Basal Levels of Salivary Alpha-Amylase Are Associated with Preference for Foods High in Sugar and Anthropometric Markers of Cardiovascular Risk
Abstract
1. Introduction
2. Material and Methods
2.1. Participants and Procedure
2.2. Body Composition
2.3. Evaluation of Food Preference
2.4. Salivary Alpha-Amylase Determination
2.5. Data Analysis
3. Results
3.1. Participant Characteristics
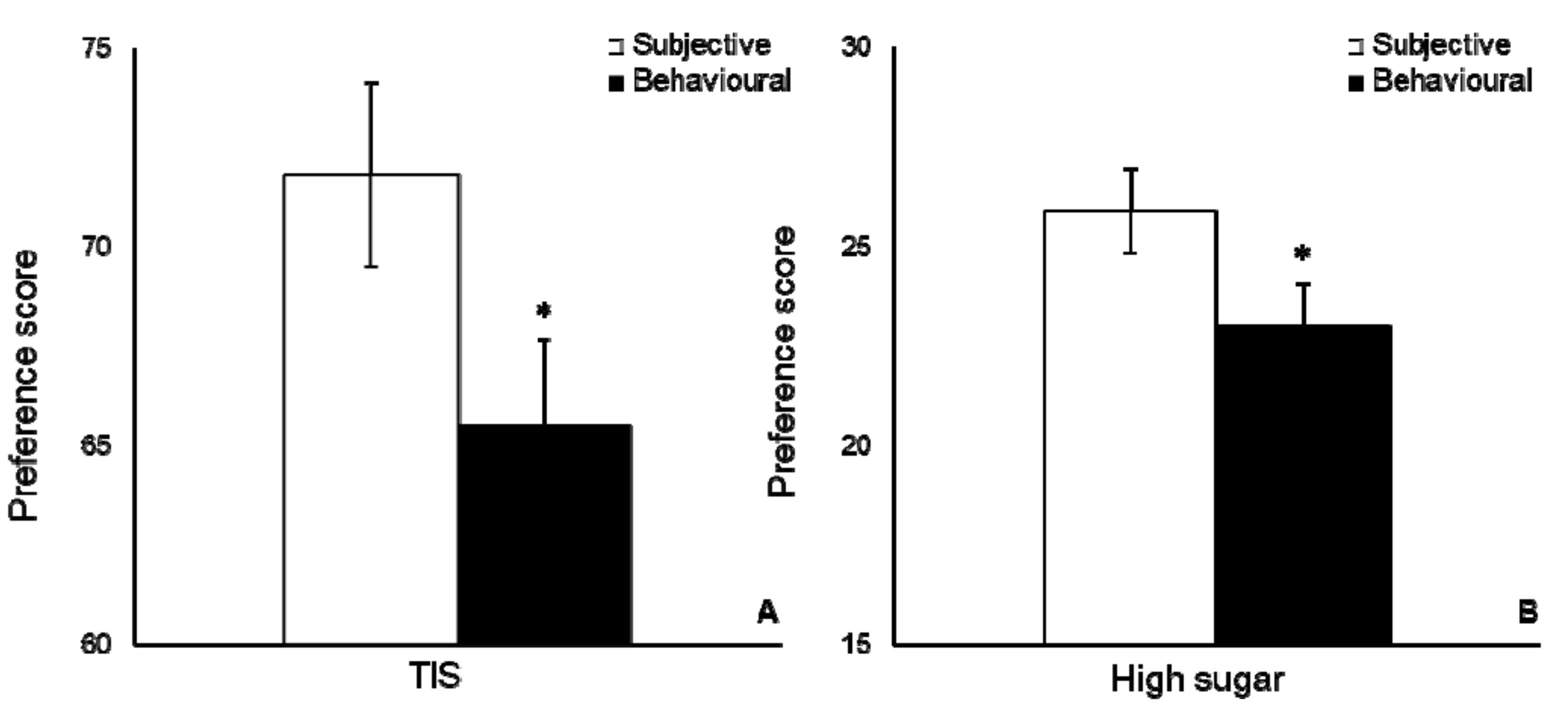
3.2. Food Preference
3.3. Food Preference and Anthropometric Markers of Health Risk
3.4. Effect of Time in sAA Levels
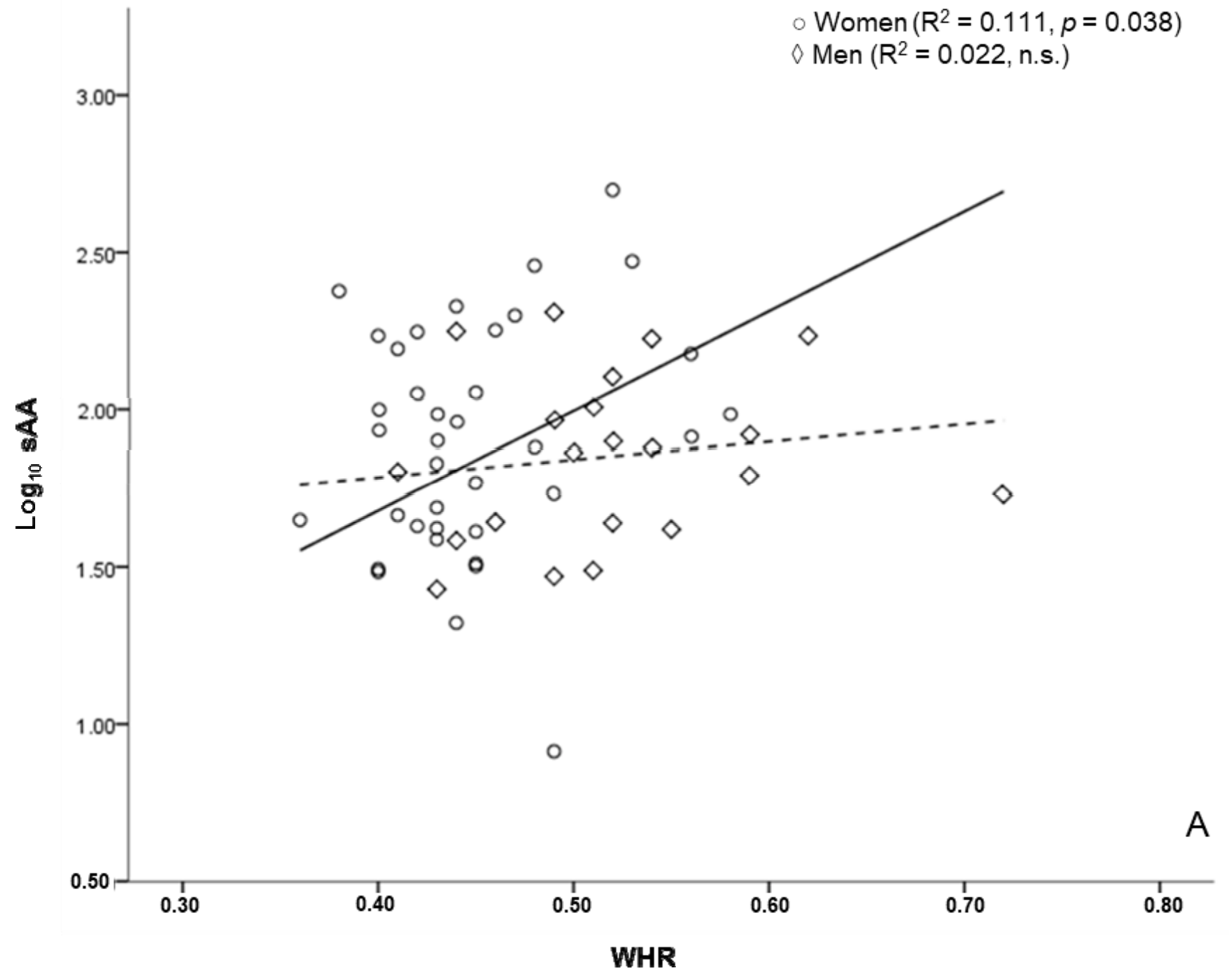
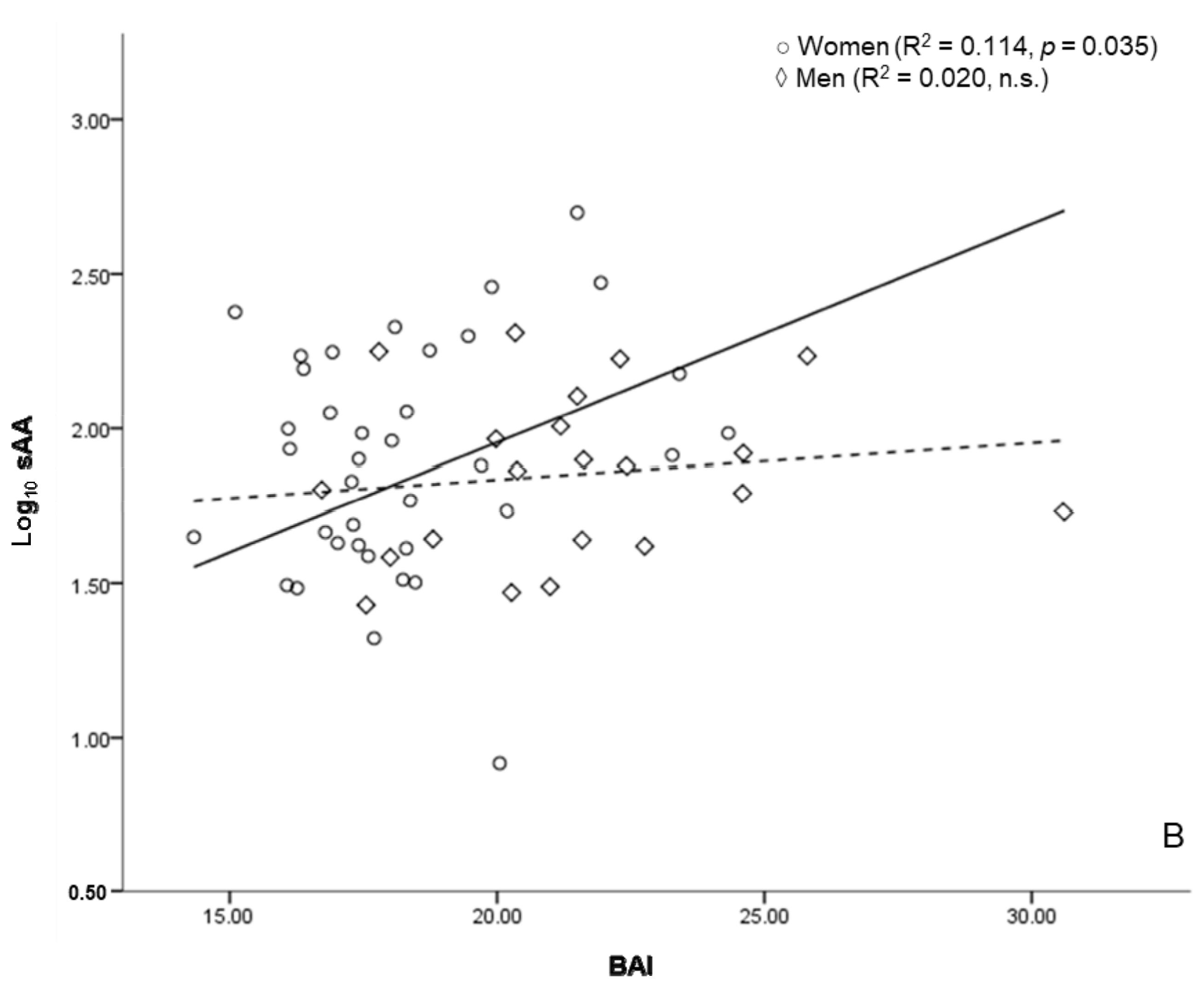
3.5. sAA and Anthropometric Markers of Health Risk
3.6. sAA and Food Preference
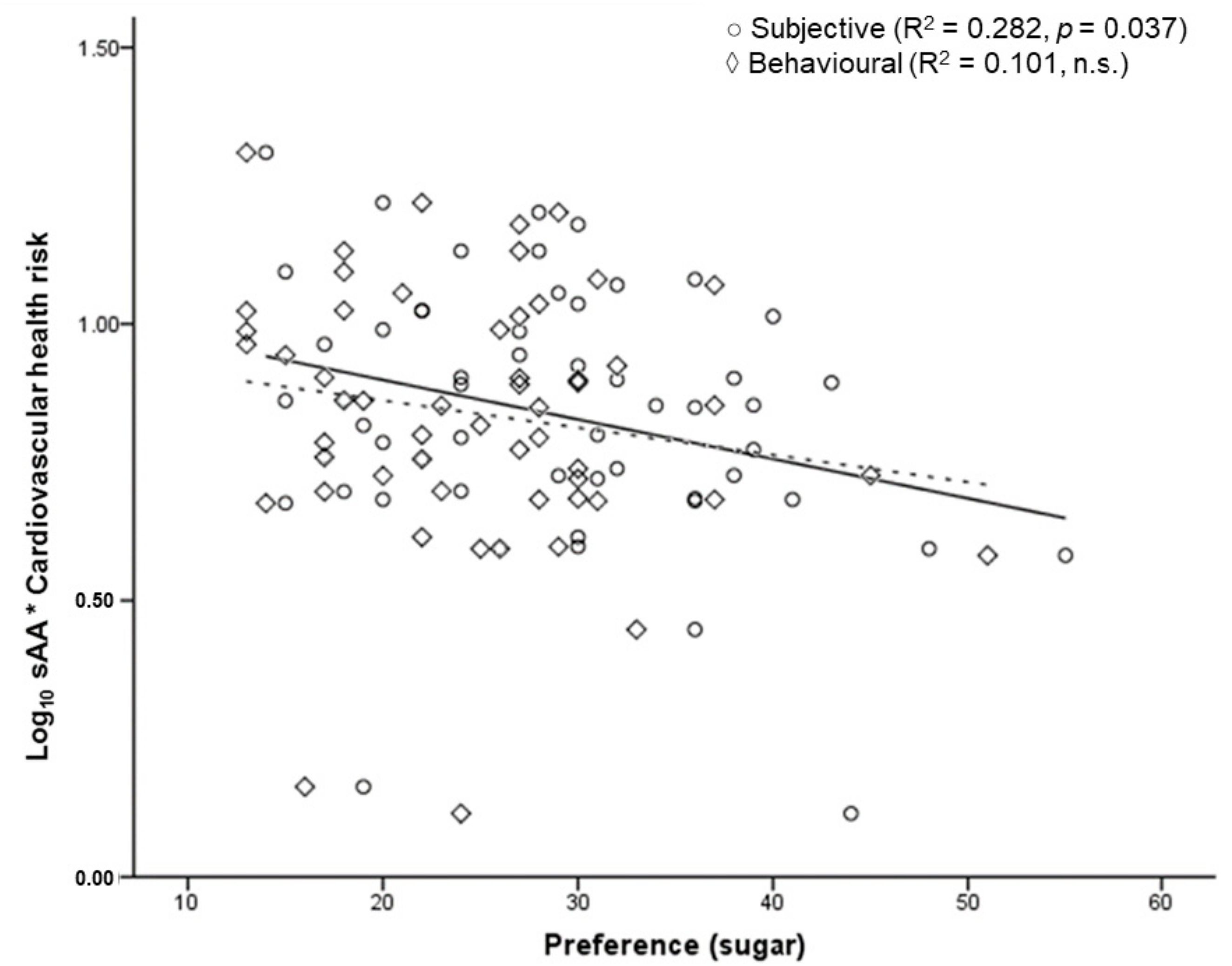
3.7. SAA, Body Composition, and Eating Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Food Item Inventory. Subjective Scale
| Nie | Selten | Manchmal | Häufig | Immer/Fast Immer | |
|---|---|---|---|---|---|
| Backfisch | □ | □ | □ | □ | □ |
| Frühstücksspeck (Bacon) | □ | □ | □ | □ | □ |
| Belegte Brötchen | □ | □ | □ | □ | □ |
| Brezeln | □ | □ | □ | □ | □ |
| Brötchen | □ | □ | □ | □ | □ |
| Brownies | □ | □ | □ | □ | □ |
| Chips | □ | □ | □ | □ | □ |
| Cracker | □ | □ | □ | □ | □ |
| Döner | □ | □ | □ | □ | □ |
| Donuts | □ | □ | □ | □ | □ |
| Eiscreme | □ | □ | □ | □ | □ |
| Frikadellen | □ | □ | □ | □ | □ |
| Gesalzene Erdnüsse | □ | □ | □ | □ | □ |
| Haferflocken | □ | □ | □ | □ | □ |
| Hamburger | □ | □ | □ | □ | □ |
| Honig | □ | □ | □ | □ | □ |
| Kekse | □ | □ | □ | □ | □ |
| Kuchen | □ | □ | □ | □ | □ |
| Marzipan | □ | □ | □ | □ | □ |
| Müsli | □ | □ | □ | □ | □ |
| Nudeln | □ | □ | □ | □ | □ |
| Nussnougatcreme | □ | □ | □ | □ | □ |
| Ofenkartoffeln | □ | □ | □ | □ | □ |
| Pfannkuchen | □ | □ | □ | □ | □ |
| Pizza | □ | □ | □ | □ | □ |
| Pommes frites | □ | □ | □ | □ | □ |
| Reis | □ | □ | □ | □ | □ |
| Schokolade, Pralinen | □ | □ | □ | □ | □ |
| Steak | □ | □ | □ | □ | □ |
| Süße Backwaren | □ | □ | □ | □ | □ |
| Süßigkeiten | □ | □ | □ | □ | □ |
| Toastbrot | □ | □ | □ | □ | □ |
| Waffeln | □ | □ | □ | □ | □ |
| Wurst | □ | □ | □ | □ | □ |
Appendix B. Food Item Inventory. Behavioural Scale
| Nie | Selten | Manchmal | Häufig | Immer/Fast Immer | |
|---|---|---|---|---|---|
| Backfisch | □ | □ | □ | □ | □ |
| Bacon (Frühstücksspeck) | □ | □ | □ | □ | □ |
| Belegte Brötchen | □ | □ | □ | □ | □ |
| Brezel | □ | □ | □ | □ | □ |
| Brötchen | □ | □ | □ | □ | □ |
| Brownies | □ | □ | □ | □ | □ |
| Chips | □ | □ | □ | □ | □ |
| Cracker | □ | □ | □ | □ | □ |
| Döner | □ | □ | □ | □ | □ |
| Donuts | □ | □ | □ | □ | □ |
| Eiscreme | □ | □ | □ | □ | □ |
| Frikadellen | □ | □ | □ | □ | □ |
| Gesalzene Erdnüsse | □ | □ | □ | □ | □ |
| Haferflocken | □ | □ | □ | □ | □ |
| Hamburger | □ | □ | □ | □ | □ |
| Honig | □ | □ | □ | □ | □ |
| Keks | □ | □ | □ | □ | □ |
| Kuchen | □ | □ | □ | □ | □ |
| Marzipan | □ | □ | □ | □ | □ |
| Müsli | □ | □ | □ | □ | □ |
| Nudeln | □ | □ | □ | □ | □ |
| Nussnougatcreme | □ | □ | □ | □ | □ |
| Ofenkartoffeln | □ | □ | □ | □ | □ |
| Pfannkuchen | □ | □ | □ | □ | □ |
| Pizza | □ | □ | □ | □ | □ |
| Pommes frites | □ | □ | □ | □ | □ |
| Reis | □ | □ | □ | □ | □ |
| Schokolade, Pralinen | □ | □ | □ | □ | □ |
| Steak | □ | □ | □ | □ | □ |
| Süße Backwaren | □ | □ | □ | □ | □ |
| Süßigkeiten | □ | □ | □ | □ | □ |
| Toastbrot | □ | □ | □ | □ | □ |
| Waffel | □ | □ | □ | □ | □ |
| Wurst | □ | □ | □ | □ | □ |
References
- Dsamou, M.; Palicki, O.; Septier, C.; Chabanet, C.; Lucchi, G.; Ducoroy, P.; Chagnon, M.C.; Morzel, M. Salivary protein profiles and sensitivity to the bitter taste of caffeine. Chem. Senses 2012, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Salles, C.; Chagnon, M.-C.; Feron, G.; Guichard, E.; Laboure, H.; Morzel, M.; Semon, E.; Tarrega, A.; Yven, C. In-mouth mechanisms leading to flavor release and perception. Crit. Rev. Food Sci. Nutr. 2011, 51, 67–90. [Google Scholar] [CrossRef] [PubMed]
- De Wijk, R.A.; Prinz, J.F.; Engelen, L.; Weenen, H. The role of α-amylase in the perception of oral texture and flavour in custards. Physiol. Behav. 2004, 83, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Prindiville, E.A.; Marshall, R.T.; Heymann, H. Effect of Milk Fat on the Sensory Properties of Chocolate Ice Cream. J. Dairy Sci. 1999, 82, 1425–1432. [Google Scholar] [CrossRef]
- Prindiville, E.A.; Marshall, R.T.; Heymann, H. Effect of Milk Fat, Cocoa Butter, and Whey Protein Fat Replacers on the Sensory Properties of Lowfat and Nonfat Chocolate Ice Cream. J. Dairy Sci. 2000, 83, 2216–2223. [Google Scholar] [CrossRef]
- Berthoud, H.R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Falchi, M.; El-Sayed Moustafa, J.S.; Takousis, P.; Pesce, F.; Bonnefond, A.; Andersson-Assarsson, J.C.; Sudmant, P.H.; Dorajoo, R.; Al-Shafai, M.N.; Bottolo, L.; et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat. Genet. 2014, 46, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Viljakainen, H.; Andersson-Assarsson, J.C.; Armenio, M.; Pekkinen, M.; Pettersson, M.; Valta, H.; Lipsanen-Nyman, M.; Mäkitie, O.; Lindstrand, A. Low Copy Number of the AMY1 Locus Is Associated with Early-Onset Female Obesity in Finland. PLoS ONE 2015, 10, e0131883. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary Sugar and Body Weight: Have We Reached a Crisis in the Epidemic of Obesity and Diabetes? Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Incledon, E.; Wake, M.; Hay, M. Psychological predictors of adiposity: Systematic review of longitudinal studies. Int. J. Pediatr. Obes. 2011, 6, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Obesity—A disease with many aetiologies disguised in the same oversized phenotype: Has the overeating theory failed? Nephrol. Dial. Transplant. 2015, 30, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M.; Anderssen, S.; Gunnarsdottir, I.; Lahti-Koski, M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: A systematic literature review. Food Nutr. Res. 2012, 56, 19103. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Rouhani, M.H.; Haghighatdoost, F.; Surkan, P.J.; Azadbakht, L. Associations between dietary energy density and obesity: A systematic review and meta-analysis of observational studies. Nutrition 2016, 32, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2015, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.; Van Baak, M.; Jebb, S.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Holst, C.; et al. The diet, obesity and genes (diogenes) dietary study in eight European countries—A comprehensive design for long-term intervention. Obes. Rev. 2010, 11, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hobl, L.P.; Hoffmann, G. Effects of low glycaemic index/low glycaemic load vs. high glycaemic index/high glycaemic load diets on overweight/obesity and associated risk factors in children and adolescents: A systematic review and meta-analysis. Nutr. J. 2015, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.K.; van Strien, T.; Eisinga, R.; Herman, C.P.; Engels, R.C.M.E. Dietary restraint: Intention versus behavior to restrict food intake. Appetite 2007, 49, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Anschutz, D.J.; Engels, R.C.M.E.; van der Zwaluw, C.S.; Van Strien, T. Sex differences in young adults’ snack food intake after food commercial exposure. Appetite 2011, 56, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A Better Index of Body Adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Farran, A.; Zamora, R.; Cervera, P. Tablas de Composición de ALIMENTOs del CESNID; Reixach, M., Ed.; McGraw-Hill Interamericana: Barcelona, Spain, 2004; ISBN 84-486-0590-X. [Google Scholar]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Selya, A.S.; Rose, J.S.; Dierker, L.C.; Hedeker, D.; Mermelstein, R.J. A practical guide to calculating Cohen’s f 2, a measure of local effect size, from PROC MIXED. Front. Psychol. 2012, 3, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.Y.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Peyrot des Gachons, C.; Plank, K.L.; Alarcon, S.; Breslin, P.A.S. Individual differences in AMY1 gene copy number, salivary α-amylase levels, and the perception of oral starch. PLoS ONE 2010, 5, e13352. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Breslin, P.A.S. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and validation of the food-craving inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Grilo, C.M. Psychometric properties of the Food Craving Inventory among obese patients with binge eating disorder. Eat. Behav. 2005, 6, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Dhar, S.; Mitchell, L.M.; Fu, B.; Tyson, J.; Shwan, N.A.A.; Yang, F.; Thomas, M.G.; Armour, J.A.L. Obesity, starch digestion and amylase: Association between copy number variants at human salivary (AMY1) and pancreatic (AMY2) amylase genes. Hum. Mol. Genet. 2015, 24, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Kahlhöfer, J.; Lagerpusch, M.; Enderle, J.; Eggeling, B.; Braun, W.; Pape, D.; Müller, M.J. Bosy-Westphal, a Carbohydrate intake and glycemic index affect substrate oxidation during a controlled weight cycle in healthy men. Eur. J. Clin. Nutr. 2014, 68, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Glaesmer, H.; Klotsche, J.; Böhler, S.; Lehnert, H.; Zeiher, A.M.; März, W.; Pittrow, D.; Stalla, G.K.; Wittchen, H.-U. Accuracy of Anthropometric Indicators of Obesity to Predict Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2007, 92, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Spahillari, A.; Mukamal, K.; DeFilippi, C.; Kizer, J.R.; Gottdiener, J.S.; Djoussé, L.; Lyles, M.F.; Bartz, T.M.; Murthy, V.L.; Shah, R.V. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Stein, A.; Marcadenti, A. Visceral adiposity index and prognosis among patients with ischemic heart failure. Sao Paulo Med. J. 2016, 134, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.R.J.; Bizais, Y.; Bruley des Varaness, S.; Pouliquen, B.; Delort Laval, J.; Galmiche, J.P. α-Amylase (EC 3.2.1.1) susceptibility rather than viscosity or Gastric Emptying Rate Controls Plasma Responses to Starch in Healthy Humans. Br. J. Nutr. 1990, 63, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Sniehotta, F.F.; Scholz, U.; Schwarzer, R. Bridging the intention–behaviour gap: Planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol. Health 2005, 20, 143–160. [Google Scholar] [CrossRef]
- Ortega, F.B.; Sui, X.; Lavie, C.J.; Blair, S.N. Body Mass Index, the Most Widely Used but Also Widely Criticized Index Would a Criterion Standard Measure of Total Body Fat Be a Better Predictor of Cardiovascular Disease Mortality? Mayo Clin. Proc. 2016, 91, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Segheto, W.; Coelho, F.A.; Cristina Guimarães da Silva, D.; Hallal, P.C.; Marins, J.C.B.; Ribeiro, A.Q.; Pessoa, M.C.; Morais, S.H.O.; Longo, G.Z. Validity of body adiposity index in predicting body fat in Brazilians adults. Am. J. Hum. Biol. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Despreś, J.P. What is “metabolically healthy obesity”? From epidemiology to pathophysiological insights. J. Clin. Endocrinol. Metab. 2012, 97, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef] [PubMed]


| Men | Women | |
|---|---|---|
| (n = 21) | (n = 37) | |
| Mean ± s.d., Asymmetry ± d.e. | ||
| Weight | 82.62 ± 12.69, 0.95 ± 0.501 | 62.58 ± 13.16, 0.13 ± 0.388 |
| Body Mass Index | 25.34 ± 3.62, 1.52 ± 0.501 | 21.94 ± 4.73, 0.49 ± 0.388 |
| Body fat % | 20.03 ± 5.40, 0.93 ± 0.501 | 25.23 ± 5.13, 1.01 ± 0.388 |
| Body Adiposity Index | 21.43 ± 3.18, 1.12 ± 0.501 | 18.07 ± 2.27, 1.05 ± 0.388 |
| Waist to Hip Ratio | 0.52 ± 0.07, 1.06 ± 0.501 | 0.44 ± 0.05, 1.04 ± 0.388 |
| sAA | 85.06 ± 11.80, 1.01 ± 0.501 | 95.85 ± 12.50, 1.09 ± 0.388 |
| Log10 sAA | ||
|---|---|---|
| Women | BMI | 0.154 |
| WHR | 0.323 * | |
| BAI | 0.327 * | |
| BF % | 0.209 | |
| Men | BMI | 0.119 |
| WHR | 0.149 | |
| BAI | 0.14 | |
| BF % | 0.173 |
| Sex | Preference | Category | Log10 sAA |
|---|---|---|---|
| Women | Subjective | TIS | −0.254 |
| Starch | −0.115 | ||
| Sugar | −0.323 * | ||
| GI | −0.244 | ||
| GL | −0.258 | ||
| Self-reported behaviour | TIS | −0.206 | |
| Starch | −0.081 | ||
| Sugar | −0.283 * | ||
| GI | −0.154 | ||
| GL | −0.173 | ||
| Men | Subjective | TIS | −0.072 |
| Starch | 0.105 | ||
| Sugar | −0.138 | ||
| GI | 0.046 | ||
| GL | −0.044 | ||
| Self-reported behaviour | TIS | 0.044 | |
| Starch | 0.241 | ||
| Sugar | −0.011 | ||
| GI | 0.209 | ||
| GL | 0.125 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarragon, E.; Stein, J.; Meyer, J. Basal Levels of Salivary Alpha-Amylase Are Associated with Preference for Foods High in Sugar and Anthropometric Markers of Cardiovascular Risk. Behav. Sci. 2018, 8, 94. https://doi.org/10.3390/bs8100094
Tarragon E, Stein J, Meyer J. Basal Levels of Salivary Alpha-Amylase Are Associated with Preference for Foods High in Sugar and Anthropometric Markers of Cardiovascular Risk. Behavioral Sciences. 2018; 8(10):94. https://doi.org/10.3390/bs8100094
Chicago/Turabian StyleTarragon, Ernesto, Jakob Stein, and Jobst Meyer. 2018. "Basal Levels of Salivary Alpha-Amylase Are Associated with Preference for Foods High in Sugar and Anthropometric Markers of Cardiovascular Risk" Behavioral Sciences 8, no. 10: 94. https://doi.org/10.3390/bs8100094
APA StyleTarragon, E., Stein, J., & Meyer, J. (2018). Basal Levels of Salivary Alpha-Amylase Are Associated with Preference for Foods High in Sugar and Anthropometric Markers of Cardiovascular Risk. Behavioral Sciences, 8(10), 94. https://doi.org/10.3390/bs8100094





