Comparison of the Response of Male BALB/c and C57BL/6 Mice in Behavioral Tasks to Evaluate Cognitive Function
Abstract
:1. Introduction
2. Materials and Methods
Animals and Housing Conditions
Total of Possible Alternations
Total time to visit the three arms (s)
3. Results
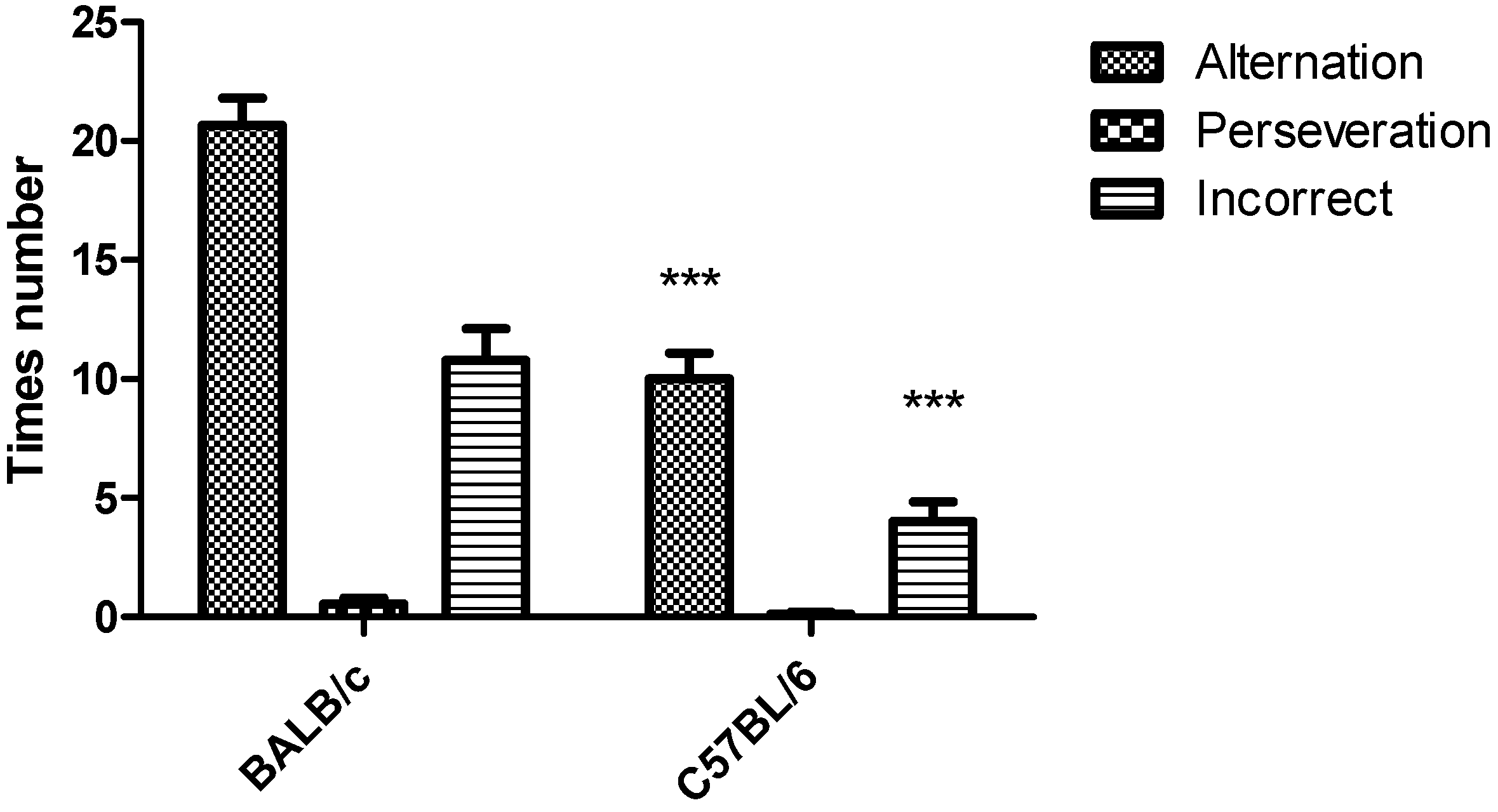
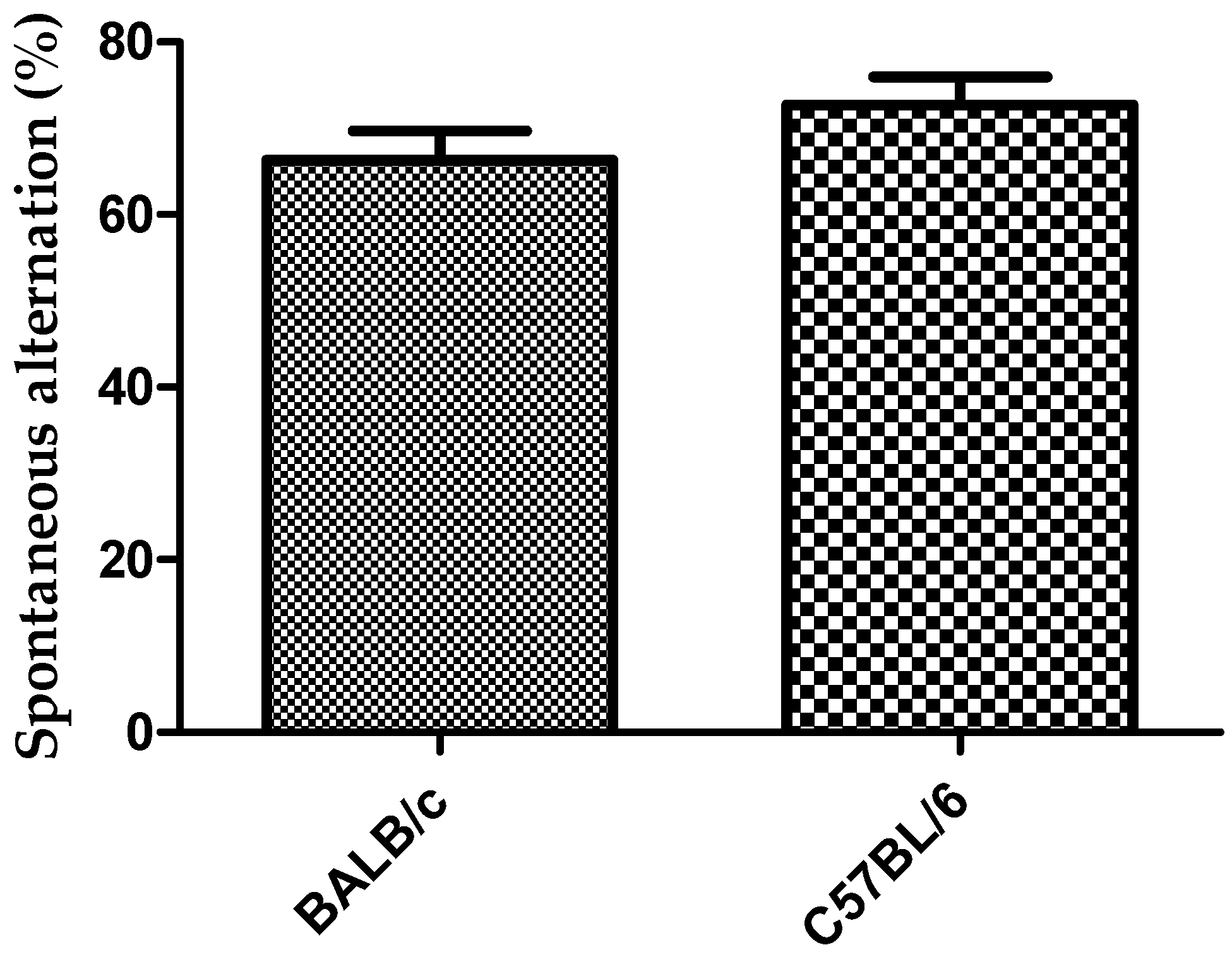
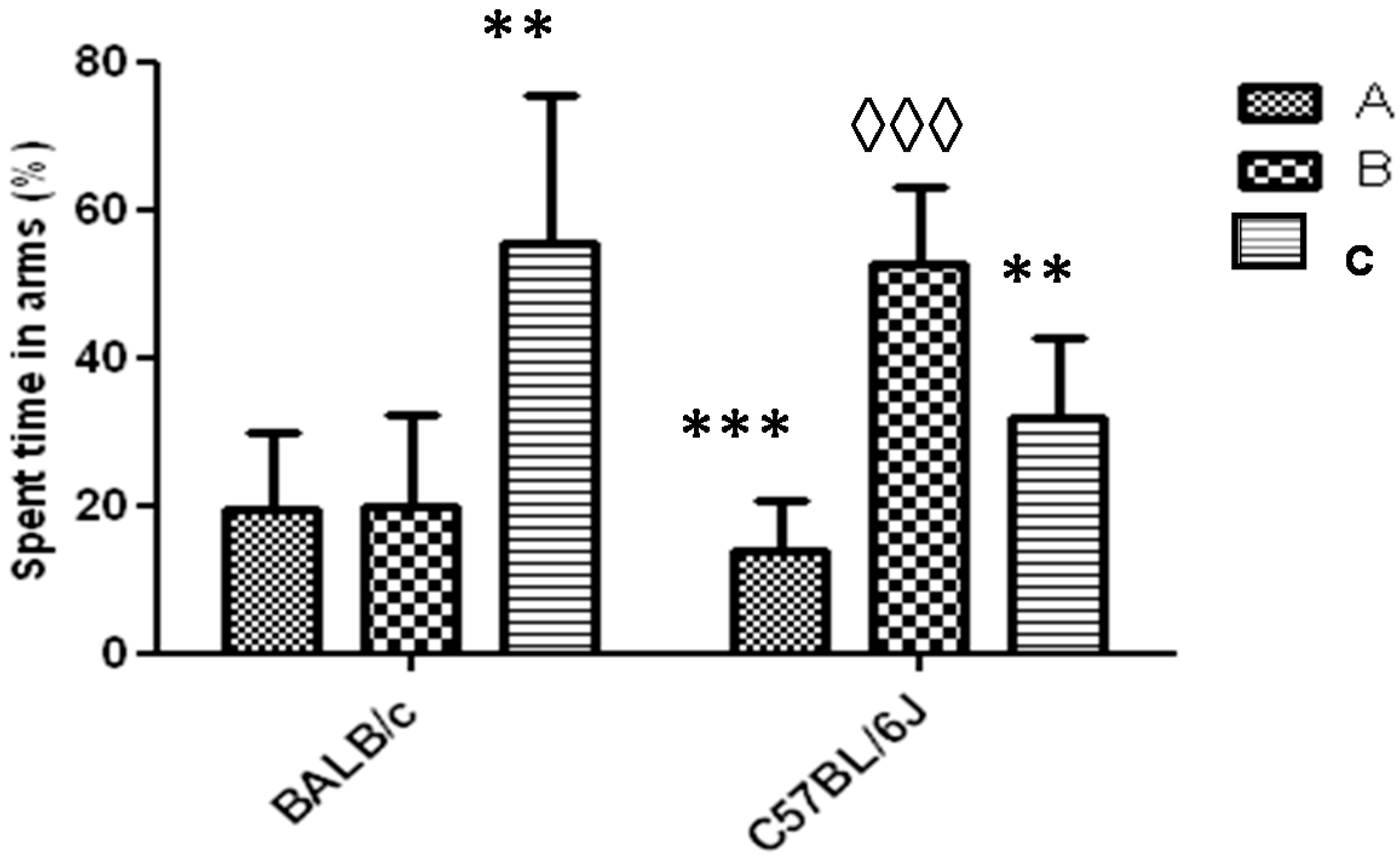
3.1. Y-Maze Task
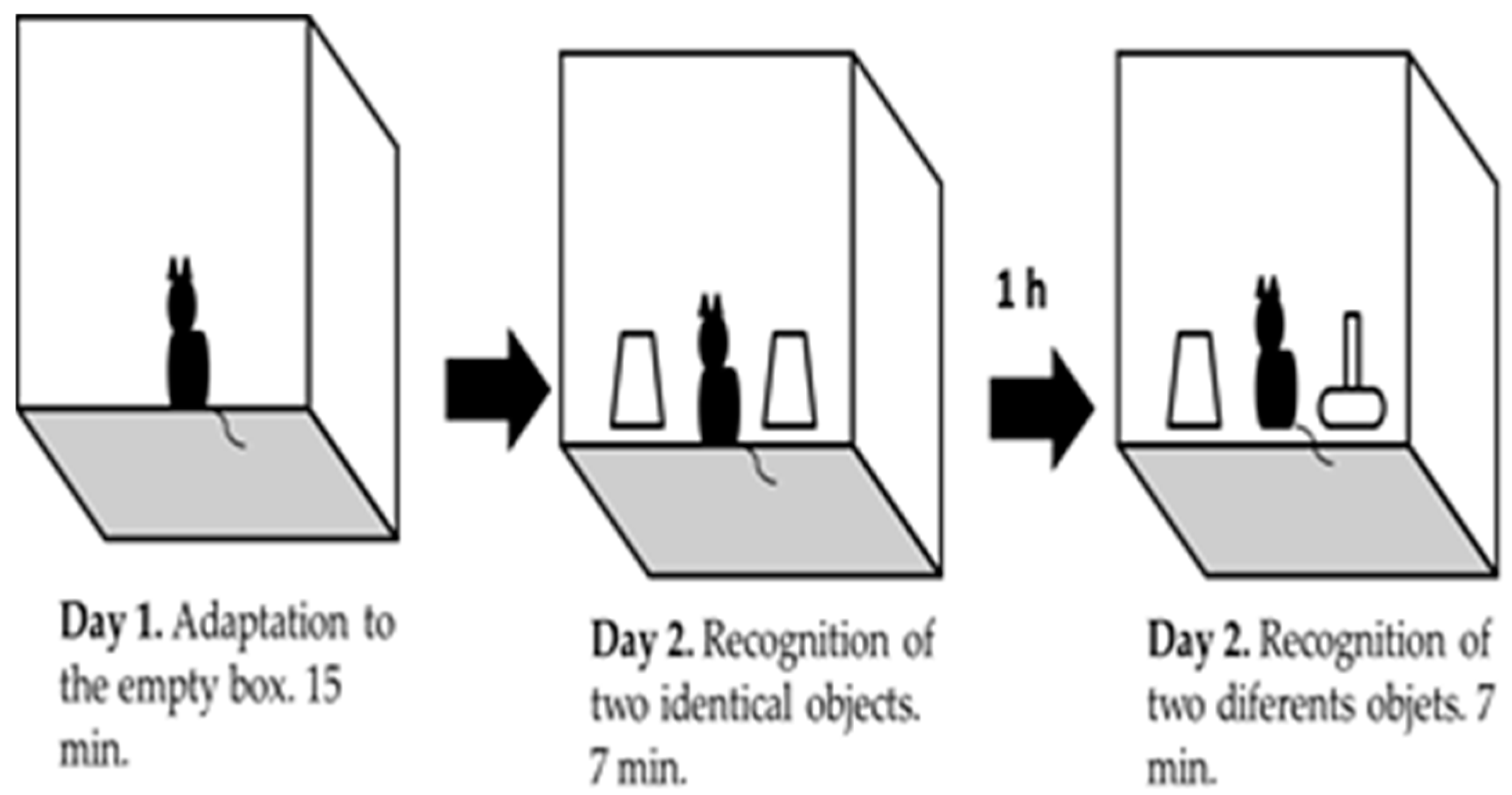
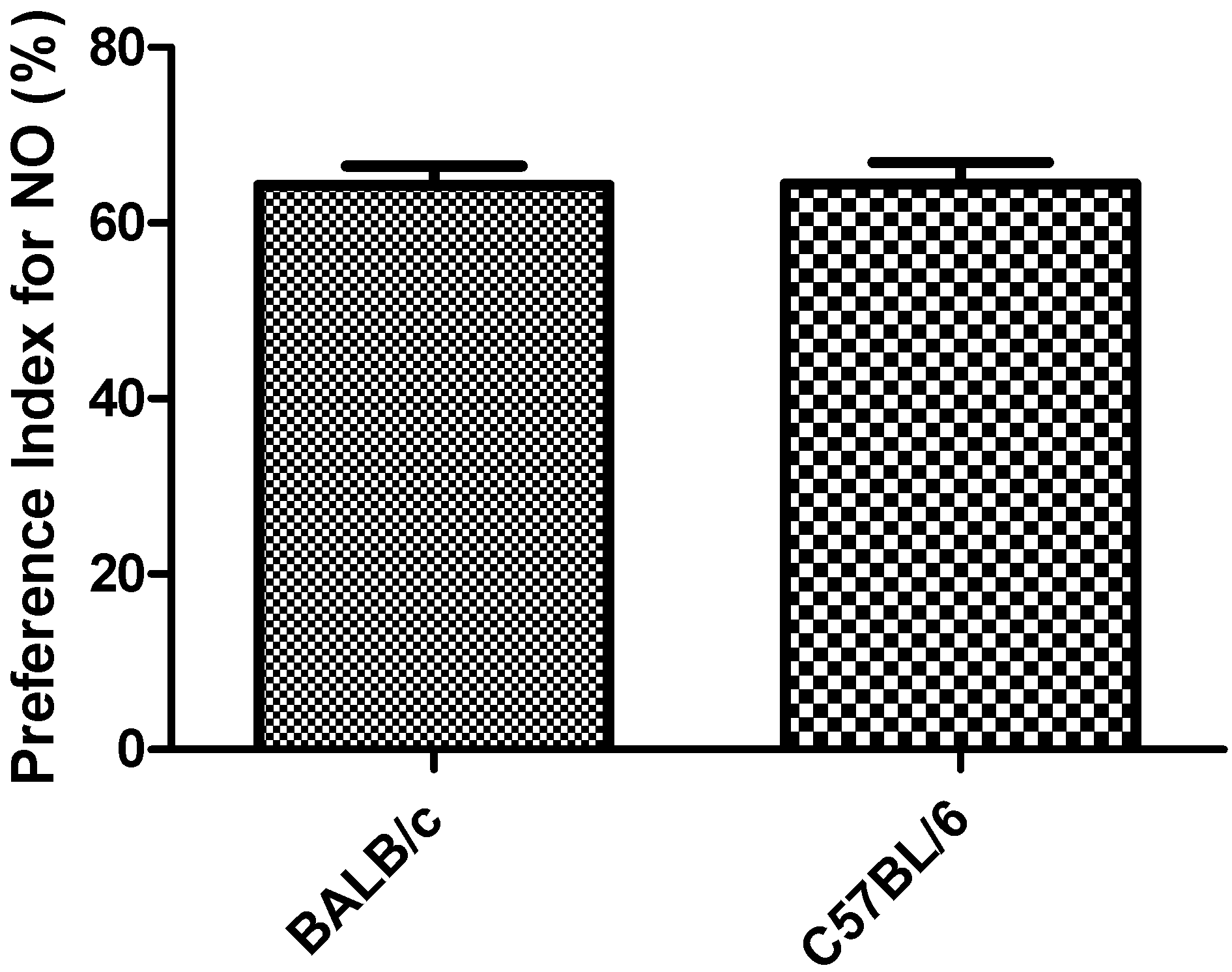
3.2. Object Recognition Task
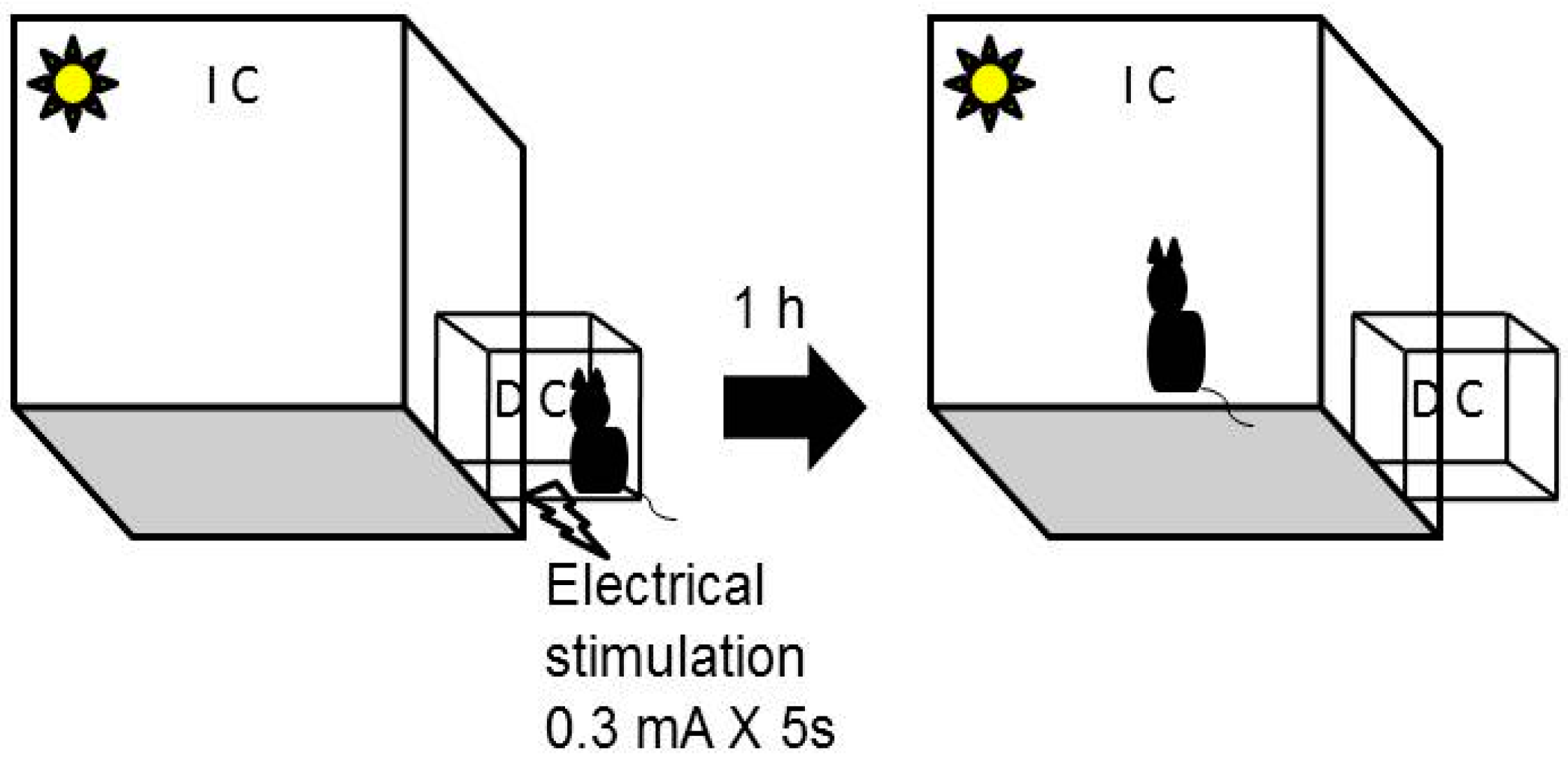
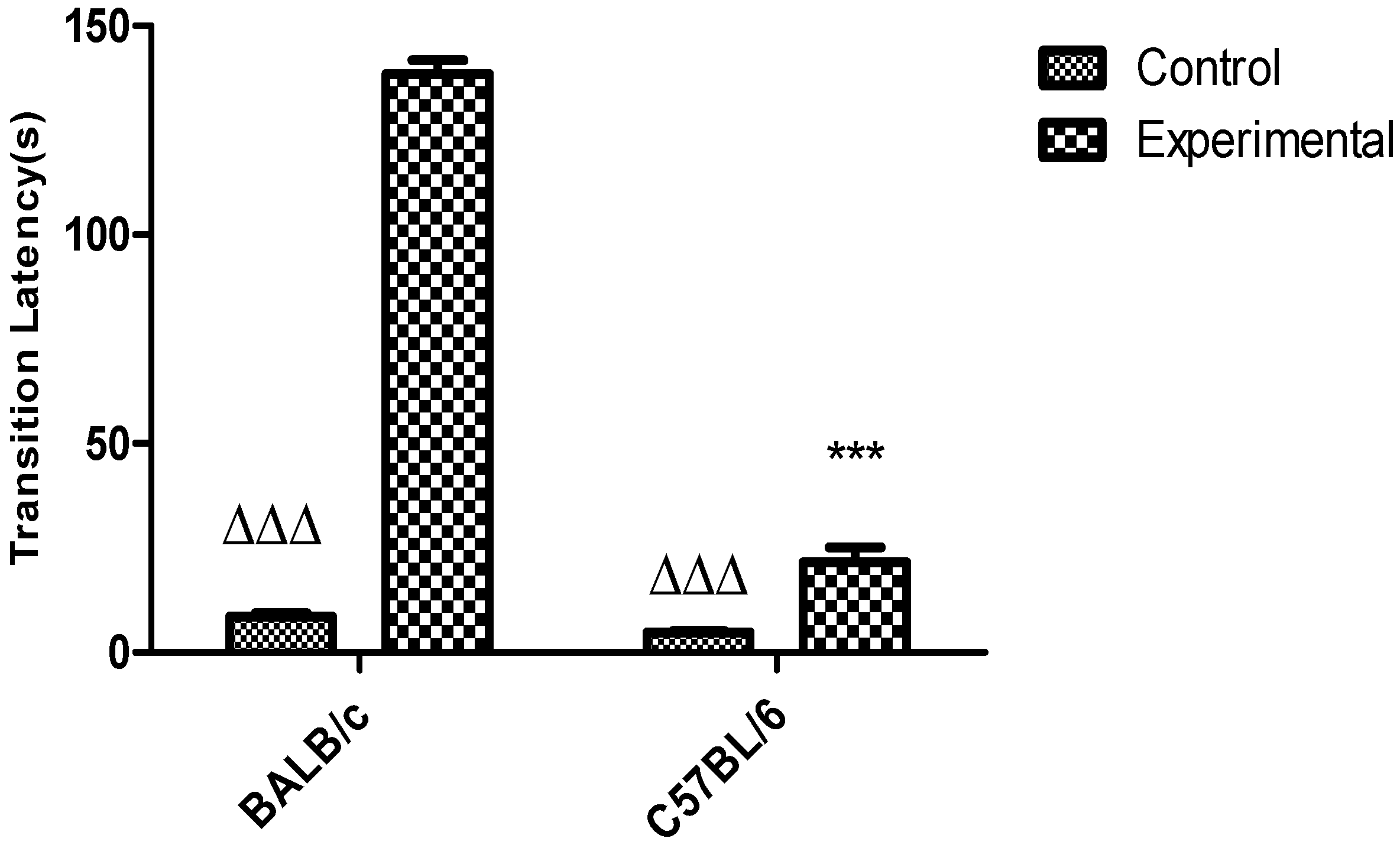
3.3. Passive Avoidance Task
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolf, A.; Bauer, B.; Abner, E.L.; Anika, M.; Hartz, S. Comprehensive behavioral test battery to assess learning and memory in 129s6/ Tg2576 mice. PLoS ONE 2016, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tanila, H. Testing cognitive functions in rodent disease models: Present pitfalls and future perspectives. Behav. Brain Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Pérez-Ortiz, J.M.; Femenía, T. Métodos de evaluación de trastornos cognitivos en modelos animales. Rev. Neurol. 2008, 47, 137–145. [Google Scholar] [PubMed]
- Dudchenko, P.A. Overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lorigados, L.; Pavón, N.; Almaguer, W. Modelos experimentales de neurodegeneración: Tratamiento restaurativo y factor de crecimiento nervioso. Rev. Mex. Neurocienc. 2009, 10, 264–273. [Google Scholar]
- Manzano, S.; González, J.L.; Marcos, A.; Payno, M. Modelos experimentales de la enfermedad de Alzheimer. Neurologia 2009, 24, 255–262. [Google Scholar] [PubMed]
- Forny-Germano, L.; Silva, N.M.; Batista, A.F. Alzheimer’s Disease-Like Pathology Induced by Amyloid-β Oligomers in Nonhuman Primates. J. Neurosci 2014, 34, 13629–13643. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Verdile, G.; Martins, R.N. Zebrafish as a tool in Alzheimer’s disease research. Biochim. Biophys. Acta 2011, 1812, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Laugero, K.D.; Adkins, Y.; Mackey, B.; Kelley, D. Emotion-based cognition in mice Is differentially influenced by dose and chemical form of dietary docosahexaenoic acid. Nutrients 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Balogh, S.A.; Wehner, J.M. Inbred mouse strain differences in the establishment of long-term fear memory. Behav. Brain Res. 2003, 140, 97–106. [Google Scholar] [CrossRef]
- Brinks, V.; van der Mark, M.; de Kloet, R.; Oitzl, M. Emotion and cognition in high and low stress sensitive mouse strains: A combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front. Behav. Neurosci. 2007, 1. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.L.; Zorrilla, M.A.; Cremaschi, G.A.; Genaro, A.M. Different effect of chronic stress on learning and memory in BALB/c and C57BL/6 inbred mice: Involvement of hippocampal NO production and PKC activity. Stress 2009, 12. [Google Scholar] [CrossRef] [PubMed]
- Akar, F. Effects of 7-NI and ODQ on memory in the passive avoidance, novel object recognition, and social transmission of food preference tests in mice. Med. Sci. Monit. Basic Res. 2014, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Magani, F.; Damianich, A.; Duaip, G.; Rojo, D.; Trigila, A.; Pimentel, J.; Federman, N. Reconocimiento de objetos en ratones Mus musculus cepa C57BL/6J. Rev. Argent Cienc. Comport. 2013, 5, 24–29. [Google Scholar]
- Brinks, V.; de Kloet, E.R.; Oitzl, M.S. Strain specific fear behaviour and glucocorticoid response to aversive events: Modelling PTSD in mice. Prog. Brain Res. 2008, 167, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Schmauss, C. Strain-Specific Cognitive Deficits in Adult Mice Exposed to Early Life Stress. Behav. Neurosci. 2011, 125, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Oriel, S.; Kofman, O. Strain dependent effects of conditioned fear in adult C57Bl/6 and Balb/C mice following postnatal exposure to chlorpyrifos: Relation to expression of brain acetylcholinesterase mRNA. Front. Behav. Neurosci. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keum, S.; Park, J.; Kim, A.; Park, J.; Kim, K.K.; Jeong, J.; Shin, S. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016, 15, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M.; Takiguchi, O.; Nishiyama, A. Cilostazol prevents amyloid b peptide25-35-induced memory impairment and oxidative stress in mice. Br. J. Pharmacol. 2010, 161, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Liang-Wen, J.; Chun-Chieh, L.; Wen-Sung, L.; Chia-Yuan, C.; Ju-Chun, P.; Wan-Rong, W.; Chih-Min, L.; Hai-Gwo, H.; Li-Jen, L. Phenotypic characterization of C57BL/6 mice carrying the Disc1 gene from the 129S6/SvEv strain. Brain Struct. Funct. 2014, 219, 1417–1431. [Google Scholar] [CrossRef]
- Brown, R.E.; Wong, A.A. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn. Mem. 2007, 14, 134–144. [Google Scholar] [CrossRef] [PubMed]

| Aim | Reference |
|---|---|
| Inbred mouse strain differences in the establishment of long-term fear memory | Balogh et al. [11] |
| Emotion and cognition in high and low stress | Brinks et al. [12] |
| Different effect of chronic stress on learning and memory | Palumbo et al. [13] |
| Strain specific fear behavior and glucocorticoid response to aversive events | Brinks et al. [16] |
| Strain-Specific Cognitive Deficits in Adult Mice Exposed to Early Life Stress | Mehta and Schmauss [17] |
| Effects of conditioned fear in adult mice following postnatal exposure to chlorpyrifos | Oriel and Kofman [18] |
| Variability in empathic fear response among inbred strains of mice | Keum et al. [19] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, Y.; Esquivel, N. Comparison of the Response of Male BALB/c and C57BL/6 Mice in Behavioral Tasks to Evaluate Cognitive Function. Behav. Sci. 2018, 8, 14. https://doi.org/10.3390/bs8010014
Garcia Y, Esquivel N. Comparison of the Response of Male BALB/c and C57BL/6 Mice in Behavioral Tasks to Evaluate Cognitive Function. Behavioral Sciences. 2018; 8(1):14. https://doi.org/10.3390/bs8010014
Chicago/Turabian StyleGarcia, Yenela, and Nashelly Esquivel. 2018. "Comparison of the Response of Male BALB/c and C57BL/6 Mice in Behavioral Tasks to Evaluate Cognitive Function" Behavioral Sciences 8, no. 1: 14. https://doi.org/10.3390/bs8010014
APA StyleGarcia, Y., & Esquivel, N. (2018). Comparison of the Response of Male BALB/c and C57BL/6 Mice in Behavioral Tasks to Evaluate Cognitive Function. Behavioral Sciences, 8(1), 14. https://doi.org/10.3390/bs8010014





