; Martínez-Lavanchy, P.M.; Jehmlich, N.; Pieper, D.H.; Rincón, C.A.; Harms, H.; Junca, H.; Heipieper, H.J. Delftia sp. LCW, a strain isolated from a constructed wetland shows novel properties for dimethylphenol isomers degradation. BMC Microbiol. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Coon, C.M.; Doherty, M.E.; McHugh, E.A.; Warner, M.C.; Walters, C.L.; Orahood, O.M.; Loesch, A.E.; Hatfield, D.C.; Sitko, J.C.; et al. Engineering and characterization of dehalogenase enzymes from Delftia acidovorans in bioremediation of perfluorinated compounds. Synth. Syst. Biotechnol. 2022, 7, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Alamri, S.A.; Al-Zomyh, S.S.A.A.; Alrumman, S.A. Biodegradation and detoxification of aliphatic and aromatic hydrocarbons by new yeast strains. Ecotoxicol. Environ. Saf. 2018, 151, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Morshed, M.; Tavani, H. Effects of Enzymatic Hydrolysis on Drawn Polyester Filament Yarns. Iran. Polym. J. 2001, 10, 363–370. [Google Scholar]
- Zafar, U.; Houlden, A.; Robson, G.D. Fungal communities associated with the biodegradation of polyester polyurethane buried under compost at different temperatures. Appl. Environ. Microbiol. 2013, 79, 7313–7324. [Google Scholar] [CrossRef] [PubMed]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Virulence of Clinical Candida Isolates. Pathogens 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- Devi, R.; Kannan, V.; Natarajan, K.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A. The Role of Microbes in Plastic Degradation. In Environmental Waste Management; Chandra, R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 355–384. [Google Scholar] [CrossRef]
- Singh, B.; Christina, E. Chapter 13—Indigenous microorganisms as an effective tool for in situ bioremediation. In Relationship between Microbes and the Environment for Sustainable Ecosystem Services; Samuel, J., Kumar, A., Singh, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 273–295. [Google Scholar] [CrossRef]
- Magan, N. Fungi in Extreme Environments. In Environmental and Microbial Relationships; Kubicek, C.P., Druzhinina, I.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 85–103. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.R.; He, H.W.; Sang, B.; Yu, D.L.; Feng, J.T.; Zhang, X. Effects of Agitation, Aeration and Temperature on Production of a Novel Glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and Scale-Up Based on Volumetric Oxygen Transfer Coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef]
- Valdez-Cruz, N.A.; Reynoso-Cereceda, G.I.; Pérez-Rodriguez, S.; Restrepo-Pineda, S.; González-Santana, J.; Olvera, A.; Zavala, G.; Alagón, A.; Trujillo-Roldán, M.A. Production of a recombinant phospholipase A2 in Escherichia coli using resonant acoustic mixing that improves oxygen transfer in shake flasks. Microb. Cell Fact. 2017, 16, 129. [Google Scholar] [CrossRef]
- Seth, M.; Chand, S. Biosynthesis of tannase and hydrolysis of tannins to gallic acid by Aspergillus awamori—Optimisation of process parameters. Process Biochem. 2000, 36, 39–44. [Google Scholar] [CrossRef]
- Ibrahim, D.; Weloosamy, H.; Lim, S.-H. Effect of agitation speed on the morphology of Aspergillus niger HFD5A-1 hyphae and its pectinase production in submerged fermentation. World. J. Biol. Chem. 2015, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahhaf, Z.Y. Hematological and biochemical effects of an air freshener in rabbits. J. Am. Sci. 2012, 8, 70–73. [Google Scholar]
- Philibert, D.; Parketon, T.; Marteinson, S.; de Jourdan, B. Assessing the Toxicity of Individual Aromatic Compounds and Mixtures to American Lobster (Homarus americanus) Larvae Using a Passive Dosing System. Environ. Toxicol. Chem. 2021, 40, 1379–1388. [Google Scholar] [CrossRef]
- Miloloža, M.; Bule, K.; Prevarić, V.; Cvetnić, M.; Ukić, Š.; Bolanča, T.; Kučić Grgić, D. Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae. Polymers 2022, 14, 1246. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2008, 127, 704–716. [Google Scholar] [CrossRef]
- Ul-Hamid, A.; Soufi, K.Y.; Al-Hadhrami, L.M.; Shemsi, A.M. Failure investigation of an underground low voltage XLPE insulated cable. Anti-Corros. Methods Mater. 2015, 62, 281–287. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectroscopy of Alkenes. Spectroscopy 2016, 31, 28–34. Available online: https://www.spectroscopyonline.com/view/infrared-spectroscopy-alkenes (accessed on 5 June 2023).
- Smith, B.C. The Infrared Spectra of Polymers IV: Rubbers. Spectroscopy 2022, 37, 8–12. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/HR/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 5 June 2023).
- IR Spectroscopy Tutorial: Alkenes. Available online: https://orgchemboulder.com/Spectroscopy/irtutor/alkenesir.shtml (accessed on 5 June 2023).
- Amar, Z.H.; Chabira, S.F.; Sebaa, M.; Ahmed, B. Structural changes undergone during thermal aging and/or processing of Unstabilized, dry-blend and rigid PVC, investigated by FTIR-ATR and curve fitting. Ann. Chim. Sci. Mater. 2019, 43, 59–68. [Google Scholar] [CrossRef]
- Ma, D.; Liang, L.; Hu, E.; Chen, H.; Wang, D.; He, C.; Feng, Q. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process Saf. Environ. Prot. 2021, 146, 108–117. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ. Sci. Pollut. Res. 2019, 26, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Subramani, M.; Sepperumal, U. FTIR analysis of bacterial mediated chemical changes in Polystyrene foam. Ann. Biol. Res. 2016, 7, 55–61. [Google Scholar]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- MassBank Record: MSBNK-Eawag-EQ363802. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-Eawag-EQ363802 (accessed on 23 November 2023).
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- MassBank Record: MSBNK-BGC_Munich-RP019801. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-BGC_Munich-RP019801&dsn=BGC_Munich (accessed on 23 November 2023).
- MassBank Record: MSBNK-Antwerp_Univ-AN112602. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-Antwerp_Univ-AN112602&dsn=Antwerp_Univ (accessed on 23 November 2023).
- Plasticisers. Available online: http://www.seepvcforum.com/en/content/16-plasticisers (accessed on 23 November 2023).
- Thew, X.E.C.; Lo, S.C.; Ramanan, R.N.; Tey, B.T.; Huy, N.D.; Wei, O.C. Enhancing plastic biodegradation process: Strategies and opportunities. Crit. Rev. Biotechnol. 2023. Online ahead of print. [Google Scholar] [CrossRef]





| For Bacteria | For Yeasts | ||
|---|---|---|---|
| Substance | Conc./g L−1 | Substance | Conc./g L−1 |
| K2HPO4 | 12.500 | K2HPO4 | 1.000 |
| KH2PO4 | 3.800 | KH2PO4 | 1.000 |
| (NH4)2SO4 | 1.000 | NH4NO3 | 1.000 |
| H3BO3 | 0.232 | NaCl | 0.500 |
| ZnSO4·7H2O | 0.174 | MgSO4·7H2O | 0.200 |
| FeSO4(NH4)2SO4·6H2O | 0.116 | CaCl2 | 0.020 |
| MgSO4·7H2O | 0.100 | FeCl3·6H2O | 0.005 |
| CoSO4·7H2O | 0.096 | ||
| (NH4)6Mo7O24·4H2O | 0.022 | ||
| CuSO4·5H2O | 0.008 | ||
| MnSO4·4H2O | 0.008 | ||
| Condition | For Bacteria | For Yeasts |
|---|---|---|
| pH | 7.0 ± 0.2 | 6.0 ± 0.2 |
| T/°C | 25.0 ± 0.2 | 25.0 ± 0.2 |
| ODinitial | 0.5 | 1.0 |
| AS/rpm | 150 | 160 |
| Microorganism | Type of MPs | ODinitial | AS/rpm | pH | log CFU * |
|---|---|---|---|---|---|
| Delftia acidovorans | PS | 0.9 | 120 | 7.95 | 8.10 |
| PVC | 1.0 | 120 | 7.45 | 8.40 | |
| Candida parapsilosis | PS | 1.0 | 156 | 5.67 | 7.24 |
| PVC | 1.0 | 136 | 4.94 | 7.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Ocelić Bulatović, V.; Mandić, V.; Bafti, A.; Ukić, Š.; Kučić Grgić, D.; Bolanča, T. Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization. Environments 2023, 10, 207. https://doi.org/10.3390/environments10120207
Bule Možar K, Miloloža M, Martinjak V, Cvetnić M, Ocelić Bulatović V, Mandić V, Bafti A, Ukić Š, Kučić Grgić D, Bolanča T. Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization. Environments. 2023; 10(12):207. https://doi.org/10.3390/environments10120207
Chicago/Turabian StyleBule Možar, Kristina, Martina Miloloža, Viktorija Martinjak, Matija Cvetnić, Vesna Ocelić Bulatović, Vilko Mandić, Arijeta Bafti, Šime Ukić, Dajana Kučić Grgić, and Tomislav Bolanča. 2023. "Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization" Environments 10, no. 12: 207. https://doi.org/10.3390/environments10120207
APA StyleBule Možar, K., Miloloža, M., Martinjak, V., Cvetnić, M., Ocelić Bulatović, V., Mandić, V., Bafti, A., Ukić, Š., Kučić Grgić, D., & Bolanča, T. (2023). Bacteria and Yeasts Isolated from the Environment in Biodegradation of PS and PVC Microplastics: Screening and Treatment Optimization. Environments, 10(12), 207. https://doi.org/10.3390/environments10120207