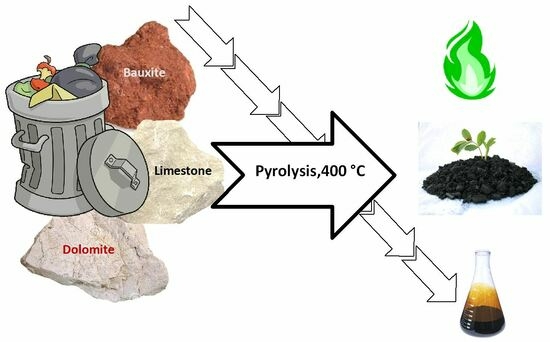
Home Trash Biomass Valorization by Catalytic Pyrolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Thermogravimetry Analysis (TGA)
3.2. Fixed Bed Reactor Experiments
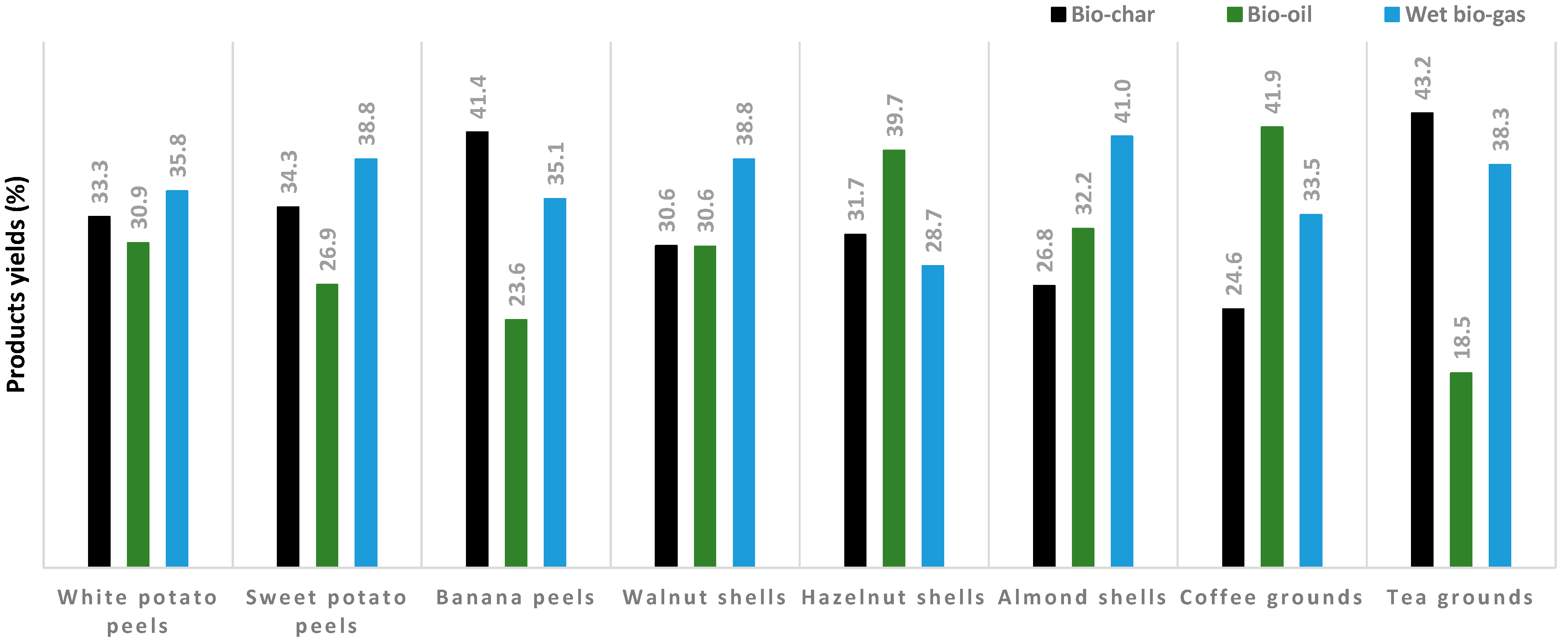
3.2.1. Pyrolysis Yields
3.2.2. Bio-Oil Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Bio-Oil Infrared Bands Attribution
| Band Position (cm−1) | Functional Group | Band Assignment |
|---|---|---|
| 1767 | C=O | Lactones |
| 1740 | C=O | Unconjugated alkyl aldehydes and alkyl esters |
| 1713 | C=O | Carboxylic acids (and fatty acids) |
| 1696 | C=O | Unsaturated aldehydes, ketones |
| 1640 | C=O | Hydroxyunsaturated ketones, aldehydes |
| 1600 | C=C | Aromatics with various types of substitution |
| 1575 | C=C | |
| 1517 | C=C | |
| 1501 | C=C | |
| 3008 | C=C | Symmetric stretching vibration of aromatics |
| 3000–2800 | C–H | Aliphatics |
| 3650–3100 | OH | Carboxylic acids, alcohols, and phenols |
| 1645 | NH | Amines |
| 3363 (broad) | OH | Water, alcohols, organic acids |
| NH | ||
| 1659 | C=C | Stretching vibration of carbon–carbon double bonds |
| C=O | Stretching vibration of carbonyl groups | |
| 1455 | CH2, CH3 | Bending vibrations of aliphatic groups |
| 1406 | C–H | Rocking vibrations of olefins |
| 1028 | C–O | Stretching vibration of ester groups |
| 880 | C–H | Out-of-plane deformation vibration of terminal olefins |
| 666 (broad) | CH2 | Overlapping of the rocking vibration and the out-of-plane vibration of cis-disubstituted olefins |
References
- Trends in Solid Waste Management, (n.d.). Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 30 September 2022).
- Estimates of European Food Waste Levels, (n.d.). Available online: https://www.eu-fusions.org/phocadownload/Publications/EstimatesofEuropeanfoodwastelevels.pdf (accessed on 3 October 2022).
- Li, H.; Xu, J.; Nyambura, S.M.; Wang, J.; Li, C.; Zhu, X.; Feng, X.; Wang, Y. Food waste pyrolysis by traditional heating and microwave heating: A review. Fuel 2022, 324, 124574. [Google Scholar] [CrossRef]
- Sridhar, A.; Kapoor, A.; Kumar, P.S.; Ponnuchamy, M.; Balasubramanian, S.; Prabhakar, S. Conversion of food waste to energy: A focus on sustainability and life cycle assessment. Fuel 2021, 302, 121069. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lin, K.-Y.A.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Cheng, F.; Bayat, H.; Jena, U.; Brewer, C.E. Impact of feedstock composition on pyrolysis of low-cost, protein- and lignin-rich biomass: A review. J. Anal. Appl. Pyrolysis 2020, 147, 104780. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Fattah, I.M.R.; Ok, Y.S.; Jang, J.-H.; Wang, C.-T. State-of-the-art of the pyrolysis and co-pyrolysis of food waste: Progress and challenges. Sci. Total. Environ. 2022, 809, 151170. [Google Scholar] [CrossRef] [PubMed]
- Amrullah, A.; Farobie, O.; Septarini, S.; Satrio, J.A. Synergetic biofuel production from co-pyrolysis of food and plastic waste: Reaction kinetics and product behavior. Heliyon 2022, 8, e10278. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Fu, M.-M.; Mo, C.-H.; Li, H.; Zhang, Y.-N.; Huang, W.-X.; Wong, M.H. Comparison of physicochemical properties of biochars and hydrochars produced from food wastes. J. Clean. Prod. 2019, 236, 117637. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Ramos, M.; de Jesus, N.; Puna, J. Catalyzed pyrolysis of coffee and tea wastes. Energy 2021, 235, 121252. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Dias, A.P.S. Almond shells: Catalytic fixed-bed pyrolysis and volatilization kinetics. Renew. Energy 2021, 180, 1380–1390. [Google Scholar] [CrossRef]
- Ly, H.V.; Tran, Q.K.; Kim, S.-S.; Kim, J.; Choi, S.S.; Oh, C. Catalytic upgrade for pyrolysis of food waste in a bubbling fluidized-bed reactor. Environ. Pollut. 2021, 275, 116023. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Rijo, B.; Ramos, M.; Casquilho, M.; Rodrigues, A.; Viana, H.; Rosa, F. Pyrolysis of burnt maritime pine biomass from forest fires. Biomass Bioenergy 2022, 163, 106535. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Wojnicki, Ł. Catalyzed pyrolysis of scrap tires rubber. J. Environ. Chem. Eng. 2022, 10, 107037. [Google Scholar] [CrossRef]
- Rijo, B.; Dias, A.P.S.; Ramos, M.; Ameixa, M. Valorization of forest waste biomass by catalyzed pyrolysis. Energy 2022, 243, 122766. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Wang, X.; Zhang, Y.; Lu, H.; Duan, N.; Li, B.; Zhang, D.; Dong, T.; Liu, Z. Biogas liquid digestate grown Chlorella sp. for biocrude oil production via hydrothermal liquefaction. Sci. Total. Environ. 2018, 635, 70–77. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; Elhassan, O.; Mansour, S.; McKay, G. Biochar development from thermal TGA studies of individual food waste vegetables and their blended systems. Biomass Convers. Biorefinery 2022, 1, 1–18. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Fernandez, A.; Palacios, C.; Echegaray, M.; Mazza, G.; Rodriguez, R. Pyrolysis and Combustion of Regional Agro-Industrial Wastes: Thermal Behavior and Kinetic Parameters Comparison. Combust. Sci. Technol. 2017, 190, 114–135. [Google Scholar] [CrossRef]
- Chen, X.; Wu, R.; Sun, Y.; Jian, X. Synergistic Effects on the Co-pyrolysis of Agricultural Wastes and Sewage Sludge at Various Ratios. ACS Omega 2022, 7, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Wahia, H.; Zhang, L.; Zhou, C.; Ma, H. State-of-the-art co-pyrolysis of lignocellulosic and macroalgae biomass feedstocks for improved bio-oil production—A review. Fuel 2023, 332, 126071. [Google Scholar] [CrossRef]
- Surahmanto, F.; Saptoadi, H.; Sulistyo, H.; A Rohmat, T. Investigation of the pyrolysis characteristics and kinetics of oil-palm solid waste by using Coats–Redfern method. Energy Explor. Exploit. 2020, 38, 298–309. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; E, J.; Xue, Y. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Qing, M.; Long, Y.; Liu, L.; Yi, Y.; Li, W.; He, R.; Yin, Y.; Tian, H.; He, J.; Cheng, S.; et al. Pyrolysis of the food waste collected from catering and households under different temperatures: Assessing the evolution of char structure and bio-oil composition. J. Anal. Appl. Pyrolysis 2022, 164, 105543. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Jo, J.-H.; Kim, S.-S.; Shim, J.-W.; Lee, Y.-E.; Yoo, Y.-S. Pyrolysis Characteristics and Kinetics of Food Wastes. Energies 2017, 10, 1191. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Ji, Y.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y. Thermal decomposition of castor oil, corn starch, soy protein, lignin, xylan, and cellulose during fast pyrolysis. Bioresour. Technol. 2019, 278, 287–295. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, J.; Yu, G. Understanding the Effect of Different Biomass Ash Additions on Pyrolysis Product Distribution, Char Physicochemical Characteristics, and Char Gasification Reactivity of Bituminous Coal. Energy Fuels 2019, 33, 3068–3076. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Rego, F.; Fonseca, F.; Casquilho, M.; Rosa, F.; Rodrigues, A. Catalyzed pyrolysis of SRC poplar biomass. Alkaline carbonates and zeolites catalysts. Energy 2019, 183, 1114–1122. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent Modeling Approaches to Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Y. Catalytic pyrolysis of cellulose and chitin with calcined dolomite—Pyrolysis kinetics and products analysis. Fuel 2022, 312, 122875. [Google Scholar] [CrossRef]
- Ly, H.V.; Lim, D.-H.; Sim, J.W.; Kim, S.-S.; Kim, J. Catalytic pyrolysis of tulip tree (Liriodendron) in bubbling fluidized-bed reactor for upgrading bio-oil using dolomite catalyst. Energy 2018, 162, 564–575. [Google Scholar] [CrossRef]
- Prabhakara, H.M.; Bramer, E.; Brem, G. Role of dolomite as an in-situ CO2 sorbent and deoxygenation catalyst in fast pyrolysis of beechwood in a bench scale fluidized bed reactor. Fuel Process. Technol. 2021, 224, 107029. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Cheng, Y.; Liu, Y.; Cobb, K.; Chen, P.; et al. Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification—A review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, J.; Liu, L.; Wang, J. The evolution of catalytically active calcium catalyst during steam gasification of lignite char. Carbon 2021, 172, 162–173. [Google Scholar] [CrossRef]
- Cai, R.; Pei, X.; Pan, H.; Wan, K.; Chen, H.; Zhang, Z.; Zhang, Y. Biomass Catalytic Pyrolysis over Zeolite Catalysts with an Emphasis on Porosity and Acidity: A State-of-the-Art Review. Energy Fuels 2020, 34, 11771–11790. [Google Scholar] [CrossRef]
- Modak, S.; Jain, S.; Katiyar, P.; Yadav, S. Compositional study of bio-oil obtained from torrefaction of mixed food waste using H-NMR, 13C-NMR, DEPT 90 & 135, and GC-MS. Biofuels 2022, 14, 183–189. [Google Scholar] [CrossRef]
- Kraiem, T.; Ben Hassen, A.; Belayouni, H.; Jeguirim, M. Production and characterization of bio-oil from the pyrolysis of waste frying oil. Environ. Sci. Pollut. Res. 2017, 24, 9951–9961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Bi, D.; Wang, T.; Gao, Z.; Liu, J. Co-pyrolysis of cellulose and urea blend: Nitrogen conversion and effects of parameters on nitrogenous compounds distributions in bio-oil. J. Anal. Appl. Pyrolysis 2021, 157, 105177. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, X.; Zhu, S.; Hu, L.; Zhang, Q. Upgrading of bio-oil from catalytic pyrolysis of pretreated rice husk over Fe-modified ZSM-5 zeolite catalyst. Fuel Process. Technol. 2018, 175, 17–25. [Google Scholar] [CrossRef]
- Pawar, A.; Panwar, N.L.; Salvi, B.L. Comprehensive review on pyrolytic oil production, upgrading and its utilization. J. Mater. Cycles Waste Manag. 2020, 22, 1712–1722. [Google Scholar] [CrossRef]
- Cai, Q.; Yu, T.; Zhang, S. Enhanced aromatic hydrocarbon production from bio-oil hydrotreating-cracking by Mo-Ga modified HZSM-5. Fuel 2020, 269, 117386. [Google Scholar] [CrossRef]
- Lievens, C.; Mourant, D.; He, M.; Gunawan, R.; Li, X.; Li, C.-Z. An FT-IR spectroscopic study of carbonyl functionalities in bio-oils. Fuel 2011, 90, 3417–3423. [Google Scholar] [CrossRef]
- Du, H.; Deng, F.; Kommalapati, R.R.; Amarasekara, A.S. Iron based catalysts in biomass processing. Renew. Sustain. Energy Rev. 2020, 134, 110292. [Google Scholar] [CrossRef]
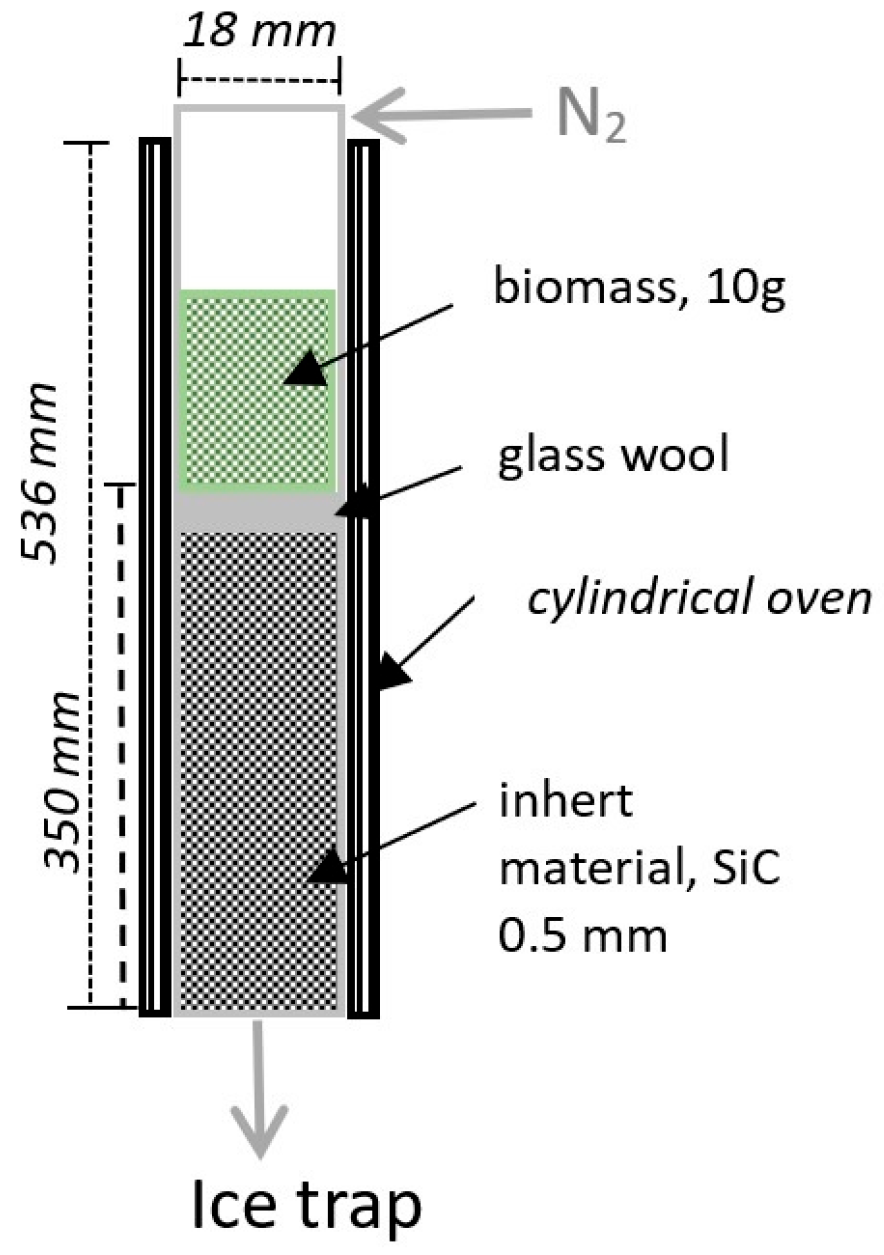


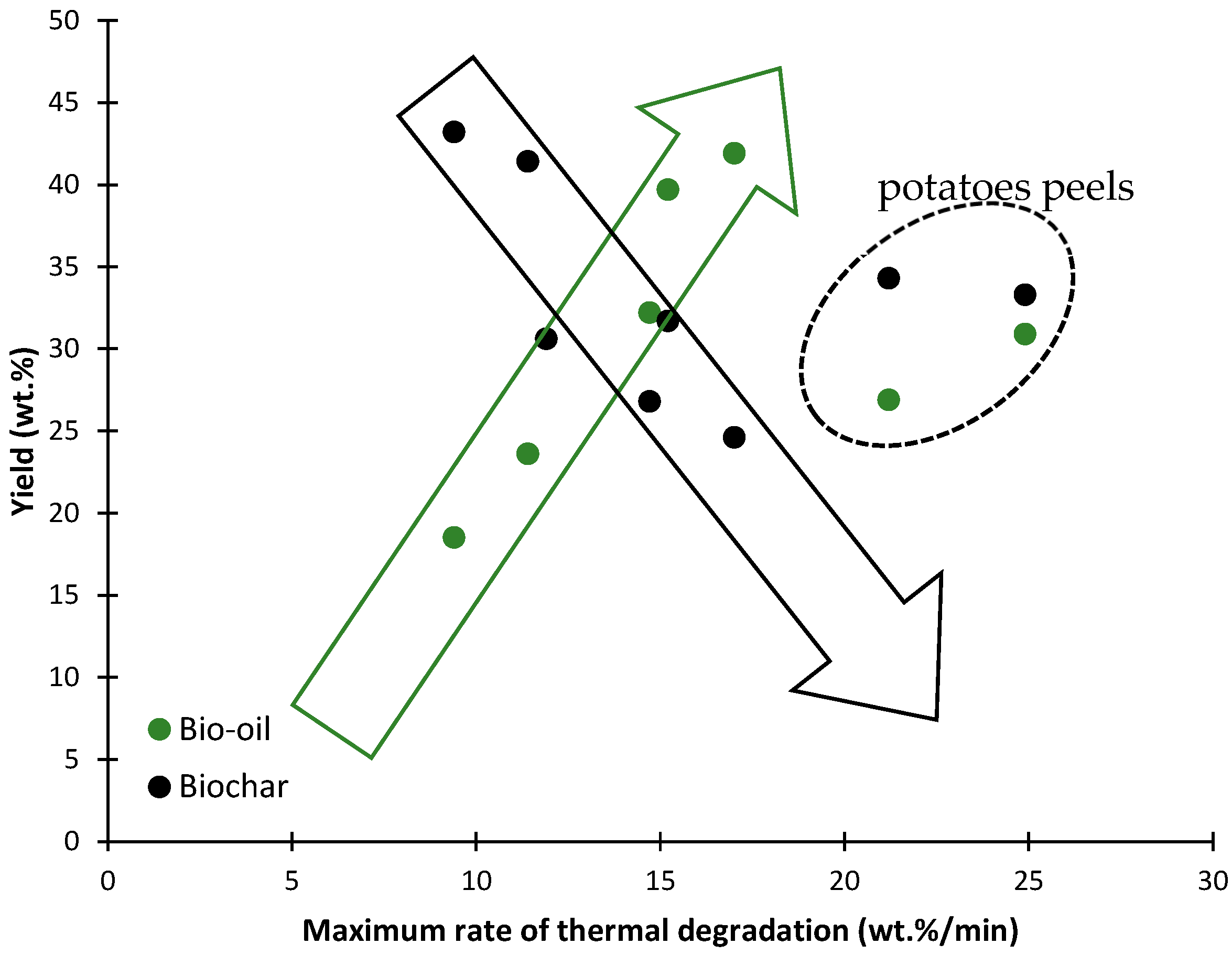





| Type | Main Features | Major Product |
|---|---|---|
| Slow | Slow heating rate < 1 °C/min 400 °C–500 °C | Biochar |
| Fast | High heating rate 600 °C–1000 °C | Bio-oil |
| Catalytic | 300 °C–600 °C | Biochar or bio-oil depending on the used catalyst |
| Microwave assisted | 400 °C–800 °C | Syngas and bio-oil |
| Hydropyrolysis | 300 °C–450 °C Hydrogen instead of nitrogen | Bio-oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijo, B.; Soares Dias, A.P.; de Jesus, N.; Pereira, M.F. Home Trash Biomass Valorization by Catalytic Pyrolysis. Environments 2023, 10, 186. https://doi.org/10.3390/environments10100186
Rijo B, Soares Dias AP, de Jesus N, Pereira MF. Home Trash Biomass Valorization by Catalytic Pyrolysis. Environments. 2023; 10(10):186. https://doi.org/10.3390/environments10100186
Chicago/Turabian StyleRijo, Bruna, Ana Paula Soares Dias, Nicole de Jesus, and Manuel Francisco Pereira. 2023. "Home Trash Biomass Valorization by Catalytic Pyrolysis" Environments 10, no. 10: 186. https://doi.org/10.3390/environments10100186
APA StyleRijo, B., Soares Dias, A. P., de Jesus, N., & Pereira, M. F. (2023). Home Trash Biomass Valorization by Catalytic Pyrolysis. Environments, 10(10), 186. https://doi.org/10.3390/environments10100186