Investigating Neural Reward Sensitivity in the School Grade Incentive Delay Task and Its Relation to Academic Buoyancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
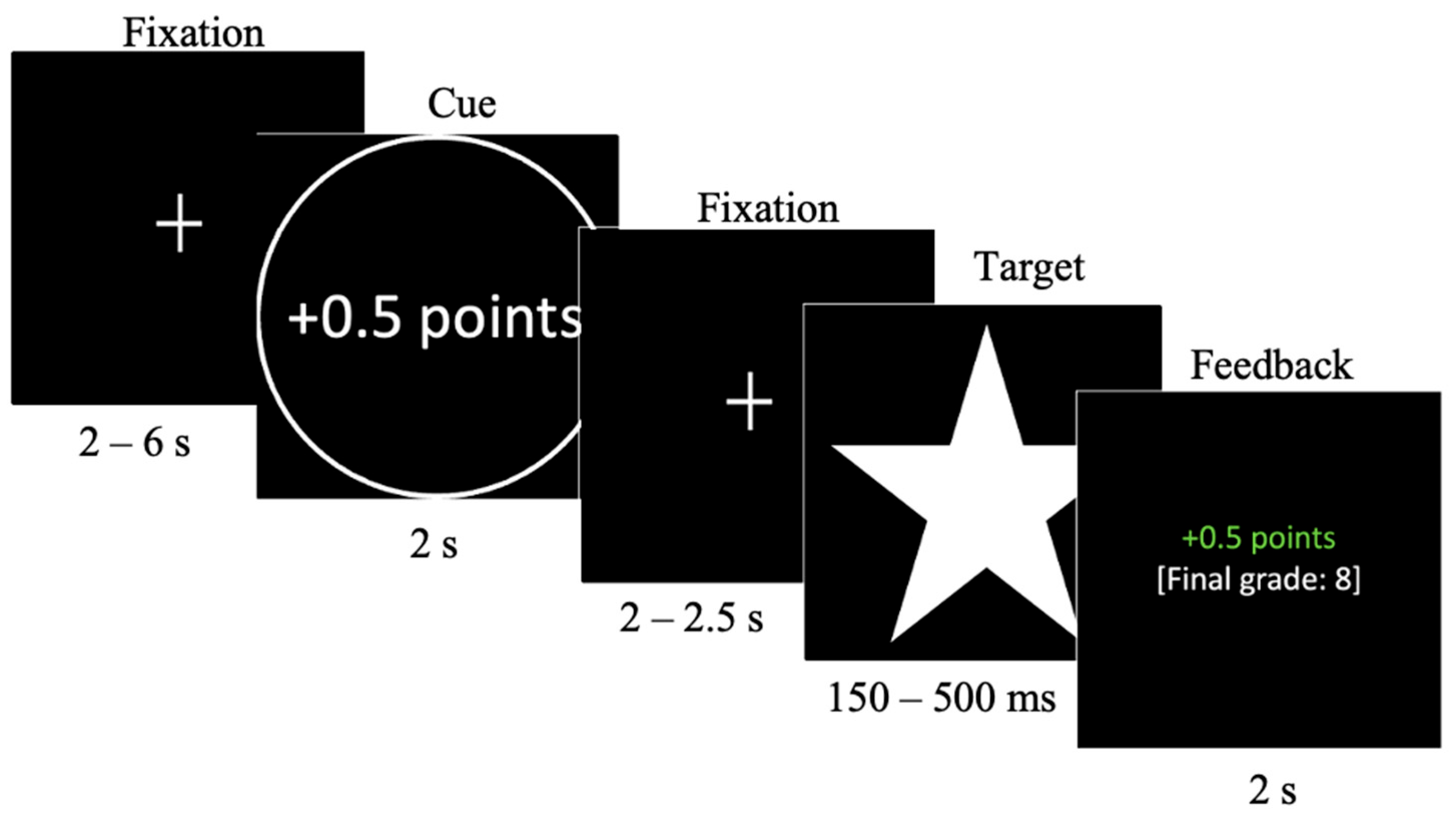
2.2.1. School Grade Incentive Delay Task
2.2.2. Academic Buoyancy Scale
2.3. Procedure
2.4. Data Acquisition
2.4.1. Behavioral Data
2.4.2. MRI Data
2.5. Data Analysis
3. Results
3.1. Descriptive Statistics
3.2. Behavioral Findings
3.3. fMRI Findings
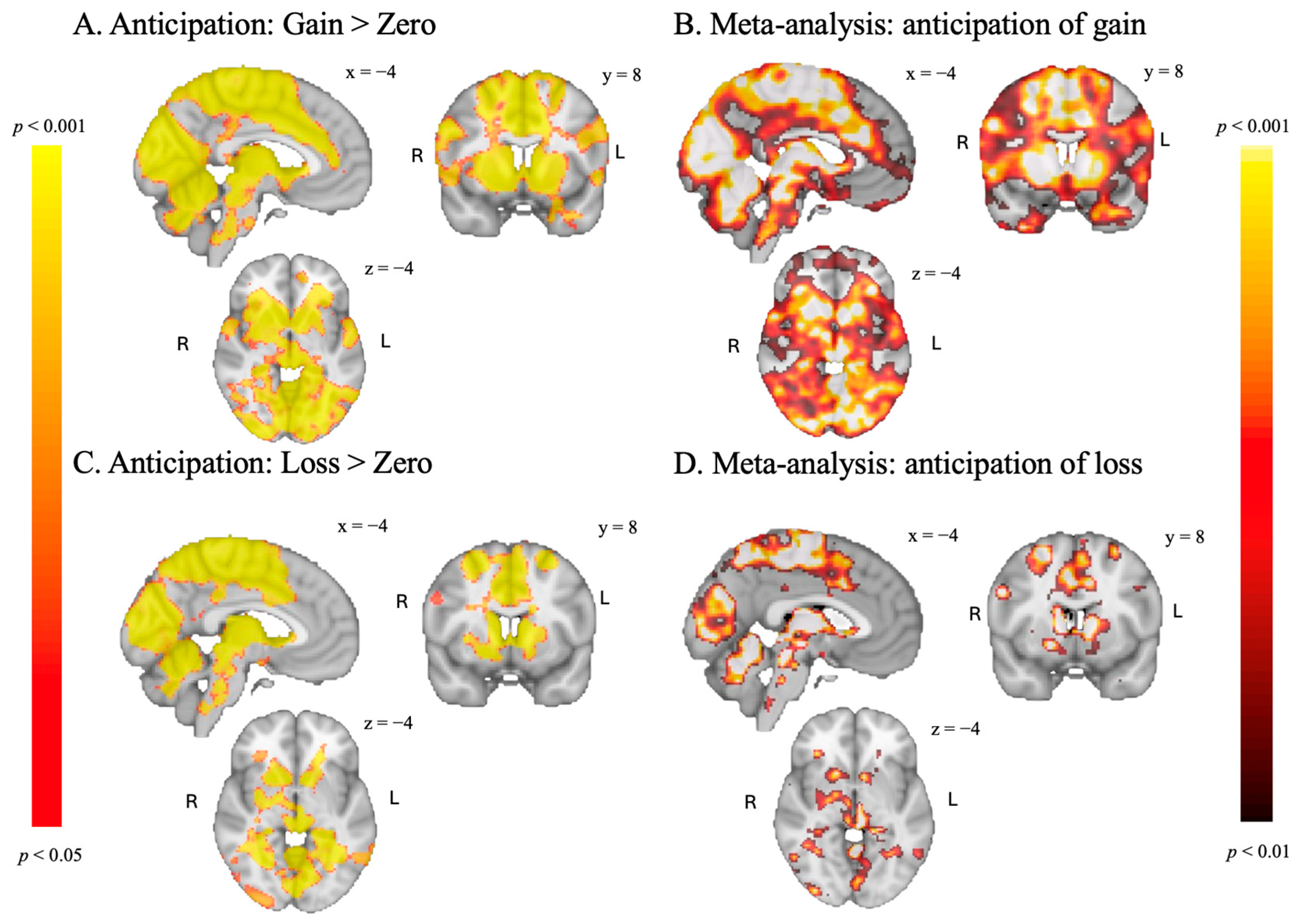
3.3.1. Anticipation Phase
3.3.2. Feedback Phase
3.3.3. Association Between Academic Buoyancy and Anticipation of Reward and Loss
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SGID | School Grade Incentive Delay (task) |
| MID | Monetary Incentive Delay (task) |
| Nacc | Nucleus accumbens |
| dlPFC | Dorsolateral prefrontal cortex |
| dACC | Dorsal anterior cingulate cortex |
| GUTS | Growing Up Together in Society |
| ABS | Academic Buoyancy Scale |
| EV | Explanatory variable |
| TFCE | Threshold-Free Cluster Enhancement |
| AI | Anterior insula |
Appendix A
| Contrast | Region of Local Maximum | MNI (X Y Z) | Corrected p-Value |
|---|---|---|---|
| Anticipation | |||
| Gain > Zero | |||
| Precuneus cortex | −8 −70 22 | 0.001 | |
| Central Opercular Cortex | −52 −22 14 | 0.003 | |
| Brain stem | 6 −38 −40 | 0.004 | |
| Superior Temporal Gyrus, anterior division | 62 4 0 | 0.009 | |
| Right Hippocampus | 28 −38 −2 | 0.003 | |
| Supramarginal Gyrus, anterior division | −50 −30 38 | 0.002 | |
| Superior Temporal Gyrus, anterior division | −54 −8 −10 | 0.003 | |
| Insular Cortex | −34 −8 12 | 0.003 | |
| Middle Temporal Gyrus, temporooccipital part | 42 −48 10 | 0.009 | |
| Lateral Occipital Cortex, inferior division | 54 −62 −6 | 0.003 | |
| Precentral Gyrus | −60 6 36 | 0.008 | |
| Cingulate Gyrus, posterior division | 14 −18 38 | 0.002 | |
| Inferior Frontal Gyrus, pars opercularis | −42 12 22 | 0.008 | |
| Precentral Gyrus | −42 4 30 | 0.008 | |
| Temporal Pole | −54 6 −8 | 0.004 | |
| Temporal Pole | −32 8 −32 | 0.011 | |
| Cingulate Gyrus, anterior division | −4 42 8 | 0.002 | |
| Loss > Zero | |||
| Precentral Gyrus | 30 −18 50 | <0.001 | |
| Cerebellum | 0 −62 −22 | <0.001 | |
| Occipital Pole | 34 −92 −6 | 0.014 | |
| Supramarginal Gyrus, posterior division | 36 −42 36 | 0.004 | |
| Cerebellum | −26 −48 −48 | 0.030 | |
| Lateral Occipital Cortex, superior division | 38 −74 34 | 0.003 | |
| Brain-Stem | −2 −32 −34 | 0.003 | |
| Cerebellum | −42 −44 −32 | 0.023 | |
| Right Cerebral White Matter | 30 28 20 | 0.008 | |
| Precentral Gyrus | 54 8 30 | 0.039 | |
| Cingulate Gyrus, posterior division | 14 −18 40 | 0.002 | |
| Cerebellum | 16 −54 −34 | 0.012 | |
| Left Cerebral White Matter | −28 −52 26 | <0.001 | |
| Inferior Temporal Gyrus, temporooccipital part | −46 −54 −20 | 0.003 | |
| Frontal Orbital Cortex | 34 32 −2 | 0.018 | |
| Left Cerebral White Matter | −16 26 36 | 0.009 | |
| Lateral Occipital Cortex, inferior division | 54 −60 −4 | 0.031 | |
| Middle Temporal Gyrus, temporooccipital part | −64 −60 −2 | 0.003 | |
| Left Hippocampus | −26 −24 −16 | 0.003 | |
| (no structure available in Harvard-Oxford or MNI atlases) | 4 −4 −14 | <0.001 | |
| Feedback | |||
| Gain > No Gain | |||
| Precentral Gyrus | −12 −32 68 | 0.004 | |
| Frontal Medial Cortex | −14 46 −4 | 0.010 | |
| Middle Frontal Gyrus | −28 32 46 | 0.018 | |
| Cerebellum | 34 −60 −42 | 0.018 | |
| Left Putamen | −14 6 −12 | 0.010 | |
| Temporal Fusiform Cortex, posterior division | −38 −26 −14 | 0.021 | |
| Cerebellum | 22 −68 −50 | 0.020 | |
| Left Cerebral White Matter | −22 38 −6 | 0.014 | |
| Cerebellum | −6 −66 −18 | 0.020 | |
| Juxtapositional Lobule Cortex (formerly known as Supplementary Motor Cortex) | 16 4 44 | 0.006 | |
| Left Hippocampus | −20 −10 −20 | 0.029 | |
| Frontal Pole | 18 64 0 | 0.018 | |
| Postcentral Gyrus | −54 −20 42 | 0.017 | |
| Inferior Temporal Gyrus, posterior division | −42 −12 −24 | 0.031 | |
| Right Cerebral White Matter | 30 26 24 | 0.020 | |
| Left Cerebral White Matter | −16 40 28 | 0.026 | |
| Superior Frontal Gyrus | 28 6 56 | 0.048 | |
| Lateral Occipital Cortex, superior division | −24 −62 56 | 0.021 | |
| Interior Temporal Gyrus, temporooccipital part | −52 −46 −32 | 0.030 | |
| Postcentral Gyrus | −52 −16 28 | 0.018 | |
| Left Cerebral White Matter | −20 −36 4 | 0.050 | |
| No Loss > Loss | |||
| Right Cerebral White Matter | 18 −8 28 | 0.011 | |
| Precentral Gyrus | 16 −26 62 | 0.011 | |
| Left Cerebral White Matter | 55 60 57 | 0.031 | |
| Left Cerebral White Matter | −20 −6 42 | 0.050 | |
| Postcentral Gyrus | 42 −32 44 | 0.032 | |
| Precentral Gyrus | 32 −16 66 | 0.021 | |
| Right Cerebral White Matter | 18 20 20 | 0.022 | |
| Right Cerebral White Matter | 18 −42 20 | 0.047 | |
| Postcentral Gyrus | 44 −22 38 | 0.044 |
References
- Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. [Google Scholar] [CrossRef]
- Ben-Zion, Z., Shany, O., Admon, R., Keynan, N. J., Avisdris, N., Balter, S. R., Shalev, A. Y., Liberzon, I., & Hendler, T. (2022). Neural responsivity to reward versus punishment shortly after trauma predicts long-term development of posttraumatic stress symptoms. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 7(2), 150–161. [Google Scholar] [CrossRef]
- Bjork, J. M., Knutson, B., Fong, G. W., Caggiano, D. M., Bennett, S. M., & Hommer, D. W. (2004). Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. The Journal of Neuroscience, 24(8), 1793–1802. [Google Scholar] [CrossRef]
- Carter, R. M., Macinnes, J. J., Huettel, S. A., & Adcock, R. A. (2009). Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience, 3, 21. [Google Scholar] [CrossRef]
- Chen, Y., Chaudhary, S., & Li, C.-S. R. (2022). Shared and distinct neural activity during anticipation and outcome of win and loss: A meta-analysis of the monetary incentive delay task. NeuroImage, 264, 119764. [Google Scholar] [CrossRef]
- Cohen, M. X. (2007). Individual differences and the neural representations of reward expectation and reward prediction error. Social Cognitive and Affective Neuroscience, 2(1), 20–30. [Google Scholar] [CrossRef]
- Corral-Frías, N. S., Nadel, L., Fellous, J.-M., & Jacobs, W. J. (2016). Behavioral and self-reported sensitivity to reward are linked to stress-related differences in positive affect. Psychoneuroendocrinology, 66, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Corral-Frías, N. S., Nikolova, Y. S., Michalski, L. J., Baranger, D. A. A., Hariri, A. R., & Bogdan, R. (2015). Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychological Medicine, 45(12), 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Crone, E. A., Bol, T., Braams, B. R., De Rooij, M., Franke, B., Franken, I., Gazzola, V., Güroğlu, B., Huizenga, H., Hulshoff Pol, H., Keijsers, L., Keysers, C., Krabbendam, L., Jansen, L., Popma, A., Stulp, G., Van Atteveldt, N., Van Duijvenvoorde, A., & Veenstra, R. (2024). Growing Up Together in Society (GUTS): A team science effort to predict societal trajectories in adolescence and young adulthood. Developmental Cognitive Neuroscience, 67, 101403. [Google Scholar] [CrossRef]
- Daw, N. D., O’Doherty, J. P., Dayan, P., Seymour, B., & Dolan, R. J. (2006). Cortical substrates for exploratory decisions in humans. Nature, 441(7095), 876–879. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J. M., & Creswell, J. D. (2018). The role of brain reward pathways in stress resilience and health. Neuroscience and Biobehavioral Reviews, 95, 559–567. [Google Scholar] [CrossRef]
- Elman, I., Lowen, S., Frederick, B. B., Chi, W., Becerra, L., & Pitman, R. K. (2009). Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in Posttraumatic Stress Disorder. Biological Psychiatry, 66(12), 1083–1090. [Google Scholar] [CrossRef]
- Ethridge, P., Ali, N., Racine, S. E., Pruessner, J. C., & Weinberg, A. (2020). Risk and resilience in an acute stress paradigm: Evidence from salivary cortisol and time-frequency analysis of the reward positivity. Clinical Psychological Science, 8(5), 872–889. [Google Scholar] [CrossRef]
- Fassett-Carman, A. N., Moser, A. D., Ruzic, L., Neilson, C., Jones, J., Barnes-Horowitz, S., Schneck, C. D., & Kaiser, R. H. (2023). Amygdala and nucleus accumbens activation during reward anticipation moderates the association between life stressor frequency and depressive symptoms. Journal of Affective Disorders, 330, 309–318. [Google Scholar] [CrossRef]
- Garrison, J., Erdeniz, B., & Done, J. (2013). Prediction error in reinforcement learning: A meta-analysis of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(7), 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N., Peeters, F., Jacobs, N., Delespaul, P., Derom, C., Thiery, E., van Os, J., & Wichers, M. (2010). Meeting risk with resilience: High daily life reward experience preserves mental health. Acta Psychiatrica Scandinavica, 122(2), 129–138. [Google Scholar] [CrossRef]
- Gray, J. A. (1987). The psychology of fear and stress (2nd ed.). Cambridge University Press. [Google Scholar]
- Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, R., Baker, D. G., Basten, U., Boks, M. P., Bonanno, G. A., Brummelman, E., Chmitorz, A., Fernàndez, G., Fiebach, C. J., Galatzer-Levy, I., Geuze, E., Groppa, S., Helmreich, I., Hendler, T., Hermans, E. J., Jovanovic, T., Kubiak, T., Lieb, K., Lutz, B., … Kleim, B. (2017). The resilience framework as a strategy to combat stress-related disorders. Nature Human Behaviour, 1(11), 784–790. [Google Scholar] [CrossRef]
- Kampa, M., Schick, A., Yuen, K., Sebastian, A., Chmitorz, A., Saase, V., Wessa, M., Tüscher, O., & Kalisch, R. (2018). A combined behavioral and neuroimaging battery to test positive appraisal style theory of resilience in longitudinal studies. BioRxiv. [Google Scholar] [CrossRef]
- Kasparek, S. W., Jenness, J. L., & McLaughlin, K. A. (2020). Reward processing modulates the association between trauma exposure and externalizing psychopathology. Clinical Psychological Science, 8(6), 989–1006. [Google Scholar] [CrossRef]
- Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21(16), RC159. [Google Scholar] [CrossRef]
- Knutson, B., Bhanji, J. P., Cooney, R. E., Atlas, L. Y., & Gotlib, I. H. (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–692. [Google Scholar] [CrossRef]
- Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). FMRI visualization of brain activity during a Monetary Incentive Delay task. NeuroImage, 12(1), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koenka, A. C., Linnenbrink-Garcia, L., Moshontz, H., Atkinson, K. M., Sanchez, C. E., & Cooper, H. (2021). A meta-analysis on the impact of grades and comments on academic motivation and achievement: A case for written feedback. Educational Psychology, 41(7), 922–947. [Google Scholar] [CrossRef]
- Kumar, P., Berghorst, L. H., Nickerson, L. D., Dutra, S. J., Goer, F. K., Greve, D. N., & Pizzagalli, D. A. (2014). Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience, 266, 1–12. [Google Scholar] [CrossRef]
- Liu, X., Hairston, J., Schrier, M., & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Martin, A. J., & Marsh, H. W. (2008a). Academic buoyancy: Towards an understanding of students’ everyday academic resilience. Journal of School Psychology, 46(1), 53–83. [Google Scholar] [CrossRef]
- Martin, A. J., & Marsh, H. W. (2008b). Workplace and academic buoyancy: Psychometric assessment and construct validity amongst school personnel and students. Journal of Psychoeducational Assessment, 26, 168–184. [Google Scholar] [CrossRef]
- Martin, A. J., & Marsh, H. W. (2009). Academic resilience and academic buoyancy: Multidimensional and hierarchical conceptual framing of causes, correlates and cognate constructs. Oxford Review of Education, 35(3), 353–370. [Google Scholar] [CrossRef]
- Must, A., Szabó, Z., Bódi, N., Szász, A., Janka, Z., & Kéri, S. (2006). Sensitivity to reward and punishment and the prefrontal cortex in major depression. Journal of Affective Disorders, 90(2), 209–215. [Google Scholar] [CrossRef]
- Oldham, S., Murawski, C., Fornito, A., Youssef, G., Yücel, M., & Lorenzetti, V. (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39, 3398–3418. [Google Scholar] [CrossRef]
- Pegg, S., & Kujawa, A. (2024). The effects of stress on reward responsiveness: A systematic review and preliminary meta-analysis of the event-related potential literature. Cognitive, Affective & Behavioral Neuroscience, 24(1), 42–59. [Google Scholar] [CrossRef]
- Pizzagalli, D. A., Holmes, A. J., Dillon, D. G., Goetz, E. L., Birk, J. L., Bogdan, R., Dougherty, D. D., Iosifescu, D. V., Rauch, S. L., & Fava, M. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with Major Depressive Disorder. The American Journal of Psychiatry, 166(6), 702–710. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, A. J., Lewis, A. H., & Delgado, M. R. (2012). Acute stress influences neural circuits of reward processing. Frontiers in Neuroscience, 6, 2–9. [Google Scholar] [CrossRef]
- Schultz, W., Dayan, P., & Read Montague, P. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. [Google Scholar] [CrossRef]
- Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. [Google Scholar] [CrossRef]
- Stan, E. (2012). The role of grades in motivating students to learn. Procedia, Social and Behavioral Sciences, 69, 1998–2003. [Google Scholar] [CrossRef]
- Tashjian, S. M., & Galván, A. (2018). The role of mesolimbic circuitry in buffering election-related distress. Journal of Neuroscience, 38(11), 2887–2898. [Google Scholar] [CrossRef]
- Telzer, E. H., Fuligni, A. J., Lieberman, M. D., & Galván, A. (2014). Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences of the United States of America, 111(18), 6600–6605. [Google Scholar] [CrossRef]
- Vidal-Ribas, P., Benson, B., Vitale, A. D., Keren, H., Harrewijn, A., Fox, N. A., Pine, D. S., & Stringaris, A. (2019). Bidirectional associations between stress and reward processing in children and adolescents: A longitudinal neuroimaging study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(10), 893–901. [Google Scholar] [CrossRef]
- Wackerhagen, C., Veer, I. M., van Leeuwen, J. M. C., Reppmann, Z., Riepenhausen, A., Bögemann, S. A., Mor, N., Puhlmann, L. M. C., Uściƚko, A., Zerban, M., Mituniewicz, J., Lerner, A., Yuen, K. S. L., Köber, G., Marciniak, M. A., Pooseh, S., Weermeijer, J., Arias-Vásquez, A., Binder, H., … Walter, H. (2023). Dynamic modelling of mental resilience in young adults: Protocol for a longitudinal observational study (DynaM-OBS). JMIR Research Protocols, 12, e39817. [Google Scholar] [CrossRef] [PubMed]
- Waller, L., Veer, I. M., Pizzagalli, F., Han, L. K. M., Penninx, B. W. J. H., Liao, Z., Paus, T., Strike, L. T., Quidé, Y., Green, M. J., Harrison, B., Jamieson, A. J., Davey, C., Knodt, A., Hariri, A., Hermesdorf, M., Berger, K., Dannlowski, U., Kircher, T., … Walter, H. (2023, July 22–26). Meta-analytic results for task-based fMRI in the ENIGMA consortium [Conference abstract]. OHBM 2023 Annual Meeting, Montréal, QC, Canada. [Google Scholar]
- Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. C., Samanez-Larkin, G. R., Katovich, K., & Knutson, B. (2014). Affective traits link to reliable neural markers of incentive anticipation. NeuroImage, 84, 279–289. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vel Tromp, M.J.B.; Huizenga, H.M.; Jansen, B.R.J.; van Duijvenvoorde, A.C.K.; Veer, I.M. Investigating Neural Reward Sensitivity in the School Grade Incentive Delay Task and Its Relation to Academic Buoyancy. Behav. Sci. 2025, 15, 1321. https://doi.org/10.3390/bs15101321
Vel Tromp MJB, Huizenga HM, Jansen BRJ, van Duijvenvoorde ACK, Veer IM. Investigating Neural Reward Sensitivity in the School Grade Incentive Delay Task and Its Relation to Academic Buoyancy. Behavioral Sciences. 2025; 15(10):1321. https://doi.org/10.3390/bs15101321
Chicago/Turabian StyleVel Tromp, Myrthe J. B., Hilde M. Huizenga, Brenda R. J. Jansen, Anna C. K. van Duijvenvoorde, and Ilya M. Veer. 2025. "Investigating Neural Reward Sensitivity in the School Grade Incentive Delay Task and Its Relation to Academic Buoyancy" Behavioral Sciences 15, no. 10: 1321. https://doi.org/10.3390/bs15101321
APA StyleVel Tromp, M. J. B., Huizenga, H. M., Jansen, B. R. J., van Duijvenvoorde, A. C. K., & Veer, I. M. (2025). Investigating Neural Reward Sensitivity in the School Grade Incentive Delay Task and Its Relation to Academic Buoyancy. Behavioral Sciences, 15(10), 1321. https://doi.org/10.3390/bs15101321







