Atypical Processing of Novel Distracters in a Visual Oddball Task in Autism Spectrum Disorder
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. ERP Data Acquisition, and Signal Processing
2.3. Three Stimuli Visual Oddball Test with Novel Distracters
2.4. Behavioral Measures
2.5. Event-Related Potentials (ERP)
2.5.1. Stimulus-Locked ERPs
2.5.2. Response-Locked Dependent Variables (ERN/Pe)
2.6. Statistical Data Analysis
3. Results
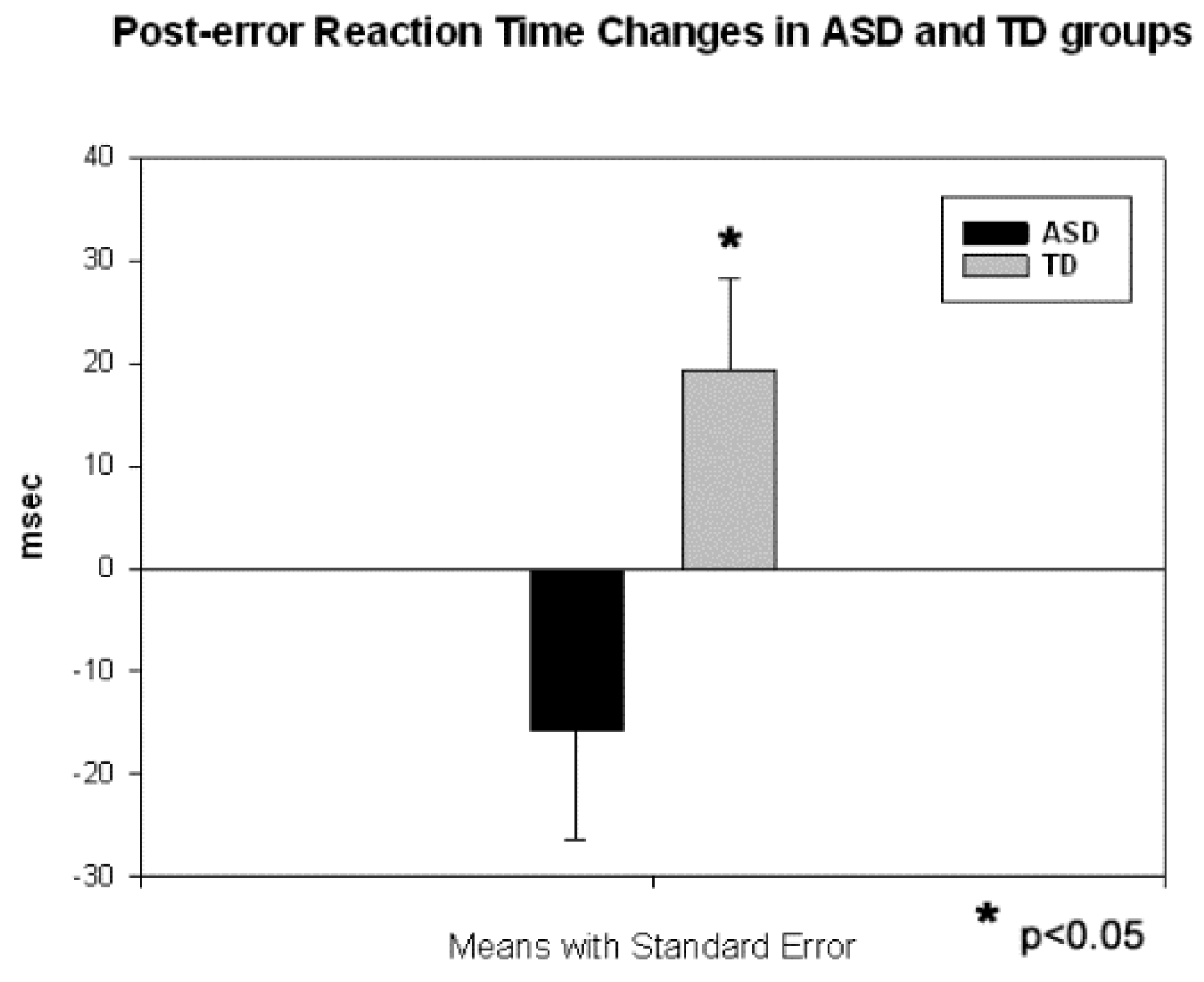
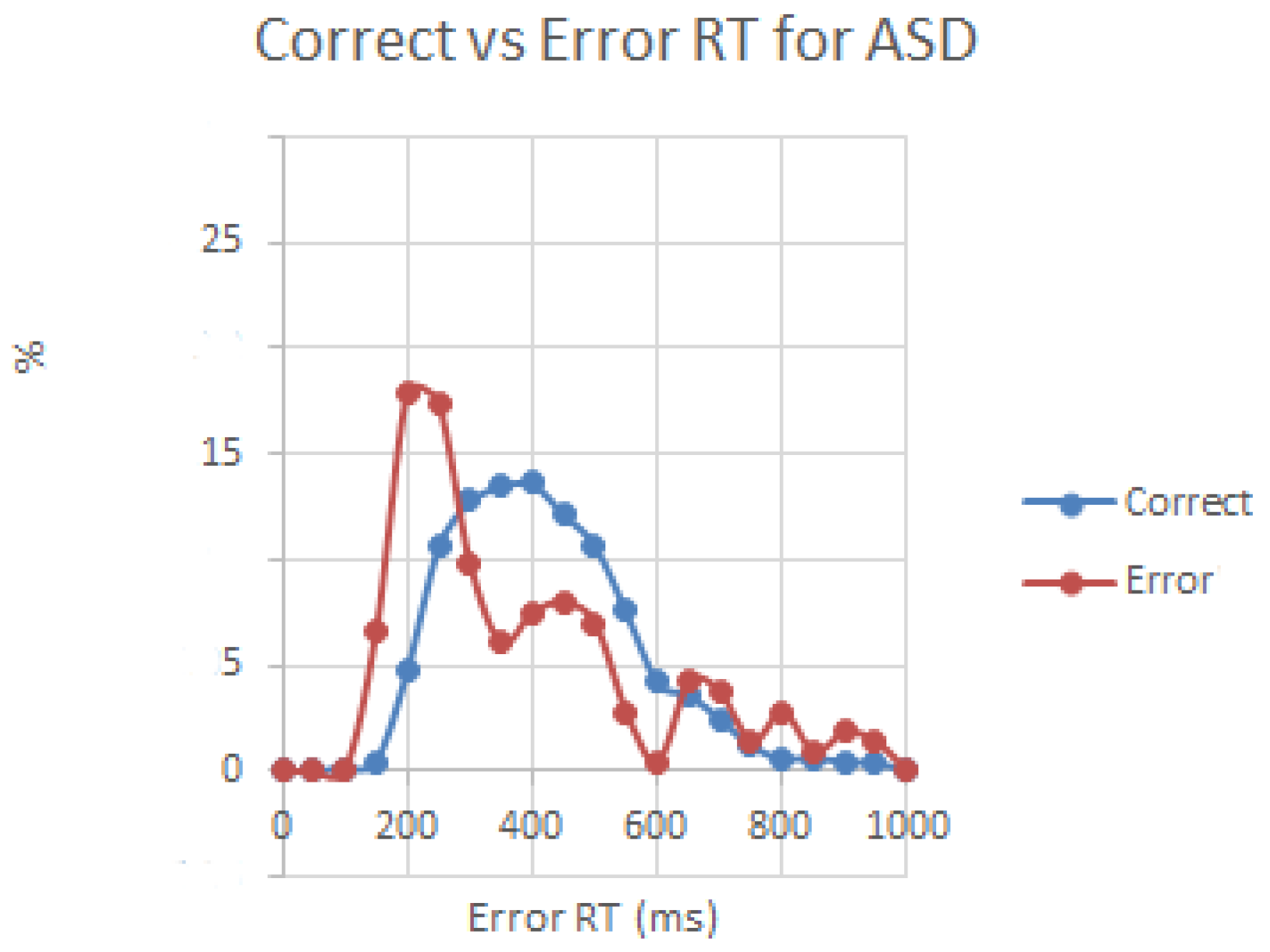
3.1. Behavioral Responses
3.2. Motor Response-Locked Fronto-Central ERPs
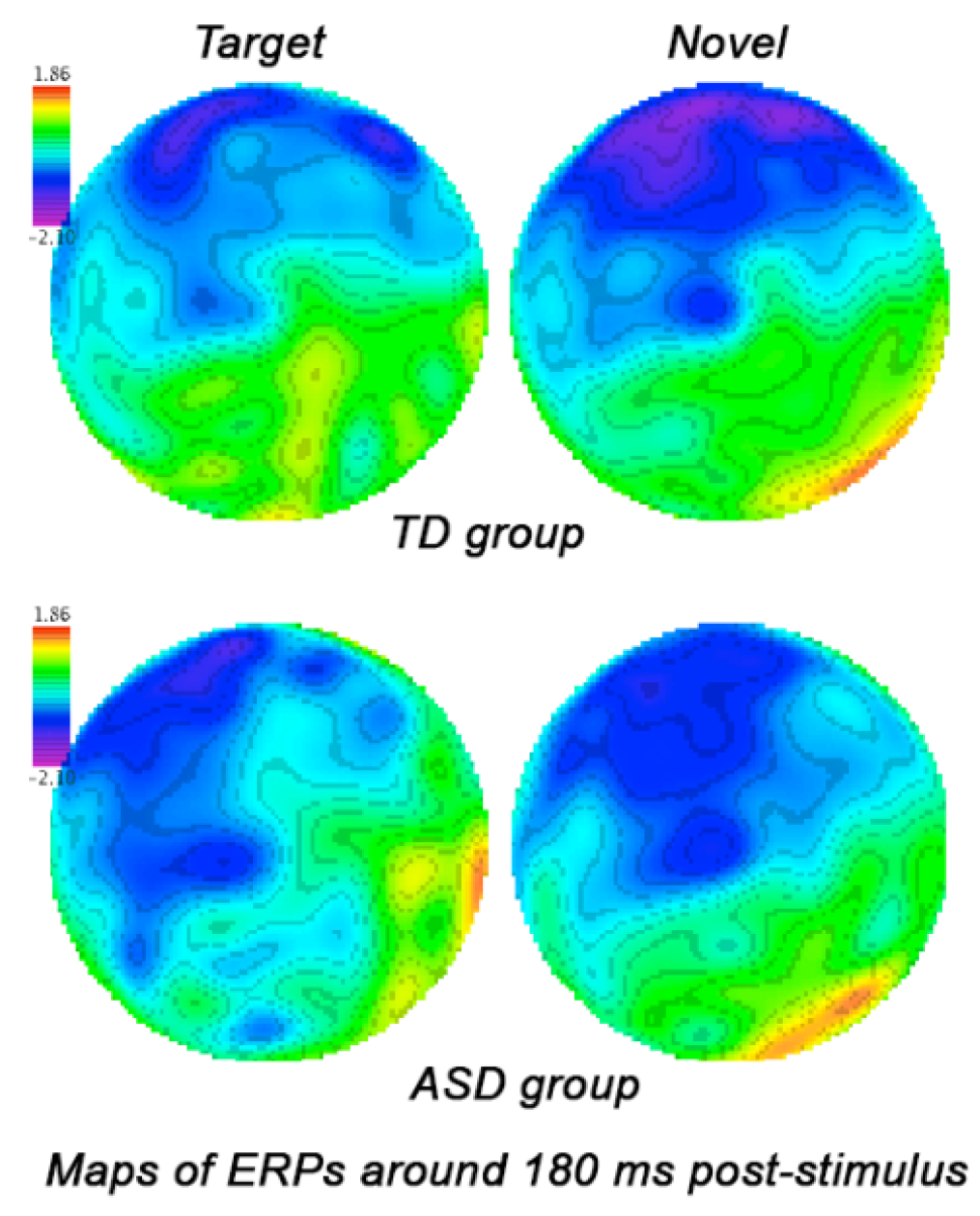
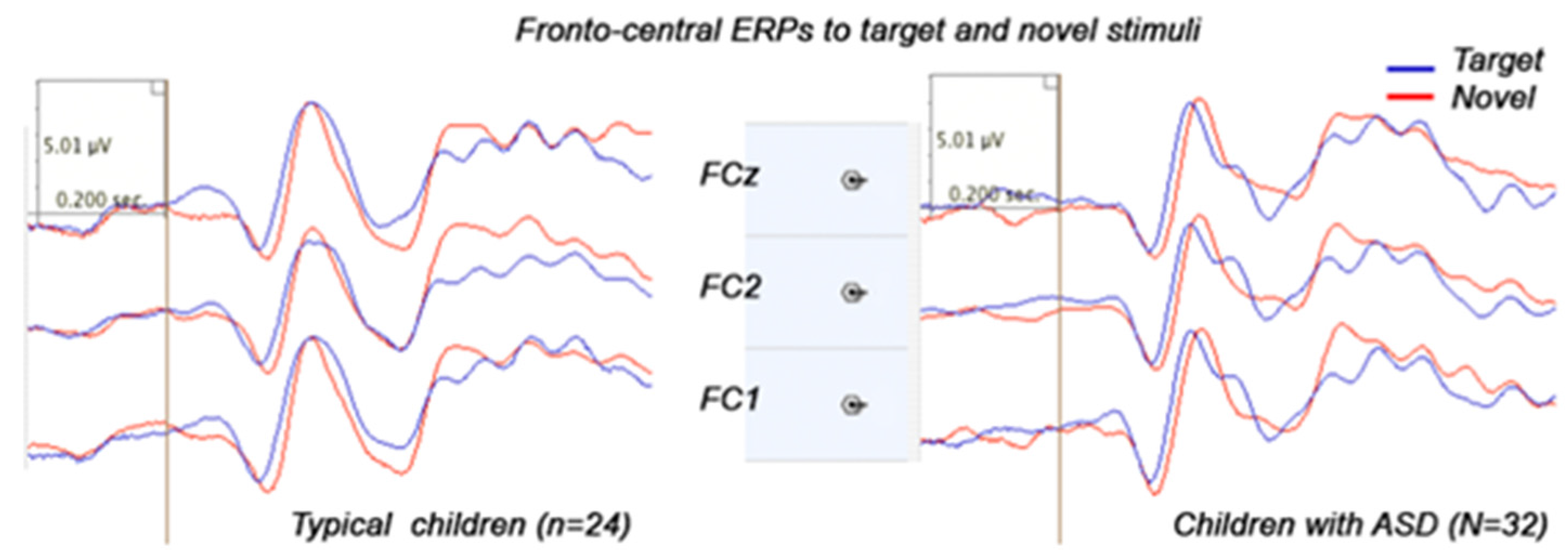
3.3. Stimulus-Locked Event-Related Potentials
3.3.1. Frontal ERPs
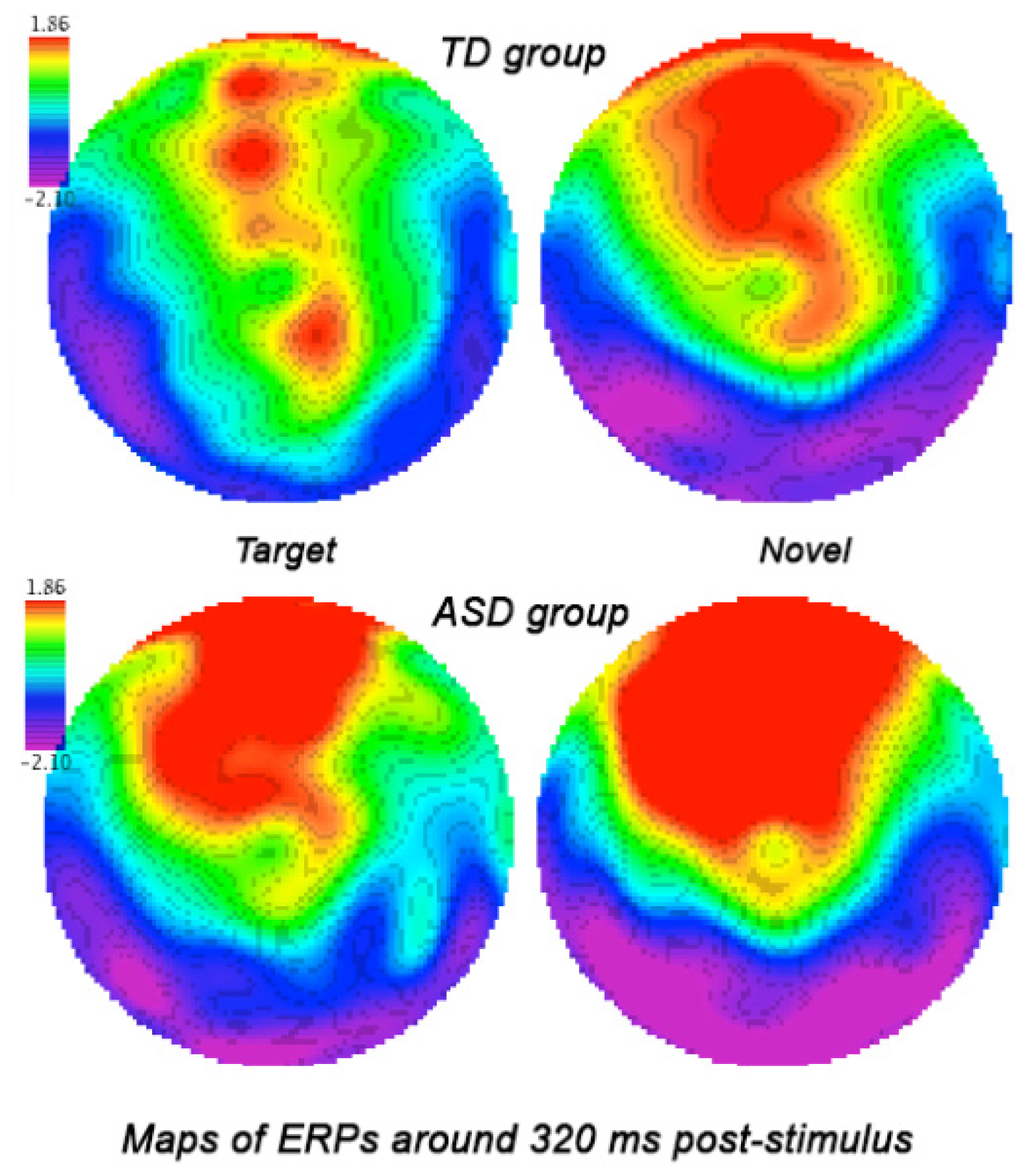
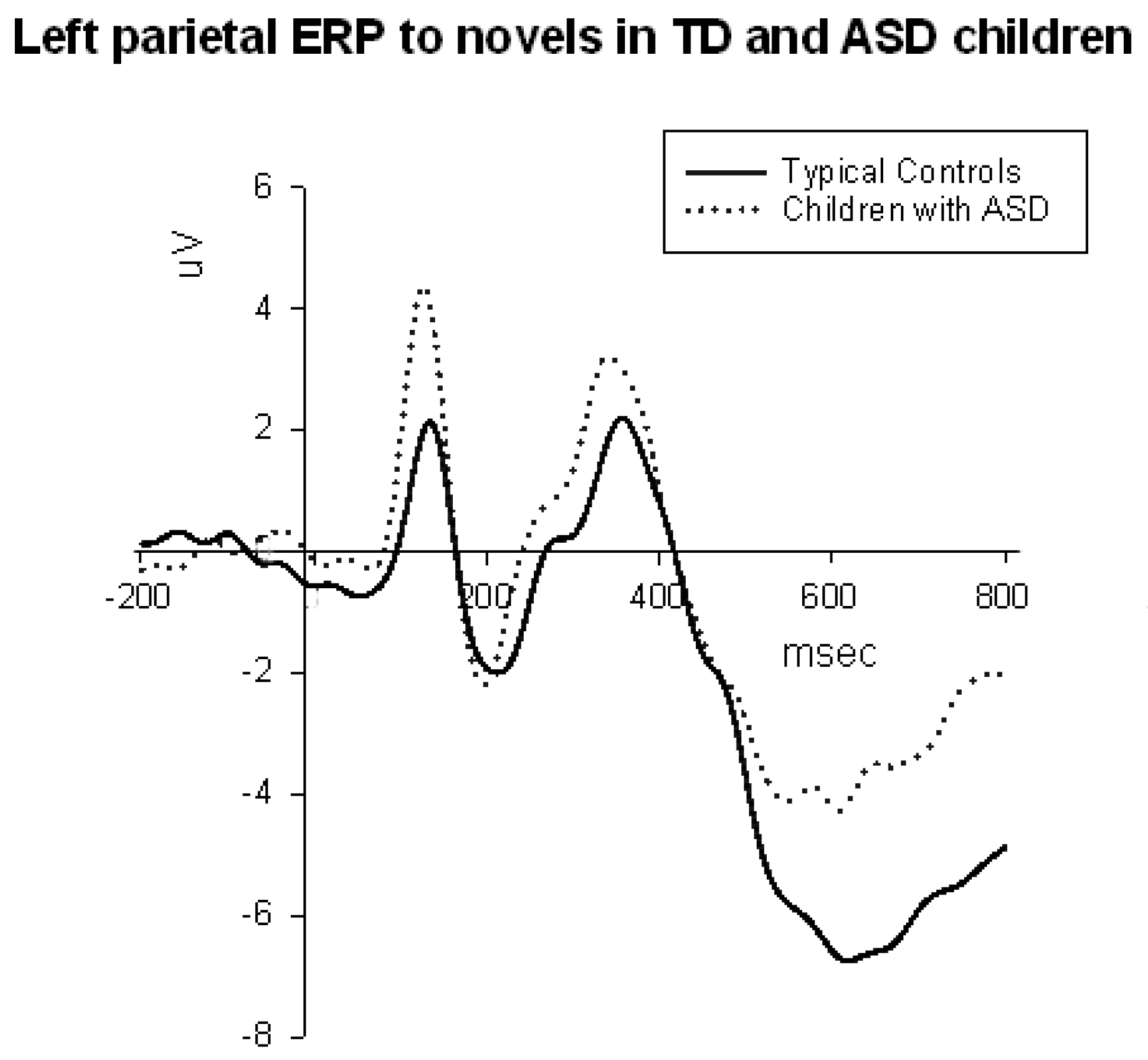
3.3.2. Parietal and Parieto-Occipital ERPs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diagnostic and Statistical Manual of Mental Disorders (DSM-V), 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013; ISBN 089042554X.
- Gomes, E.; Pedroso, F.S.; Wagner, M.B. Auditory hypersensitivity in the autistic spectrum disorder. Pro Fono 2008, 20, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Baruth, J.M.; Casanova, M.F.; Sears, L.; Sokhadze, E. Early-stage visual processing abnormalities in autism spectrum disorder (ASD). Transl. Neurosci. 2010, 1, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Baruth, J.M.; El-Baz, A.; Tasman, A.; Sears, L.; Sokhadze, E. Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. Transl. Neurosci. 2012, 3, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.; Baruth, J.; Tasman, A.; Sears, L.; Mathai, G.; El-Baz, A.; Casanova, M.F. Event-related potential study of novelty processing abnormalities in autism. Appl. Psychophysiol. Biofeedback 2009, 34, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.; Baruth, J.; Tasman, A.; Mansoor, M.; Ramaswamy, R.; Sears, L.; Mathai, G.; El-Baz, A.; Casanova, M.F. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl. Psychophysiol. Biofeedback 2010, 35, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.; Baruth, J.; El-Baz, A.; Horrell, T.; Sokhadze, G.; Carroll, T.; Tasman, A.; Sears, L.; Casanova, M. Impaired error monitoring and correction function in autism. J. Neurother. 2010, 14, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.M.; Baruth, J.M.; Sears, L.; Sokhadze, G.E.; El-Baz, A.S.; Williams, E.; Klapheke, R.; Casanova, M.F. Event related potentials study of attention regulation during illusory figure categorization task in ADHD, autism spectrum disorders, and typical children. J. Neurother. 2012, 16, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.M.; Baruth, J.; Tasman, A.; Casanova, M.F. Event-related potential studies of cognitive processing abnormalities in autism. In Imaging the Brain in Autism; Casanova, M.F., El-Baz, A.S., Suri, J.S., Eds.; Springer: New York, NY, USA, 2013; pp. 61–86. ISBN 978-1-4614-6843-1. [Google Scholar]
- Casanova, M.F.; Sokhadze, E.; Opris, I.; Wang, Y.; Li, X. Autism spectrum disorders: Linking neuropathological findings to treatment with transcranial magnetic stimulation. Acta Pediatr. 2015, 104, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Sokhadze, E.M.; Casanova, M.F.; Casanova, E.; Kelly, D.P.; Khachidze, I.; Wang, Y.; Li, X. Applications of ERPs in autism research and as functional outcomes of neuromodulation treatment. In Event-Related Potential (ERP): Methods, Outcomes and Research Insights; Harris, S.R., Ed.; NOVA Science Publishers: Hauppauge, NY, USA, 2017; pp. 27–88. ISBN 1536108057. [Google Scholar]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.W. The P300 wave of the human event-related potential. J. Clin. Neurophysiol. 1992, 9, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, W. Psychophysiology of P300. Psychol. Bull. 1981, 89, 506–540. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.G.H.; Rugg, M.D. Event-related brain potentials: An introduction. In Electrophysiology of Mind. Event-Related Brain Potentials and Cognition; Rugg, M.D., Coles, M.G.H., Eds.; Oxford University Press: Oxford, UK, 1995; pp. 1–26. ISBN 0198524161. [Google Scholar]
- Herrmann, C.S.; Knight, R.T. Mechanisms of human attention: Event related potentials and oscillations. Neurosci. Biobehav. Rev. 2001, 25, 465–476. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the event-related potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.E. Event-related potentials and controlled processes. In Event-Related Brain Potentials: Basic Issues and Applications; Rohrbaugh, J.W., Parasuraman, R., Johnson, R., Jr., Eds.; Oxford University Press: New York, NY, USA, 1990; pp. 145–157. ISBN 0195048911. [Google Scholar]
- Katayama, J.; Polich, J. Stimulus context determines P3a and P3b. Psychophysiol. 1998, 35, 23–33. [Google Scholar] [CrossRef]
- Polich, J. Theoretical overview of P3a and P3b. In Detection of Change: Event-related Potential and fMRI Findings; Polich, J., Ed.; Kluwer Academic Press: Boston, MA, USA, 2003; pp. 83–98. ISBN 978-1-4613-5008-8. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), 4th ed.; Text Revised; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2000; ISBN 0890420629. [Google Scholar]
- Le Couteur, A.; Lord, C.; Rutter, M. The Autism Diagnostic Interview—Revised (ADI-R); Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV); Harcourt Assessment, Inc.: San Antonio, TX, USA, 2003. [Google Scholar]
- Roid, G.H. Stanford-Binet Intelligence Scales Technical Manual, 5th ed.; Riverside Publishing: Itasca, IL, USA, 2003. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale for Intelligence; Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Non-Patient Edition (SCID-NP); New York State Psychiatric Institute: New York, NY, USA, 2002. [Google Scholar]
- Net Station Acquisition Technical Manual; Electrical Geodesics, Inc.: Eugene, OR, USA, 2003.
- Fletcher, E.M.; Kussmaul, C.L.; Mangun, G.R. Estimation of interpolation errors in scalp topographic mapping. Electroctoencephalogr. Clin. Neurophysiol. 1996, 98, 422–434. [Google Scholar] [CrossRef]
- Luu, P.; Tucker, D.M.; Englander, R.; Lockfeld, A.; Lutsep, H.; Oken, B. Localizing acute stroke-related EEC changes: assessing the effects of spatial undersampling. J. Clin. Neurophysiol. 2001, 18, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Perrin, E.; Pernier, J.; Bertrand, O.; Giard, M.; Echallier, J.F. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 75–81. [Google Scholar] [CrossRef]
- Srinivasan, R.; Tucker, D.M.; Murias, M. Estimating the spatial Nyquist of the human EEG. Behav. Res. Methods 1998, 30, 8–19. [Google Scholar] [CrossRef]
- Franken, H.A.; van Strien, J.W.; Franzek, E.J.; van de Wetering, B.J. Error-processing deficits in patients with cocaine dependence. Biol. Psychol. 2007, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Opris, I.; Lebedev, M.A.; Nelson, R.J. Neostriatal neuronal activity correlates better with movement kinematics under certain reward. Front. Neurosci. 2016, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.R.; Hullett, C.R. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum. Commun. Res. 2002, 28, 612–625. [Google Scholar] [CrossRef]
- Cui, T.; Wang, P.P.; Liu, S.; Zhang, X. P300 amplitude and latency in autism spectrum disorder: A meta-analysis. Eur. Child Adolesc. Psychiatry 2017, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kemner, C.; Verbaten, M.N.; Cuperus, J.M.; Camfferman, G.; Van Engeland, H. Visual and somatosensory event-related brain potentials in autistic children and three different control groups. Electroencephalogr. Clin. Neurophysiol. 1994, 92, 225–237. [Google Scholar] [CrossRef]
- Kemner, C.; Verbaten, M.N.; Cuperus, J.M.; Camfferman, G.; Van Engeland, H. Auditory event- related potentials in autistic children and three different control groups. Biol. Psychiatry 1995, 38, 150–165. [Google Scholar] [CrossRef]
- Kemner, C.; van der Gaag, R.J.; Verbaten, M.; van Engeland, H. ERP differences among subtypes of pervasive developmental disorders. Biol. Psychiatry 1999, 46, 781–789. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G. Is the p300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Townsend, J.; Westerfield, M.; Leaver, E.; Makeig, S.; Jung, T.; Pierce, K.; Courchesne, E. Event-related brain response abnormalities in autism: Evidence for impaired cerebello-frontal spatial attention networks. Brain Res. Cogn. Brain Res. 2001, 11, 127–145. [Google Scholar] [CrossRef]
- Ciesielski, K.T.; Courchesne, E.; Elmasian, R. Effects of focused attention tasks on event-related potentials in autistic and normal individuals. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 207–220. [Google Scholar] [CrossRef]
- Ferri, R.; Elia, M.; Agarwal, N.; Lanuzza, B.; Musumeci, S.A.; Pennisi, G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clin. Neurophysiol. 2003, 114, 1671–1680. [Google Scholar] [CrossRef]
- Bomba, M.D.; Pang, E.W. Cortical auditory evoked potentials in autism: A review. Int. J. Psychophysiol. 2004, 53, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Verbaten, M.N.; Roelofs, J.W.; van Engeland, H.; Kenemans, J.K.; Slangen, J.L. Abnormal visual event-related potentials of autistic children. J. Autism Dev. Disord. 1991, 21, 449–470. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Lincoln, A.J.; Yeung-Courchesne, R.; Elmasian, R.; Grillon, C. Pathophysiologic findings in nonretarded autism and receptive developmental disorder. J. Autism Dev. Disord. 1989, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, A.J.; Courchesne, E.; Harms, L.; Allen, M. Contextual probability evaluation in autistic, receptive developmental disorder and control children: Event-related potential evidence. J. Autism Dev. Disord. 1993, 23, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.K.; Yurgelun-Todd, D.A. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res. Cogn. Brain Res. 2003, 17, 651–664. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Yurgelun-Todd, D.A. Anatomic dissociation of selective and suppressive processes in visual attention. Neuroimage 2003, 19, 180–189. [Google Scholar] [CrossRef]
- Hopf, J.M.; Vogel, E.; Woodman, G.; Heinze, H.J.; Luck, S.J. Localizing visual discrimination processes in time and space. J. Neurophysiol. 2002, 88, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Heinze, H.; Mangun, G.R.; Hillyard, S.A. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 528–542. [Google Scholar] [CrossRef]
- Boutros, N.N.; Korzyukov, O.; Jansen, B.; Feingold, A.; Bell, M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004, 126, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Buxhoeveden, D.; Gomez, J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Neuroscientist 2003, 9, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, T.J.M.; Nieuwenhuis, S.; Ridderinkhof, K.R. Dissociable components of error processing. J. Psychophysiol. 2005, 19, 319–329. [Google Scholar] [CrossRef]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef] [PubMed]
- Yeung, N.; Cohen, J.D. The impact of cognitive deficits on conflict monitoring. Predictable dissociations between the error-related negativity and N2. Psychol. Sci. 2006, 17, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, V.; Carter, C.S. The timing of action-monitoring process in the anterior cingulate cortex. J. Cogn. Neurosci. 2002, 14, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Burle, B.; Vidal, F.; Tandonnet, C.; Hasbroucq, T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004, 56, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F. Minicolumnar pathology in autism. In Recent Developments in Autism Research; Casanova, M.F., Ed.; Nova Biomedical Books: New York, NY, USA, 2005; pp. 133–143. ISBN 1-59454-497-2. [Google Scholar]
- Casanova, M.F. Neuropathological and genetic findings in autism: The significance of a putative minicolumnopathy. Neuroscientist 2006, 12, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Just, M.A.; Cherkassky, V.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sequence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Casanova, M.F. Autism and dyslexia: A spectrum of cognitive styles as defined by minicolumnar morphometry. Med. Hypotheses 2009, 74, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Bogte, H.; Flamma, B.; van der Meere, J.; van Engeland, H. Post-error adaptation in adults with high functioning autism. Neuropsychologia 2007, 45, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.L.; Kemper, T.L. Structural brain anatomy in autism: What is the evidence? In The Neurobiology of Autism, 2nd ed.; Bauman, M.L., Kemper, T.L., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2005; pp. 121–135. ISBN 0801880475. [Google Scholar]
- Minshew, N.J.; Sweeney, J.A.; Bauman, M.L.; Webb, S.J. Neurological aspects of autism. In Handbook of Autism and Pervasive Developmental Disorders, Diagnosis, Development, Neurobiology, and Behavior, 3rd ed.; Volkmar, F.R., Paul, R., Klin, A., Cohen, D., Eds.; Wiley: New York, NY, USA, 2005; Volume 1, pp. 473–514. ISBN 0471716960. [Google Scholar]
- Mundy, P. Annotation: The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. J. Child Psychol. Psychiatry 2003, 44, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Henderson, H.; Schwartz, C.; Mundy, P.; Burnette, C.; Sutton, S.; Zahka, N.; Pradella, A. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006, 61, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Luciano, D. The contributions of cingulate cortex to human behavior. I. In Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook; Gabriel, M., Vogt, B.A., Eds.; Birkhauser: Boston, MA, USA, 1993; pp. 527–556. ISBN 978-1-4899-6706-0. [Google Scholar]
- Tamnes, C.K.; Walhovd, K.B.; Torstveit, M.; Sells, V.T.; Fjell, A.M. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Dev. Cogn. Neurosci. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokhadze, E.M.; Lamina, E.V.; Casanova, E.L.; Kelly, D.P.; Opris, I.; Khachidze, I.; Casanova, M.F. Atypical Processing of Novel Distracters in a Visual Oddball Task in Autism Spectrum Disorder. Behav. Sci. 2017, 7, 79. https://doi.org/10.3390/bs7040079
Sokhadze EM, Lamina EV, Casanova EL, Kelly DP, Opris I, Khachidze I, Casanova MF. Atypical Processing of Novel Distracters in a Visual Oddball Task in Autism Spectrum Disorder. Behavioral Sciences. 2017; 7(4):79. https://doi.org/10.3390/bs7040079
Chicago/Turabian StyleSokhadze, Estate M., Eva V. Lamina, Emily L. Casanova, Desmond P. Kelly, Ioan Opris, Irma Khachidze, and Manuel F. Casanova. 2017. "Atypical Processing of Novel Distracters in a Visual Oddball Task in Autism Spectrum Disorder" Behavioral Sciences 7, no. 4: 79. https://doi.org/10.3390/bs7040079
APA StyleSokhadze, E. M., Lamina, E. V., Casanova, E. L., Kelly, D. P., Opris, I., Khachidze, I., & Casanova, M. F. (2017). Atypical Processing of Novel Distracters in a Visual Oddball Task in Autism Spectrum Disorder. Behavioral Sciences, 7(4), 79. https://doi.org/10.3390/bs7040079




