Neural Adaptation Effects in Conceptual Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Phase 1: Induction of the McCollough Effect
2.2.2. Phase 2: Noun Categorization Task
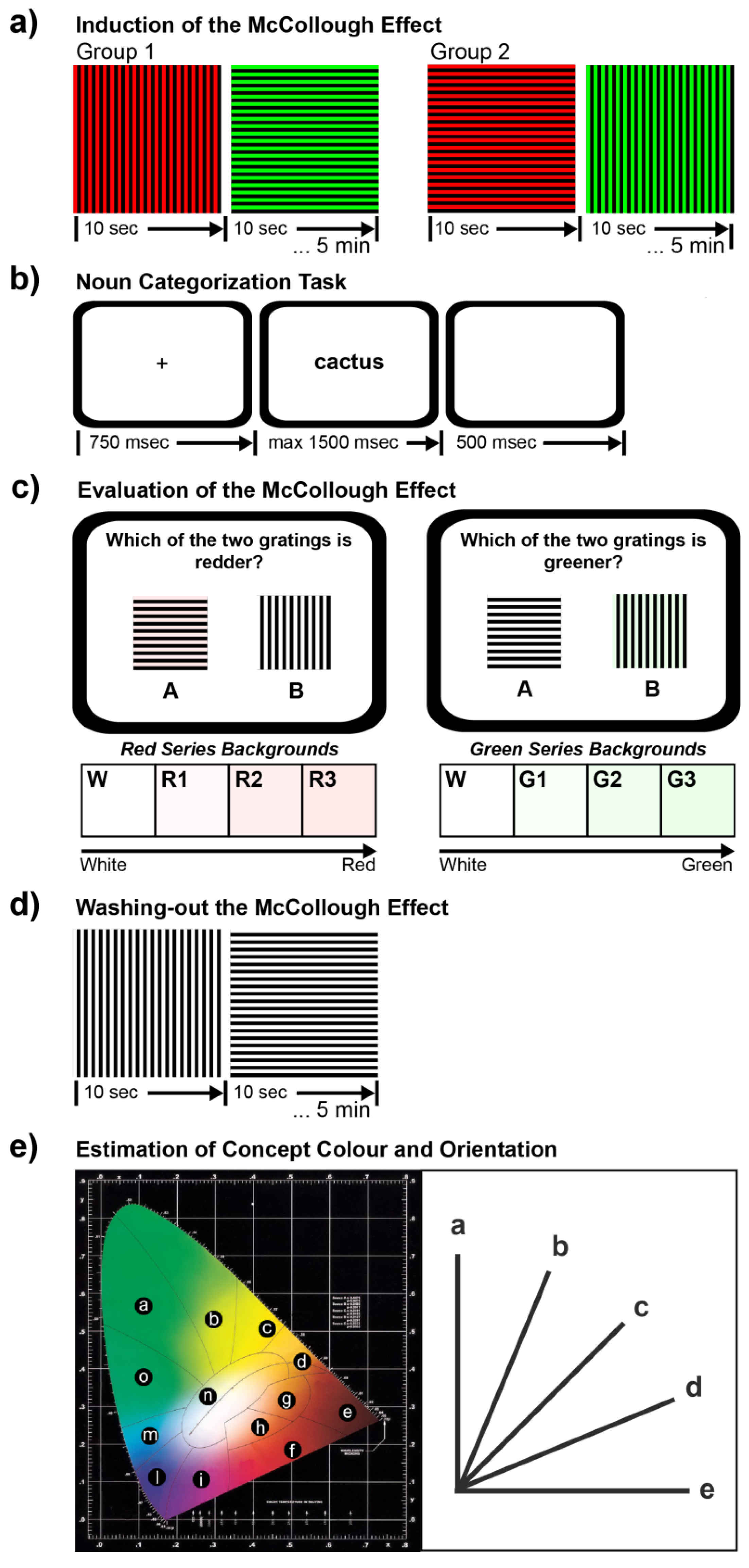
2.2.3. Phase 3: Evaluation of the McCollough Effect
2.2.4. Phase 4: Washing-Out the McCollough Effect
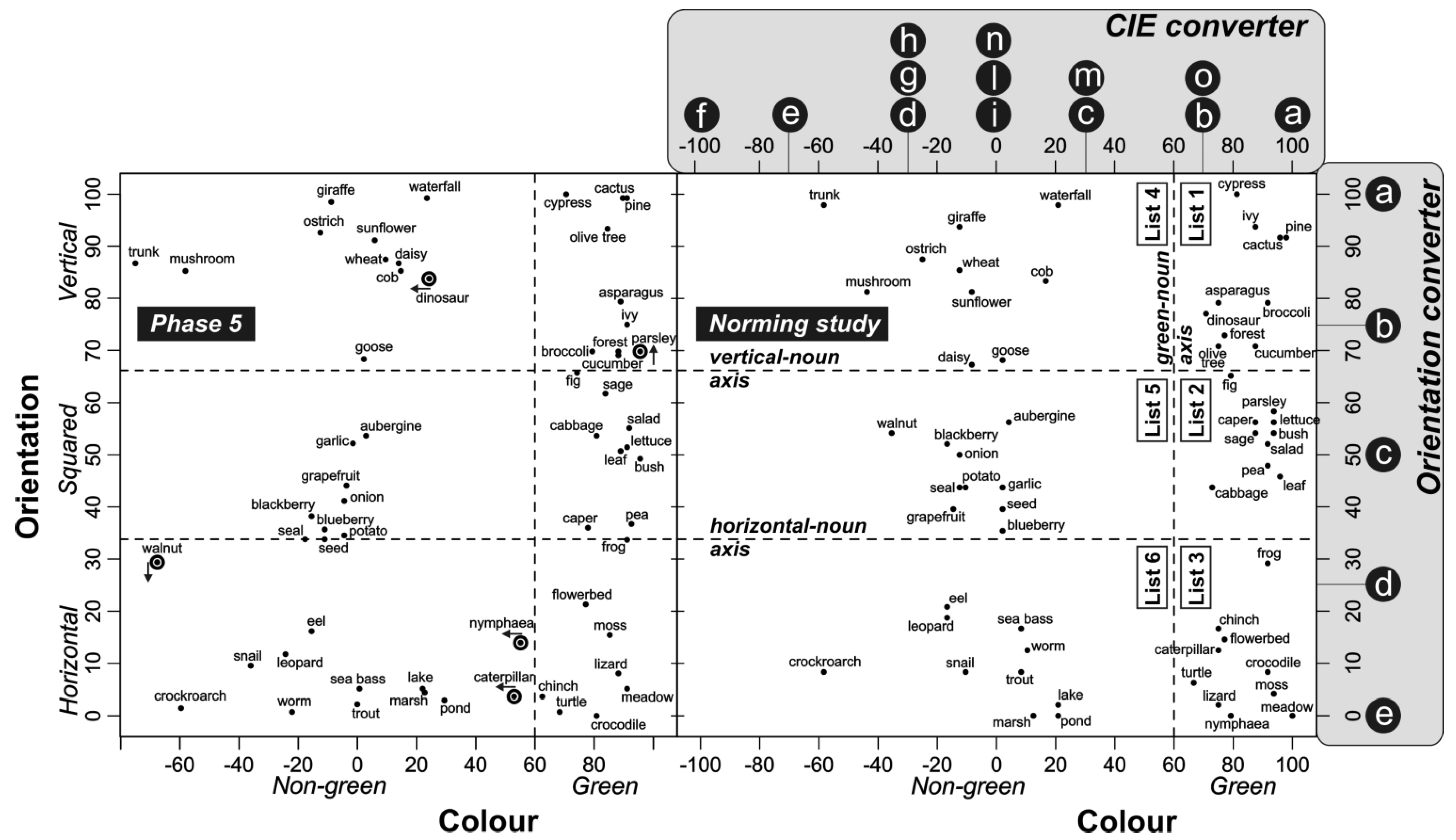
2.2.5. Phase 5: Estimation of Concept Color and Orientation
3. Results
3.1. Estimation of Concept Color and Orientation
3.2. Noun Categorization Task
| Italian Noun | English Noun | List | Word Length | Lexical Frequency | Familiarity | Imageability | Animacy |
|---|---|---|---|---|---|---|---|
| Abete | pine | 1 | 5 | 0.85 | 5.25 | 5.75 | 2.58 |
| asparago | asparagus | 1 | 8 | 0.01 | 5.33 | 5.83 | 1.00 |
| Bosco | forest | 1 | 5 | 17.8 | 4.83 | 5.25 | 2.08 |
| broccolo | broccoli | 1 | 8 | 0.01 | 5.17 | 5.25 | 1.17 |
| Cactus | cactus | 1 | 6 | 1.11 | 4.58 | 5.67 | 2.17 |
| cetriolo | cucumber | 1 | 8 | 0.28 | 5.50 | 5.67 | 1.17 |
| cipresso | cypress | 1 | 8 | 0.01 | 5.00 | 5.42 | 1.58 |
| dinosauro | dinosaur | 1 | 9 | 0.68 | 2.00 | 5.50 | 5.00 |
| Edera | ivy | 1 | 5 | 1.48 | 5.17 | 5.58 | 2.50 |
| Ulivo | olive (tree) | 1 | 5 | 0.09 | 5.25 | 5.67 | 1.83 |
| cappero | caper | 2 | 7 | 0.01 | 5.25 | 5.50 | 1.17 |
| cespuglio | bush | 2 | 9 | 1.47 | 5.17 | 5.50 | 1.92 |
| Fico | fig | 2 | 4 | 1.15 | 5.67 | 5.75 | 1.17 |
| Foglia | leaf | 2 | 6 | 6.79 | 5.75 | 5.67 | 1.83 |
| insalata | salad | 2 | 8 | 4.19 | 5.75 | 5.83 | 1.42 |
| lattuga | lettuce | 2 | 7 | 0.47 | 5.50 | 5.58 | 1.33 |
| Pisello | pea | 2 | 7 | 0.18 | 5.83 | 5.75 | 1.58 |
| prezzemolo | parsley | 2 | 10 | 3.08 | 5.50 | 5.75 | 1.92 |
| Salvia | sage | 2 | 6 | 2.26 | 5.67 | 5.83 | 1.83 |
| Verza | cabbage | 2 | 5 | 0.10 | 5.33 | 5.25 | 2.17 |
| Aiuola | flowerbed | 3 | 6 | 0.02 | 5.33 | 5.67 | 1.08 |
| Bruco | caterpillar | 3 | 5 | 0.03 | 4.75 | 5.50 | 4.33 |
| cimice | chinch | 3 | 6 | 0.12 | 4.83 | 5.08 | 5.17 |
| coccodrillo | crocodile | 3 | 11 | 1.50 | 3.42 | 5.75 | 5.08 |
| lucertola | lizard | 3 | 9 | 0.01 | 5.42 | 5.75 | 5.50 |
| muschio | Moss | 3 | 7 | 0.91 | 4.50 | 5.25 | 2.83 |
| Ninfea | nymphaea | 3 | 6 | 0.01 | 3.25 | 5.50 | 2.08 |
| Prato | meadow | 3 | 5 | 12.22 | 5.83 | 5.83 | 2.25 |
| Rana | frog | 3 | 4 | 1.13 | 4.67 | 5.67 | 5.17 |
| tartaruga | turtle | 3 | 9 | 0.83 | 4.83 | 5.67 | 5.00 |
| cascata | waterfall | 4 | 7 | 1.85 | 4.33 | 5.75 | 2.58 |
| Fungo | mushroom | 4 | 5 | 0.59 | 5.25 | 5.75 | 2.50 |
| giraffa | giraffe | 4 | 7 | 0.01 | 3.92 | 5.75 | 5.00 |
| girasole | sunflower | 4 | 8 | 0.01 | 5.17 | 5.83 | 2.67 |
| Grano | wheat | 4 | 5 | 4.95 | 4.58 | 5.58 | 1.17 |
| margherita | daisy | 4 | 10 | 0.01 | 5.58 | 5.83 | 1.67 |
| Oca | goose | 4 | 3 | 4.94 | 4.58 | 5.67 | 5.17 |
| pannocchia | corncob | 4 | 10 | 0.01 | 5.17 | 5.67 | 1.58 |
| struzzo | ostrich | 4 | 7 | 1.07 | 3.83 | 5.67 | 5.42 |
| Tronco | trunk | 4 | 6 | 4.51 | 5.25 | 5.67 | 2.08 |
| Aglio | garlic | 5 | 5 | 3.69 | 5.58 | 5.75 | 0.75 |
| cipolla | onion | 5 | 7 | 4.10 | 5.75 | 5.67 | 1.08 |
| Foca | seal | 5 | 4 | 0.25 | 3.33 | 5.50 | 4.83 |
| melanzana | aubergine | 5 | 9 | 0.12 | 5.50 | 5.67 | 1.50 |
| mirtillo | blueberry | 5 | 8 | 0.37 | 5.58 | 5.83 | 1.83 |
| Mora | blackberry | 5 | 4 | 1.10 | 5.42 | 5.50 | 1.67 |
| Noce | walnut | 5 | 4 | 2.84 | 5.83 | 5.83 | 1.33 |
| Patata | potato | 5 | 6 | 13.32 | 5.67 | 5.67 | 1.83 |
| pompelmo | grapefruit | 5 | 8 | 0.03 | 5.08 | 5.67 | 1.17 |
| Seme | seed | 5 | 4 | 6.39 | 5.58 | 5.67 | 1.67 |
| branzino | (sea) bass | 6 | 8 | 0.02 | 3.92 | 4.25 | 4.00 |
| Lago | lake | 6 | 4 | 16.26 | 5.50 | 5.83 | 2.17 |
| leopardo | leopard | 6 | 8 | 1.13 | 3.25 | 5.58 | 5.50 |
| lumaca | snail | 6 | 6 | 0.13 | 5.33 | 5.58 | 4.83 |
| murena | eel | 6 | 6 | 0.01 | 2.92 | 5.33 | 4.67 |
| palude | marsh | 6 | 6 | 1.33 | 2.42 | 5.00 | 2.00 |
| scarafaggio | cockroach | 6 | 11 | 0.12 | 4.42 | 5.33 | 5.33 |
| Stagno | pond | 6 | 6 | 2.30 | 4.25 | 5.50 | 2.08 |
| Trota | trout | 6 | 5 | 0.66 | 5.00 | 5.67 | 5.33 |
| Verme | worm | 6 | 5 | 1.26 | 5.33 | 5.50 | 5.17 |
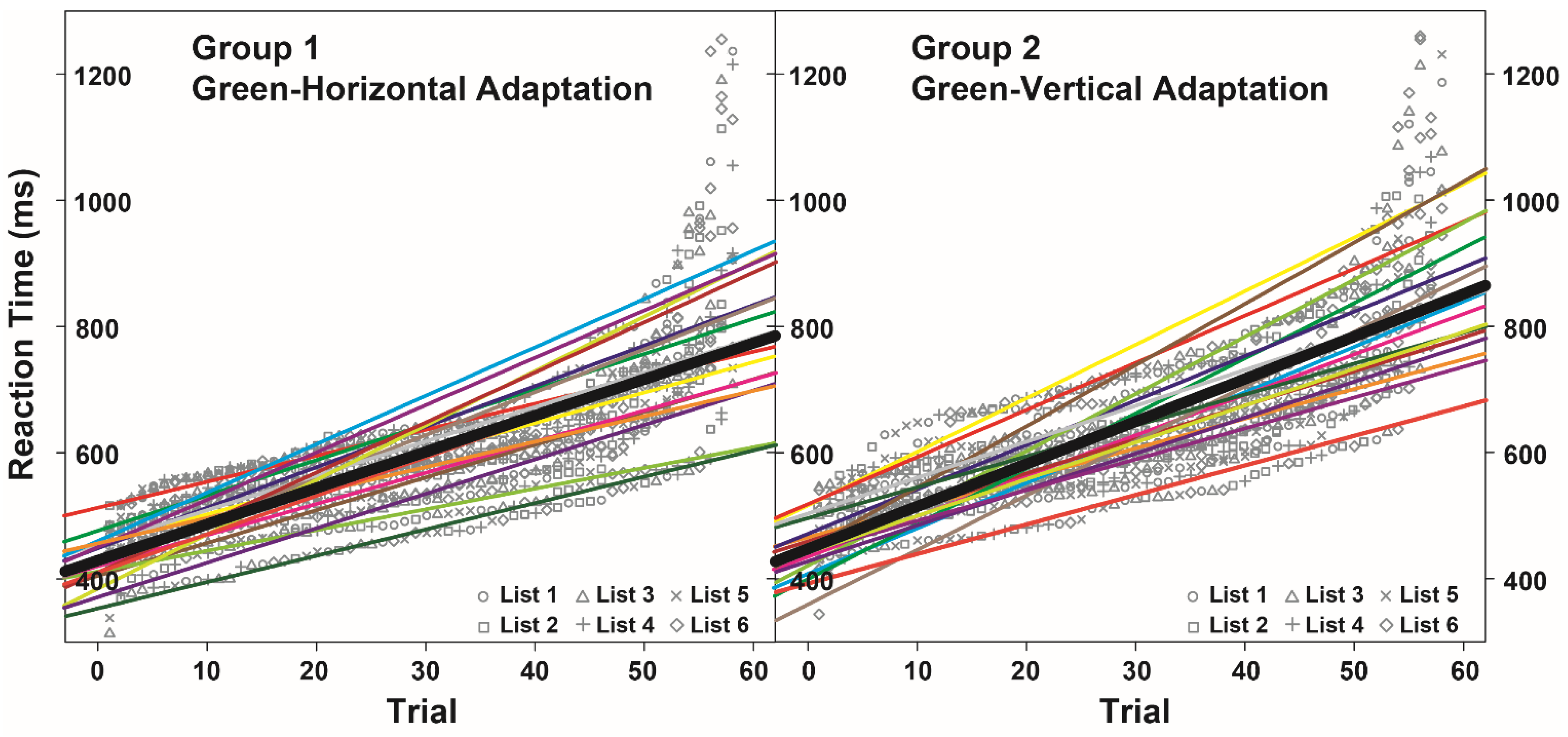
| Fixed Effect | Estimate | SE | Df | t Value | P |
|---|---|---|---|---|---|
| Intercept | 625.981 | 44.180 | 54.6 | 14.169 | 0.0001 * |
| Adaptation: Group2 | 48.381 | 19.826 | 53.5 | 2.440 | 0.018 * |
| Noun Type: 2 | 27.814 | 15.710 | 72.0 | 1.770 | 0.081 |
| Noun Type: 3 | 31.857 | 16.857 | 67.8 | 1.890 | 0.063 |
| Noun Type: 4 | 26.765 | 16.072 | 70.6 | 1.665 | 0.101 |
| Noun Type: 5 | 6.694 | 15.574 | 72.3 | 0.430 | 0.669 |
| Noun Type: 6 | 33.671 | 17.685 | 64.6 | 1.904 | 0.061 |
| Adaptation: Group2 * | |||||
| Noun Type: 2 | −24.230 | 14.309 | 1753.1 | −1.693 | 0.091 |
| Adaptation: Group 2 * | |||||
| Noun Type: 3 | −25.708 | 14.639 | 1752.1 | −1.756 | 0.079 |
| Adaptation: Group 2 * | |||||
| Noun Type: 4 | −2.851 | 14.241 | 1752.4 | −0.200 | 0.841 |
| Adaptation: Group 2 * | |||||
| Noun Type: 5 | −3.105 | 14.242 | 1752.1 | −0.218 | 0.827 |
| Adaptation: Group 2 * | |||||
| Noun Type: 6 | 6.968 | 14.410 | 1752.1 | 0.484 | 0.629 |
| Word Animacy | −9.810 | 3.496 | 45.9 | −2.806 | 0.007 * |
| Word Imageability | −67.318 | 19.940 | 52.7 | −3.376 | 0.001 * |
| Word Familiarity | −16.266 | 6.210 | 46.3 | −2.619 | 0.012 * |
| Word Length | 5.534 | 2.221 | 46.2 | 2.492 | 0.016 * |
| Lexical Frequency | −1.557 | 1.526 | 46.2 | −1.020 | 0.313 |
3.3. Evaluation of the McCollough Effect

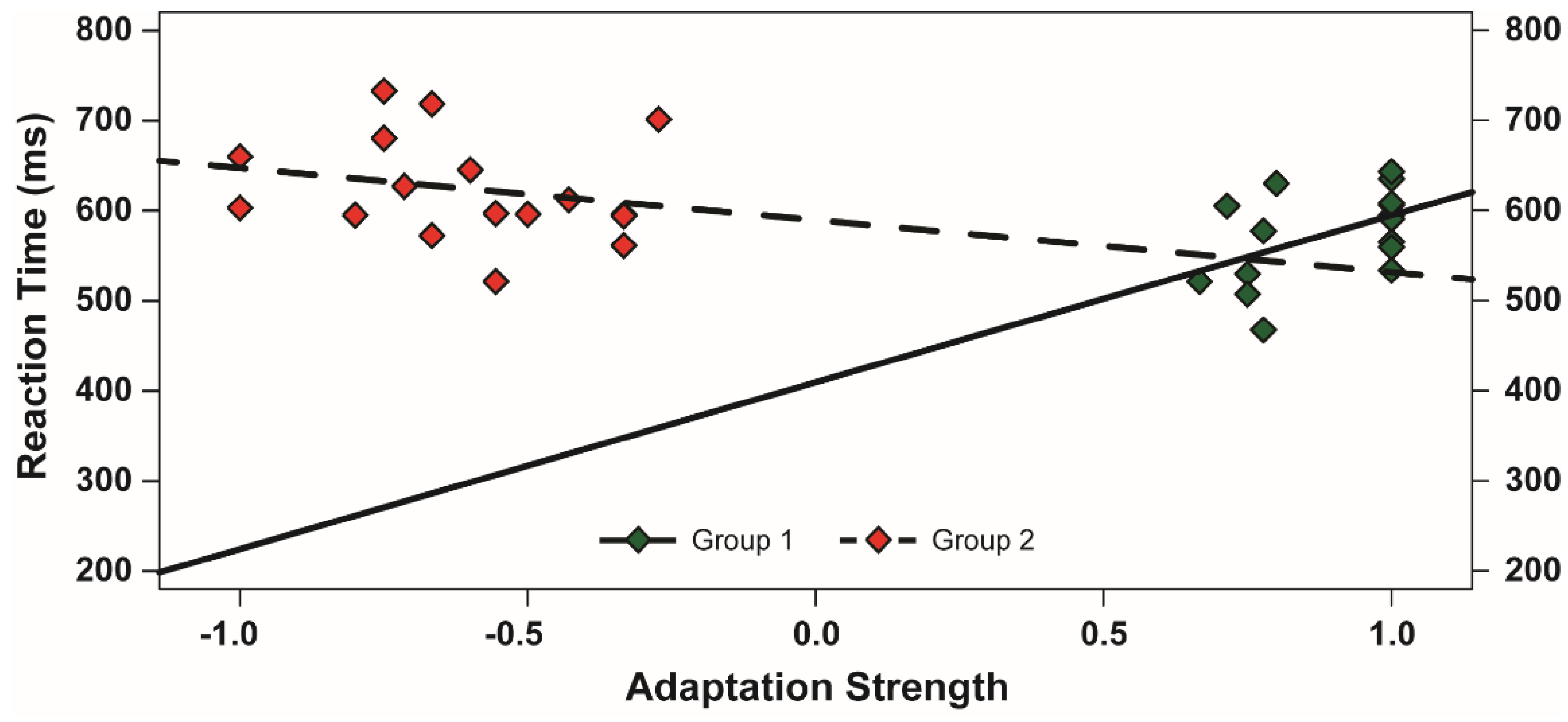
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barsalou, L.W. Perceptual symbol systems. Behav. Brain Sci. 1999, 22, 577–660. [Google Scholar] [CrossRef] [PubMed]
- Barsalou, L.W. Grounded Cognition. Annu. Rev. Psychol. 2008, 59, 617–645. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.H.; Zwaan, R.A. Embodied language: A review of the role of the motor system in language comprehension. Q. J. Exp. Psychol. 2008, 61, 825–850. [Google Scholar] [CrossRef] [PubMed]
- Glenberg, A.M.; Gallese, V. Action-based language: A theory of language acquisition, comprehension, and production. Cortex 2012, 48, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Jirak, D.; Menz, M.M.; Buccino, G.; Borghi, A.M.; Binkofski, F. Grasping language—A short story on embodiment. Conscious.Cogn. 2010, 19, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.Z.; Caramazza, A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cogn. Neuropsychol. 2005, 22, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.Z.; Caramazza, A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J. Physiol. (Paris) 2008, 102, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Martin, A. The representation of object concepts in the brain. Annu. Rev. Psychol. 2007, 58, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Meteyard, L.; Cuadrado, S.R.; Bahrami, B.; Vigliocco, G. Coming of age: A review of embodiment and the neuroscience of semantics. Cortex 2012, 48, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Toni, I.; De Lange, F.P.; Noordzij, M.L.; Hagoort, P. Language beyond action. J. Physiol. (Paris) 2008, 102, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pulvermüller, F.; Shtyrov, Y.; Ilmoniemi, R. Brain signatures of meaning access in action word recognition. J. Cogn. Neurosci. 2005, 17, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.F.; Gough, P.M.; Gallese, V.; Riggio, L.; Buccino, G. How the motor system handles nouns: A behavioral study. Psychol. Res. 2013, 77, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.F.; Sirianni, M.; Volta, R.; Magliocco, F.; Silipo, F.; Quattrone, A.; Buccino, G. Viewing photos and reading nouns of natural graspable objects similarly modulate motor responses. Front. Hum. Neurosci. 2014, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Barros-Loscertales, A.; Pulvermüller, F.; Meseguer, V.; Sanjuán, A.; Belloch, V.; Avila, C. Reading cinnamon activates olfactory brain regions. Neuroimage 2006, 32, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.K.; Ramjee, V.; Beauchamp, M.S.; McRae, K.; Martin, A.; Barsalou, L.W. A common neural substrate for perceiving and knowing about color. Neuropsychologia 2007, 45, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Pecher, D.; Zeelenberg, R.; Barsalou, L.W. Verifying different-modality properties for concepts produces switching costs. Psychol. Sci. 2003, 14, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Disembodying cognition. Lang. Cogn. 2010, 2, 79–116. [Google Scholar] [CrossRef] [PubMed]
- Dove, G. On the need for embodied and dis-embodied cognition. Front. Psychol. 2011, 1, 242. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Henson, R.; Martin, A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006, 10, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kochiyama, T.; Okada, T.; Yonekura, Y.; Matsumura, M.; Sadato, N. The neural substrates of conscious color perception demonstrated using fMRI. Neuroimage 2004, 21, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.D.; Holding, D.H. Extremely long-term persistence of the McCollough effect. J. Exp. Psychol.: Hum. Percept. Perform. 1975, 1, 323–327. [Google Scholar] [CrossRef]
- McCollough, C. Color adaptation of edge-detectors in the human visual system. Science 1965, 149, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Howard, R.; Senior, C.; Brammer, M.; Bullmore, E.T.; Simmons, A.; David, A.S. The functional anatomy of the McCollough contingent colour after-effect. Neuroreport 1999, 10, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kurby, C.A.; Wiemer-Hastings, K. Adaptation effects on world recognition times: Evidence for perceptual representations. In Proceedings of the 26th Annual Conference of the Cognitive Science Society, Chicago, IL, USA, 5–7 August 2004; Forbus, K., Gentner, D., Regier, T., Eds.; Cognitive Science Society: Austin, TX, USA, 2004; p. 1583. [Google Scholar]
- Wiemer-Hastings, K.; Kurby, C.A. Access to perceptual features during world recognition. In Proceedings of the 27th Annual Conference of the Cognitive Science Society, Stresa, Italy, 21–23 July 2005; Bara, B.G., Barsalou, L.W., Bucciarelli, M., Eds.; Cognitive Science Society: Austin, TX, USA, 2005; p. 27. [Google Scholar]
- Ishihara, S. Tests for Colour-Blindness; Kanehara Shuppan: Tokio, Japan, 1971. [Google Scholar]
- E-Prime, version 1.1; Psychology Software Tools: Pittsburgh, PA, USA, 1996.
- Laudanna, A.; Thorton, A.; Brown, G.; Burani, C.; Marconi, L. Un corpus dell’italiano scritto contemporaneo dalla parte del ricevente. In III Giornate Internazionali di Analisi Statistica dei dati Testuali, 1st ed.; Bolasco, S., Lebart, L., Salem, A., Eds.; Cisu: Roma, Italy, 1995; Volume 1, pp. 103–109. (In Italian) [Google Scholar]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Memory Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Baayen, R.H.; Milin, P. Analyzing reaction times. Int. J. Psychol. Res. 2010, 3, 12–28. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in Linear Mixed Effects Models. Available online: http://CRAN.R-project.org/package=lmerTest (accessed on 13 July 2015).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 13 July 2015).
- Guilford, J.P. The method of pair comparisons. In Psychometric Methods, 2nd ed.; Guilford, J.P., Ed.; McGraw-Hill: New York, NY, USA, 1954; Chapter 7. [Google Scholar]
- Warrington, E.K. The selective impairment of semantic memory. Q. J. Exp. Psychol. 1975, 27, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Warrington, E.K.; McCarthy, R.A. Categories of knowledge. Further fractionations and an attempted integration. Brain 1987, 110, 1273–1296. [Google Scholar] [CrossRef] [PubMed]
- Crutch, S.J.; Warrington, E.K. The selective impairment of fruit and vegetable knowledge: A multiple processing channels account of fine-grain category specificity. Cogn. Neuropsychol. 2003, 20, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.M.; Caramelli, N.; Setti, A. Conceptual information on objects’ locations. Brain Lang. 2005, 93, 140–151. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, E.; Vanoverberghe, V.; Storms, G.; de Boeck, P. The instantiation principle re-evaluated. Memory 2003, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Heit, E.; Barsalou, L.W. The instantiation principle in natural categories. Memory 1996, 4, 413–451. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.E.; Meredith, M.A. The Merging of Senses; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Massaro, D.W.; Friedman, D. Models of integration given multiple sources of information. Psychol. Rev. 1990, 97, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.O.; Bülthoff, H.H. Merging the senses into a robust percept. Trends Cogn. Sci. 2004, 8, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Tversky, A. Features of similarity. Psychol. Rev. 1977, 84, 327–352. [Google Scholar] [CrossRef]
- Hampton, J.A. Similarity-based categorization and fuzziness of natural categories. Cognition 1998, 65, 137–165. [Google Scholar] [CrossRef]
- Goodhew, S.C.; Kendall, W.; Ferber, S.; Pratt, J. Setting semantics: Conceptual set can determine the physical properties that capture attention. Atten. Percept. Psychophys. 2014, 76, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, B.F.M.; Borghi, A.M.; Gemmi, L.; Cacciari, C.; Riggio, L. Neural Adaptation Effects in Conceptual Processing. Behav. Sci. 2015, 5, 353-371. https://doi.org/10.3390/bs5030353
Marino BFM, Borghi AM, Gemmi L, Cacciari C, Riggio L. Neural Adaptation Effects in Conceptual Processing. Behavioral Sciences. 2015; 5(3):353-371. https://doi.org/10.3390/bs5030353
Chicago/Turabian StyleMarino, Barbara F. M., Anna M. Borghi, Luca Gemmi, Cristina Cacciari, and Lucia Riggio. 2015. "Neural Adaptation Effects in Conceptual Processing" Behavioral Sciences 5, no. 3: 353-371. https://doi.org/10.3390/bs5030353
APA StyleMarino, B. F. M., Borghi, A. M., Gemmi, L., Cacciari, C., & Riggio, L. (2015). Neural Adaptation Effects in Conceptual Processing. Behavioral Sciences, 5(3), 353-371. https://doi.org/10.3390/bs5030353





