Fusiform Correlates of Facial Memory in Autism
Abstract
:1. Introduction
1.1. Facial Processing and Memory as a Deficit in Autism
1.2. Neuroanatomical Correlates of Facial Processing and Memory
2. Method
2.1. Ascertainment
2.2. Subject Groups
2.3. Idiopathic Autism Sample
2.4. Control Sample
2.5. IQ
2.6. Head Circumference and Handedness
2.7. Neuroimaging
2.8. Volumetric Image Analysis
2.8.1. Memory
2.8.2. Statistical Analysis
3. Results
3.1. Sample Characteristics
| ASD (n = 56) | Typically-developing (n = 31) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | p | |
| Age in years | 12.00 | 4.37 | 5.00–19.75 | 11.98 | 4.01 | 5.25–19.33 | 0.02 | 0.98 |
| Head Circumference (cm) | 54.71 | 5.81 | 50.70–60.50 | 55.34 | 2.13 | 51.80–60.50 | –0.00 | 0.99 |
| Total Intracranial Volume (TICV,cm3) | 1672.32 | 157.84 | 1268.66–2063.84 | 1675.94 | 176.45 | 1399.97–2171.21 | 0.10 | 0.92 |
| Handedness Inventory | 61.45 | 54.78 | –100–100 | 65.28 | 44.97 | –80–100 | 0.01 | 0.99 |
| Wechsler FIQ | 98.26 | 16.63 | 61–137 | 115.24 | 15.57 | 87–152 | 4.53 ** | < 0.001 |
| Wechsler PIQ | 102.41 | 16.00 | 66–138 | 116.13 | 15.46 | 90–155 | 3.88 ** | < 0.001 |
| Wechsler VIQ | 95.57 | 20.92 | 55–145 | 110.94 | 16.14 | 74–140 | 3.54 ** | < 0.001 |
3.2. Facial Memory Performance in Autism and Controls
| Measure | Mean (SD) ASD n = 56 | Mean (SD) TDC n = 31 | F | p | ρη2 |
|---|---|---|---|---|---|
| Facial Memory | 7.29 (2.36) | 10.52 (2.53) | 32.76 | < 0.001 | 0.28 |
| Facial Memory Delayed | 7.64 (2.73) | 9.83 (2.17) | 14.32 | < 0.001 | 0.15 |
| Object Recall | 5.98 (3.45) | 9.37 (2.70) | 21.57 | < 0.001 | 0.21 |
| Visual Selective Reminding (Immediate) | 7.48 (3.30) | 9.87 (2.56) | 11.76 | < 0.001 | 0.12 |
| Visual Selective Reminding (Delayed) | 8.75 (2.03) | 10.10 (1.52) | 10.06 | < 0.001 | 0.11 |
3.3. ROI Volumes in Autism and Controls
| Structure | Mean (SD) ASD n = 56 | Mean (SD) TDC n = 31 | F | P | ρη2 |
|---|---|---|---|---|---|
| center Fusiform | 12.12 (1.89) | 12.95 (2.03) | 4.91 * | 0.03 | 0.06 |
| Right Fusiform | 11.81 (1.79) | 11.64 (1.67) | 0.25 | 0.62 | 0.00 |
| center Amygdala | 1.78 (0.30) | 1.67 (0.24) | 4.07 * | 0.049 | 0.05 |
| Right Amygdala | 1.78 (0.28) | 1.72 (0.23) | 1.21 | 0.27 | 0.01 |
| center Hippocampus | 4.62 (0.61) | 4.39 (0.66) | 4.15 * | 0.048 | 0.05 |
| Right Hippocampus | 4.61 (0.65) | 4.56 (0.46) | 0.40 | 0.53 | 0.01 |
3.4. Relationship between ROI Volume and Facial Memory Performance
| Structure | Facial Memory | Facial Memory Delayed | ||
|---|---|---|---|---|
| ASD | TDC | ASD | TDC | |
| center Fusiform | 0.10 | 0.05 | –0.28 * | –0.21 |
| Right Fusiform | 0.13 | 0.12 | –0.15 | –0.09 |
| center Amygdala | 0.04 | –0.01 | –0.02 | 0.09 |
| Right Amygdala | 0.07 | –0.04 | –0.11 | –0.07 |
| center Hippocampus | –0.05 | 0.06 | –0.11 | 0.08 |
| Right Hippocampus | –0.17 | 0.17 | –0.28 * | 0.14 |
| Structure | Object Recall | Visual Selective Reminding (Immediate) | Visual Selective Reminding (Delayed) | |||
|---|---|---|---|---|---|---|
| ASD | TDC | ASD | TDC | ASD | TDC | |
| Left Fusiform | 0.08 | –0.08 | 0.13 | –0.24 | 0.11 | 0.13 |
| Right Fusiform | –0.01 | 0.01 | –0.14 | –0.07 | 0.10 | 0.26 |
| Left Amygdala | –0.14 | 0.01 | –0.13 | –0.11 | 0.06 | 0.10 |
| Right Amygdala | 0.03 | –0.04 | –0.14 | –0.14 | 0.14 | 0.12 |
| Left Hippocampus | –0.20 | 0.19 | –0.02 | –0.18 | 0.07 | –0.16 |
| Right Hippocampus | –0.16 | 0.17 | –0.24 | –0.18 | 0.08 | 0.26 |
4. Discussion
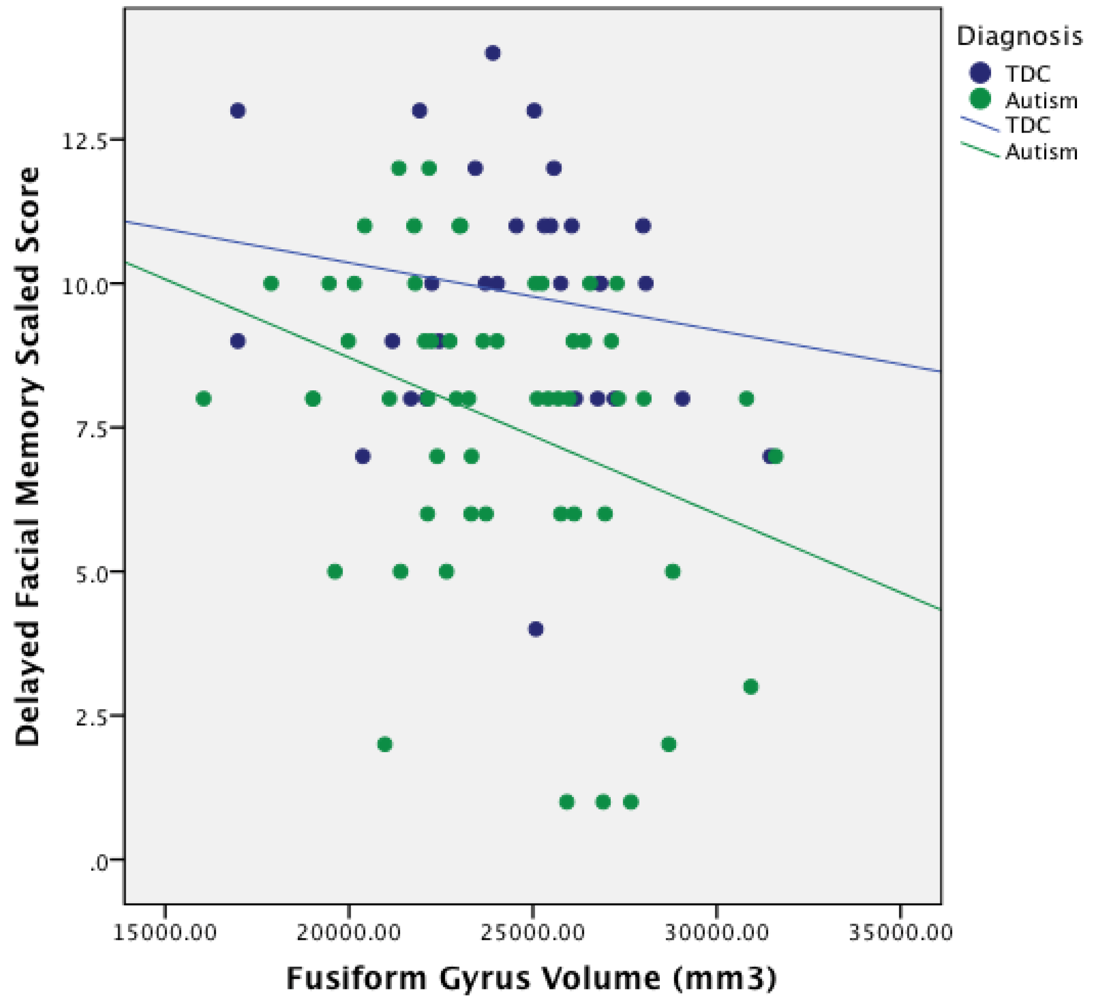

4.1. Fusiform Gyrus Volumes in Autism
4.2. Atypical Structure–Function Relationship of Fusiform Gyrus Morphometry in Autism
4.3. Relationship between Structure Size and Connectivity
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Bowler, D.M.; Gardiner, J.M.; Grice, S.J. Episodic memory and remembering in adults with Asperger syndrome. J. Autism Dev. Disord. 2000, 30, 295–304. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Bowler, D.M.; Grice, S.J. Further evidence of preserved priming and impaired recall in adults with Asperger’s syndrome. J. Autism Dev. Disord. 2003, 33, 259–269. [Google Scholar] [CrossRef]
- Millward, C.; Powell, S.; Messer, D.; Jordan, R. Recall for self and other in autism: Children’s memory for events experienced by themselves and their peers. J. Autism Dev. Disord. 2000, 30, 15–28. [Google Scholar] [CrossRef]
- Russell, J.; Jarrold, C.; Henry, L. Working memory in children with autism and with moderate learning difficulties. J. Child. Psychol. Psyc. 1996, 37, 673–686. [Google Scholar] [CrossRef]
- Southwick, J.S.; Bigler, E.D.; Froehlich, A.; Dubray, M.B.; Alexander, A.L.; Lange, N.; Lainhart, J.E. Memory functioning in children and adolescents with autism. Neuropsychology 2011, 25, 702–710. [Google Scholar] [CrossRef]
- Williams, D.L.; Goldstein, G.; Minshew, N.J. The profile of memory function in children with autism. Neuropsychology 2006, 20, 21–29. [Google Scholar] [CrossRef]
- Lange, N.; DuBray, M.B.; Lee, J.E.; Froimowitz, M.P.; Froehlich, A.; Adluru, N.; Wright, B.; Ravichandran, C.; Fletcher, P.T.; Bigler, E.D.; et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010, 3, 350–358. [Google Scholar] [CrossRef]
- Jeneson, A.; Squire, L.R. Working memory, long-term memory, and medial temporal lobe function. Learn. Mem. 2011, 19, 15–25. [Google Scholar] [CrossRef]
- Lajiness-O’Neill, R.R.; Beaulieu, I.; Titus, J.B.; Asamoah, A.; Bigler, E.D.; Bawle, E.V.; Pollack, R. Memory and learning in children with 22q11.2 deletion syndrome: Evidence for ventral and dorsal stream disruption? Child. Neuropsychol. 2005, 11, 55–71. [Google Scholar] [CrossRef]
- Reynolds, C.R.; Bigler, E.D. Test of Memory and Learning; Pro-ed: Austin, TX, USA, 1994. [Google Scholar]
- Gastgeb, H.Z.; Wilkinson, D.A.; Minshew, N.J.; Strauss, M.S. Can individuals with autism abstract prototypes of natural faces? J. Autism Dev. Disord. 2011, 41, 1609–1618. [Google Scholar] [CrossRef]
- Weiner, K.S.; Grill-Spector, K. The improbable simplicity of the fusiform face area. Trends Cog. Sci. 2012, 16, 251–254. [Google Scholar] [CrossRef]
- Hauck, M.; Fein, D.; Maltby, N.; Waterhouse, L.; Feinstein, C. Memory for faces in children with autism. Child. Neuropsychol. 1998, 4, 187–198. [Google Scholar] [CrossRef]
- Williams, D.L.; Goldstein, G.; Minshew, N.J. Impaired memory for faces and social scenes in autism: Clinical implications of memory dysfunction. Arch. Clin. Neuropsychol. 2005, 20, 1–15. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Best, C.A.; Minshew, N.J.; Strauss, M.S. Memory awareness for faces in individuals with autism. J. Autism Dev. Disord. 2010, 40, 1371–1377. [Google Scholar] [CrossRef]
- Adolphs, R.; Spezio, M.L.; Parlier, M.; Piven, J. Distinct face-processing strategies in parents of autistic children. Curr. Biol. 2008, 18, 1090–1093. [Google Scholar] [CrossRef]
- Dawson, G.; Webb, S.J.; McPartland, J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 2005, 27, 403–424. [Google Scholar] [CrossRef]
- Kleinhans, N.M.; Richards, T.; Johnson, L.C.; Weaver, K.E.; Greenson, J.; Dawson, G.; Aylward, E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 2011, 54, 697–704. [Google Scholar] [CrossRef]
- Hobson, R.P. The autistic child’s appraisal of expressions of emotion: A further study. J. Child. Psychol. Psychiatry 1986, 27, 671–680. [Google Scholar] [CrossRef]
- Hobson, R.P.; Ouston, J.; Lee, A. What’s in a face? The case of autism. Br. J. Psychol. 1988, 79, 441–453. [Google Scholar] [CrossRef]
- Bormann-Kischkel, C.; Vilsmeier, M.; Baude, B. The development of emotional concepts in autism. J. Child. Psychol. Psychiatry 1995, 36, 1243–1259. [Google Scholar] [CrossRef] [Green Version]
- Baron-Cohen, S.; Baldwin, D.A.; Crowson, M. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child. Dev. 1997, 68, 48–57. [Google Scholar] [CrossRef]
- Phillips, W.; Baron-Cohen, S.; Rutter, M. The role of eye contact in goal detection: Evidence from normal infants and children with autism or mental handicap. Dev. Psychopathol. 1992, 4, 8. [Google Scholar]
- Hobson, R.P.; Lee, A. Hello and goodbye: A study of social engagement in autism. J. Autism Dev. Disord. 1998, 28, 117–127. [Google Scholar] [CrossRef]
- Dalton, K.M.; Nacewicz, B.M.; Johnstone, T.; Schaefer, H.S.; Gernsbacher, M.A.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005, 8, 519–526. [Google Scholar]
- Klin, A.; Jones, W.; Schultz, R.; Volkmar, F.; Cohen, D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry 2002, 59, 809–816. [Google Scholar] [CrossRef]
- Moore, D.J.; Heavey, L.; Reidy, J. Attentional processing of faces in ASD: A dot-probe study. J. Autism Dev. Disord. 2012, 42, 2038–2045. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Sasson, N.J.; Reznick, J.S.; Paul, G.; Goldman, B.D.; Piven, J. Visual scanning of faces in autism. J. Autism Dev. Disord. 2002, 32, 249–261. [Google Scholar] [CrossRef]
- Snow, J.; Ingeholm, J.E.; Levy, I.F.; Caravella, R.A.; Case, L.K.; Wallace, G.L.; Martin, A. Impaired visual scanning and memory for faces in high-functioning autism spectrum disorders: It’s not just the eyes. J. Int. Neuropsychol. Soc. 2011, 17, 1021–1029. [Google Scholar] [CrossRef]
- Wright, B.; Alderson-Day, B.; Prendergast, G.; Bennett, S.; Jordan, J.; Whitton, C.; Gouws, A.; Jones, N.; Attur, R.; Tomlinson, H.; et al. Gamma activation in young people with autism spectrum disorders and typically-developing controls when viewing emotions on faces. PLoS One 2012, 7, e41326. [Google Scholar] [CrossRef] [Green Version]
- Weeks, S.J.; Hobson, R. The salience of facial expression for autistic children. J. Child. Psychol. Psychiatry 1987, 28, 137–151. [Google Scholar] [CrossRef]
- Tantam, D.; Monaghan, L.; Nicholson, H.; Stirling, J. Autistic children’s ability to interpret faces: A research note. J. Child. Psychol. Psychiatry 1989, 30, 623–630. [Google Scholar] [CrossRef]
- Blair, R.J.; Frith, U.; Smith, N.; Abell, F.; Cipolotti, L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia 2002, 40, 108–118. [Google Scholar]
- Boucher, J.; Lewis, V.; Collis, G. Familiar face and voice matching and recognition in children with autism. J. Child. Psychol. Psychiatry 1998, 39, 171–181. [Google Scholar] [CrossRef]
- Kuusikko-Gauffin, S.; Jansson-Verkasalo, E.; Carter, A.; Pollock-Wurman, R.; Jussila, K.; Mattila, M.; Rahko, J.; Ebeling, H.; Pauls, D.; Moilanen, I. Face memory and object recognition in children with high-functioning autism or asperger syndrome and in their parents. Res. Autism Spectr. Disord. 2011, 5, 622–628. [Google Scholar] [CrossRef]
- Weigelt, S.; Koldewyn, K.; Kanwisher, N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci. Biobehav. Rev. 2012, 36, 1060–1084. [Google Scholar] [CrossRef]
- Hubl, D.; Bolte, S.; Feineis-Matthews, S.; Lanfermann, H.; Federspiel, A.; Strik, W.; Poustka, M.D.; Dierks, T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology 2003, 61, 1232–1237. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Wang, A.T.; Scott, A.; Sigman, M.; Dapretto, M. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. J. Int. Neuropsychol. Soc. 2008, 14, 922–932. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Joseph, R.M.; Snyder, J.; Chabris, C.F.; Clark, J.; Steele, S.; McGrath, L.; Vangel, M.; Aharon, I.; Feczko, E.; et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. NeuroImage 2004, 22, 1141–1150. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar]
- Barton, J.J.; Press, D.Z.; Keenan, J.P.; O’Connor, M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology 2002, 58, 71–78. [Google Scholar] [CrossRef]
- Harris, A.; Aguirre, G.K. Neural tuning for face wholes and parts in human fusiform gyrus revealed by FMRI adaptation. J. Neurophysiol. 2010, 104, 336–345. [Google Scholar] [CrossRef]
- Harris, A.; Aguirre, G.K. The representation of parts and wholes in face-selective cortex. J. Cogn. Neurosci. 2008, 20, 863–878. [Google Scholar] [CrossRef]
- Maurer, D.; O’Craven, K.M.; Le Grand, R.; Mondloch, C.J.; Springer, M.V.; Lewis, T.L.; Grady, C.L. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia 2007, 45, 1438–1451. [Google Scholar] [CrossRef]
- Rhodes, G.; Michie, P.T.; Hughes, M.E.; Byatt, G. The fusiform face area and occipital face area show sensitivity to spatial relations in faces. Eur. J. Neurosci. 2009, 30, 721–733. [Google Scholar] [CrossRef]
- Rotshtein, P.; Geng, J.J.; Driver, J.; Dolan, R.J. Role of features and second-order spatial relations in face discrimination, Face recognition, And individual face skills: Behavioral and functional magnetic resonance imaging data. J. Cogn. Neurosci. 2007, 19, 1435–1452. [Google Scholar] [CrossRef]
- Yovel, G.; Kanwisher, N. Face perception: Domain specific, not process specific. Neuron 2004, 44, 889–898. [Google Scholar]
- Kleinhans, N.M.; Richards, T.; Sterling, L.; Stegbauer, K.C.; Mahurin, R.; Johnson, L.C.; Greenson, J.; Dawson, G.; Aylward, E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 2008, 131, 1000–1012. [Google Scholar] [CrossRef]
- Anderson, J.S.; Druzgal, T.J.; Froehlich, A.; DuBray, M.B.; Lange, N.; Alexander, A.L.; Abildskov, T.; Nielsen, J.A.; Cariello, A.N.; Cooperrider, J.R.; et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 2011, 21, 1134–1146. [Google Scholar] [CrossRef]
- Khan, S.; Gramfort, A.; Shetty, N.R.; Kitzbichler, M.G.; Ganesan, S.; Moran, J.M.; Lee, S.M.; Gabrieli, J.D.; Tager-Flusberg, H.B.; Joseph, R.M.; et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2013, 110, 3107–3112. [Google Scholar] [CrossRef]
- Damasio, A.R.; Damasio, H.; Van Hoesen, G.W. Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology 1982, 32, 331–341. [Google Scholar] [CrossRef]
- Sergent, J.; Signoret, J.L. Varieties of functional deficits in prosopagnosia. Cereb. Cortex 1992, 2, 375–388. [Google Scholar] [CrossRef]
- Uttner, I.; Bliem, H.; Danek, A. Prosopagnosia after unilateral right cerebral infarction. J. Neurol. 2002, 249, 933–935. [Google Scholar] [CrossRef]
- Sato, W.; Toichi, M.; Uono, S.; Kochiyama, T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. Neuroscience 2012, 13, 99. [Google Scholar] [Green Version]
- Pelphrey, K.A.; Shultz, S.; Hudac, C.M.; Vander Wyk, B.C. Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J. Child. Psychol. Psychiatry 2011, 52, 631–644. [Google Scholar] [CrossRef]
- Conturo, T.E.; Williams, D.L.; Smith, C.D.; Gultepe, E.; Akbudak, E.; Minshew, N.J. Neuronal fiber pathway abnormalities in autism: An initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J. Int. Neuropsychol. Soc. 2008, 14, 933–946. [Google Scholar] [CrossRef]
- Critchley, H.; Daly, E.; Phillips, M.; Brammer, M.; Bullmore, E.; Williams, S.; Van Amelsvoort, T.; Robertson, D.; David, A.; Murphy, D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Hum. Brain Mapp. 2000, 9, 93–105. [Google Scholar] [CrossRef]
- Deeley, Q.; Daly, E.M.; Surguladze, S.; Page, L.; Toal, F.; Robertson, D.; Curran, S.; Giampietro, V.; Seal, M.; Brammer, M.J.; et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol. Psychiatry 2007, 62, 207–217. [Google Scholar] [CrossRef]
- Ishitobi, M.; Kosaka, H.; Omori, M.; Matsumura, Y.; Munesue, T.; Mizukami, K.; Shimoyama, T.; Murata, T.; Sadato, N.; Okazawa, H.; et al. Differential amygdala response to lower face in patients with autistic spectrum disorders: An fMRI study. Res. Autism Spectr. Disord. 2011, 5, 910–919. [Google Scholar] [CrossRef]
- Ashwin, C.; Baron-Cohen, S.; Wheelwright, S.; O’Riordan, M.; Bullmore, E.T. Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger Syndrome. Neuropsychologia 2006, 45, 2–14. [Google Scholar]
- Pelphrey, K.A.; Morris, J.P.; McCarthy, G.; LaBar, K.S. Perception of dynamic changes in facial affect and identity in autism. Soc. Cogn. Affect. Neurosci. 2007, 2, 140–149. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Pierce, K.; Muller, R.A.; Ambrose, J.; Allen, G.; Courchesne, E. Face processing occurs outside the fusiform “face area” in autism: Evidence from functional MRI. Brain 2001, 124, 2059–2073. [Google Scholar] [CrossRef]
- Waiter, G.D.; Williams, J.H.; Murray, A.D.; Gilchrist, A.; Perrett, D.I.; Whiten, A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 2004, 22, 619–625. [Google Scholar] [CrossRef]
- Rojas, D.C.; Peterson, E.; Winterrowd, E.; Reite, M.L.; Rogers, S.J.; Tregellas, J.R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 2006, 6, 56. [Google Scholar] [CrossRef]
- Neeley, E.S.; Bigler, E.D.; Krasny, L.; Ozonoff, S.; McMahon, W.; Lainhart, J.E. Quantitative temporal lobe differences: Autism distinguished from controls using classification and regression tree analysis. Brain Dev. 2007, 29, 389–399. [Google Scholar] [CrossRef]
- Toal, F.; Daly, E.M.; Page, L.; Deeley, Q.; Hallahan, B.; Bloemen, O.; Cutter, J.; Brammer, M.J.; Curran, S.; Robertson, D.; et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: A structural MRI study. Psychol. Med. 2010, 40, 1171–1181. [Google Scholar] [CrossRef]
- Dziobek, I.; Bahnemann, M.; Convit, A.; Heekeren, H.R. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch. Gen. Psychiatry 2010, 67, 397–405. [Google Scholar] [CrossRef]
- Wallace, G.L.; Dankner, N.; Kenworthy, L.; Giedd, J.N.; Martin, A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain 2010, 133, 3745–3754. [Google Scholar] [CrossRef]
- Raznahan, A.; Toro, R.; Daly, E.; Robertson, D.; Murphy, C.; Deeley, Q.; Bolton, P.F.; Paus, T.; Murphy, D.G. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb. Cortex 2010, 20, 1332–1340. [Google Scholar] [CrossRef]
- Cauda, F.; Geda, E.; Sacco, K.; D’Agata, F.; Duca, S.; Geminiani, G.; Keller, R. Grey matter abnormality in autism spectrum disorder: An activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1304–1313. [Google Scholar] [CrossRef]
- Murphy, C.M.; Deeley, Q.; Daly, E.M.; Ecker, C.; O’Brien, F.M.; Hallahan, B.; Toal, F.; Reed, S.; Hales, S.; Robertson, D.M.; et al. Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: An in vivo magnetic resonance imaging study of Asperger syndrome. Autism Res. 2012, 5, 3–12. [Google Scholar] [CrossRef]
- Hasan, K.M.; Walimuni, I.S.; Frye, R.E. Global cerebral and regional multimodal neuroimaging markers of the neurobiology of autism: Development and cognition. J. Child. Neurol. 2013, 28, 874–885. [Google Scholar] [CrossRef]
- Aylward, E.H.; Minshew, N.J.; Goldstein, G.; Honeycutt, N.A.; Augustine, A.M.; Yates, K.O.; Barta, P.E.; Pearlson, G.D. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999, 53, 2145–2150. [Google Scholar] [CrossRef]
- Bigler, E.D. Neurobiology and neuropathology underlie the neuropsychological deficits associated with traumatic brain injury. Arch. Clin. Neuropsychol. 2003, 18, 595–621; discussion 623–627. [Google Scholar]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Boudos, R.; DuBray, M.B.; Oakes, T.R.; Miller, J.N.; Lu, J.; Jeong, E.; McMahon, W.M.; et al. Diffusion tensor imaging of the corpus callosum in autism. NeuroImage 2007, 34, 61–73. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; APA: Washington, DC, USA, 1994. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children-III (WISC-III); The Psychological Corporation: San Antonio, TX, USA, 1991. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale-III (WAIS-III); The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence (WASI); The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Elliot, C.D. Differential Ability Scales, 2nd ed.; Harcourt Assessment: San Antonio, TX, USA, 2007. [Google Scholar]
- Dennis, M.; Francis, D.J.; Cirino, P.T.; Schachar, R.; Barnes, M.A.; Fletcher, J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc. 2009, 15, 331–343. [Google Scholar] [CrossRef]
- White, S.; O’Reilly, H.; Frith, U. Big heads, Small details and autism. Neuropsychologia 2009, 47, 1274–1281. [Google Scholar] [CrossRef]
- Lainhart, J.E.; Bigler, E.D.; Bocian, M.; Coon, H.; Dinh, E.; Dawson, G.; Deutsch, C.K.; Dunn, M.; Estes, A.; Tager-Flusberg, H.; et al. Head circumference and height in autism: A study by the collaborative program of excellence in autism. Am. J. Med. Genet. A 2006, 140, 2257–2274. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Bigler, E.D.; Abildskov, T.J.; Wilde, E.A.; McCauley, S.R.; Li, X.; Merkley, T.L.; Fearing, M.A.; Newsome, M.R.; Scheibel, R.S.; Hunter, J.V.; et al. Diffuse damage in pediatric traumatic brain injury: A comparison of automated versus operator-controlled quantification methods. Neuroimage 2010, 50, 1017–1026. [Google Scholar] [CrossRef]
- Hanson, J.L.; Suh, J.W.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Stodola, D.E.; Burghy, C.A.; Wang, H.; Avants, B.B.; Yushkevich, P.A.; et al. Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Front. Neurosci. 2012, 6, 166. [Google Scholar]
- Hau, X.; Berg, A.C.; Oh, H.; Samaras, D.; Leung, H.C. Multi-voxel pattern analysis of selective representation of visual working memory in ventral temporal and occipital regions. NeuroImage 2013, 73, 8–15. [Google Scholar] [CrossRef]
- Robinson-Long, M.; Eslinger, P.J.; Wang, J.; Meadowcroft, M.; Yang, Q.X. Functional MRI evidence for distinctive binding and consolidation pathways for face-name associations: Analysis of activation maps and BOLD response amplitudes. Top. Magn. Reson. Imag. 2009, 20, 271–278. [Google Scholar] [CrossRef]
- Taylor, M.J.; Mills, T.; Pang, E.W. The development of face recognition; hippocampal and frontal lobe contributions with MEG. Brain Topogr. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Atri, A.; O’Brien, J.L.; Sreenivasan, A.; Rastegar, S.; Salisbury, S.; DeLuca, A.N.; O’Keefe, K.M.; LaViolette, P.S.; Rentz, D.M.; Locascio, J.J.; et al. Test-retest reliability of memory task functional magnetic resonance imaging in Alzheimer disease clinical trials. Arch. Neurol. 2011, 68, 599–606. [Google Scholar] [CrossRef]
- Majerus, S.; D’Argembeau, A.; Martinez Perez, T.; Belayachi, S.; Van der Linden, M.; Collette, F.; Salmon, E.; Seurinck, R.; Fias, W.; Maquet, P. The commonality of neural networks for verbal and visual short-term memory. J. Cogn. Neurosci. 2010, 22, 2570–2593. [Google Scholar] [CrossRef]
- Koshino, H.; Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb. Cortex 2008, 18, 289–300. [Google Scholar]
- Brown, C.; Lloyd-Jones, T.J. Verbal facilitation of face recognition. Mem. Cognit. 2005, 33, 1442–1456. [Google Scholar] [CrossRef]
- Van Kooten, I.A.; Palmen, S.J.; von Cappeln, P.; Steinbusch, H.W.; Korr, H.; Heinsen, H.; Hof, P.R.; van Engeland, H.; Schmitz, C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain 2008, 131, 987–999. [Google Scholar] [CrossRef]
- Yamasaki, L. Balancing proliferation and apoptosis in vivo: The Goldilocks theory of E2F/DP action. Biochim. Biophys. Acta 1999, 1423, M9–M15. [Google Scholar]
- Little, A.G. The “Goldilocks” principle. Chest 2005, 128, 13–14. [Google Scholar] [CrossRef]
- Yun, T.J.; Bevan, M.J. The Goldilocks conditions applied to T cell development. Nat. Immunol. 2001, 2, 13–14. [Google Scholar] [CrossRef]
- Koscik, T.R.; Tranel, D. Brain evolution and human neuropsychology: The inferential brain hypothesis. J. Int. Neuropsychol. Soc. 2012, 18, 394–401. [Google Scholar] [CrossRef]
- Guatam, P.; Cherbuin, N.; Sachdev, P.S.; Wen, W.; Anstey, K.J. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: The PATH through life study. NeuroImage 2011, 55, 845–855. [Google Scholar] [CrossRef]
- Bartrés-Faz, D.; Solé-Padullés, C.; Junque, C.; Rami, L.; Bosch, B.; Bargallo, N.; Falcon, C.; Sanchez-Valle, R.; Molinuevo, J.L. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol. Psychol. 2009, 80, 256–259. [Google Scholar] [CrossRef]
- Solé-Padullés, C.; Bartrés-Faz, D.; Junque, C.; Vendrell, P.; Rami, L.; Clemente, I.C.; Bosch, B.; Villar, A.; Bargallo, N.; Jurado, M.A.; et al. Brain structure and function related to cognitive reserve variables in normal aging, Mild cognitive impairment and Alzheimer’s disease. Neurobiol. Ag. 2009, 30, 1114–1124. [Google Scholar] [CrossRef]
- Stern, Y.; Habeck, C.; Moeller, J.; Scarmeas, N.; Anderson, K.E.; Hilton, H.J.; Flynn, J.; Sackeim, H.; van Heertum, R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 2005, 15, 394–402. [Google Scholar] [CrossRef]
- Baxter, L.C.; Sparks, D.L.; Johnson, S.C.; Lenoski, B.; Lopez, J.E.; Connor, D.J.; Sabbagh, M.N. Relationship of cognitive measures and gray and white matter in Alzheimer’s disease. J. Alzheimers. Dis. 2006, 9, 253–260. [Google Scholar]
- Braak, E.; Braak, H. Alzheimer’s disease: Transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol. 1997, 93, 323–325. [Google Scholar] [CrossRef]
- Giedd, J.N.; Rapoport, J.L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron 2010, 67, 728–734. [Google Scholar] [CrossRef]
- Thomas, M.S.; Knowland, V.C.; Karmiloff-Smith, A. Mechanisms of developmental regression in autism and the broader phenotype: A neural network modeling approach. Psychol. Rev. 2011, 118, 637054. [Google Scholar]
- Hua, X.; Thompson, P.M.; Leow, A.D.; Madsen, S.K.; Caplan, R.; Alger, J.R.; O’Neill, J.; Joshi, K.; Smalley, S.L.; Toga, A.W.; et al. Brain growth rate abnormalities visualized in adolscents with autism. Brain Mapp. 2013, 34, 425–436. [Google Scholar] [CrossRef]
- Nordahl, C.W.; Lange, N.; Li, D.D.; Barnett, L.A.; Lee, A.; Buonocore, M.H.; Simon, T.J.; Rogers, S.; Ozonoff, S.; Amaral, D.G. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2011, 108, 20195–20200. [Google Scholar] [CrossRef]
- Mainy, N.; Kahane, P.; Minotti, L.; Hoffmann, D.; Bertrand, O.; Lachaux, J.P. Neural correlates of consolidation in working memory. Hum. Brain Mapp. 2007, 28, 183–193. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, T.; Yu, C.; Tian, L.; Li, J.; Liu, Y.; Zhou, Y.; Xu, L.; Song, M.; Li, K. Spontaneous activity associated with primary visual cortex: A resting-state fMRI study. Cereb. Cortex 2008, 18, 697–704. [Google Scholar]
- Van Dongen, E.V.; Takashima, A.; Barth, M.; Fernandez, G. Functional connectivity during light sleep is correlated with memory performance for face-location associations. Neuroimage 2011, 57, 8. [Google Scholar]
- Courchesne, E.; Pierce, K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005, 23, 153–170. [Google Scholar] [CrossRef]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. JAMA 2003, 290, 337–344. [Google Scholar] [CrossRef]
- Polsek, D.; Jagatic, T.; Cepanec, M.; Hof, P.R.; Simic, G. Recent Developments in neuropathology of autism spectrum disorders. Transl. Neurosci. 2011, 2, 256–264. [Google Scholar] [CrossRef]
- Lewis, J.D.; Theilmann, R.J.; Fonov, V.; Bellec, P.; Lincoln, A.; Evans, A.C.; Townsend, J. Callosal fiber length and interhemispheric connectivity in adults with autism: Brain overgrowth and underconnectivity. Hum. Brain Mapp. 2012. [Google Scholar] [CrossRef]
- Hazlett, H.C.; Poe, M.D.; Gerig, G.; Styner, M.; Chappell, C.; Smith, R.G.; Vachet, C.; Piven, J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 2011, 68, 467–476. [Google Scholar] [CrossRef]
- Boersma, M.; Kemner, C.; de Reus, M.A.; Collin, G.; Snijders, T.M.; Hofman, D.; Buitelaar, J.K.; Stam, C.J.; van den Heuvel, M.P. Disrupted functional brain networks in autistic toddlers. Brain Connect. 2013, 3, 41–49. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Allen, G.; Beckel-Mitchener, A.; Boulanger, L.M.; Carper, R.A.; Webb, S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004, 24, 9228–9231. [Google Scholar] [CrossRef]
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821. [Google Scholar] [CrossRef]
- Minshew, N.J. Brief report: Brain mechanisms in autism: Functional and structural abnormalities. J. Autism Dev. Disord. 1996, 26, 205–209. [Google Scholar] [CrossRef]
- Minshew, N.J.; Williams, D.L. The new neurobiology of autism: Cortex, Connectivity, And neuronal organization. Arch. Neurol. 2007, 64, 945–950. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.; Gomez, J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Neuroscientist 2003, 9, 496–507. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.P.; Brown, C. Clinical and macroscopic correlates of minicolumnar pathology in autism. J. Child. Neurol. 2002, 17, 692–695. [Google Scholar] [CrossRef]
- Casanova, M.F.; van Kooten, I.A.; Switala, A.E.; van Engeland, H.; Heinsen, H.; Steinbusch, H.W.; Hof, P.R.; Trippe, J.; Stone, J.; Schmitz, C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006, 112, 287–303. [Google Scholar] [CrossRef]
- Tehrani-Doost, M.; Salmanian, M.; Ghanbari-Motlagh, M.; Shahrivar, Z. Delayed face recognition in children and adolescents with autism spectrum disorders. Iran. J. Psychiatry 2012, 7, 52–56. [Google Scholar]
- Frith, C. What do imaging studies tell us about the neural basis of autism? Novartis Found. Symp. 2003, 251, 149–197. [Google Scholar] [CrossRef]
- Grossman, J.B.; Klin, A.; Carter, A.S.; Volkmar, F.R. Verbal bias in recognition of facial emotions in children with Asperger syndrome. J. Child. Psychol. Psyc. 2003, 41, 369–379. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trontel, H.G.; Duffield, T.C.; Bigler, E.D.; Froehlich, A.; Prigge, M.B.D.; Nielsen, J.A.; Cooperrider, J.R.; Cariello, A.N.; Travers, B.G.; Anderson, J.S.; et al. Fusiform Correlates of Facial Memory in Autism. Behav. Sci. 2013, 3, 348-371. https://doi.org/10.3390/bs3030348
Trontel HG, Duffield TC, Bigler ED, Froehlich A, Prigge MBD, Nielsen JA, Cooperrider JR, Cariello AN, Travers BG, Anderson JS, et al. Fusiform Correlates of Facial Memory in Autism. Behavioral Sciences. 2013; 3(3):348-371. https://doi.org/10.3390/bs3030348
Chicago/Turabian StyleTrontel, Haley G., Tyler C. Duffield, Erin D. Bigler, Alyson Froehlich, Molly B.D. Prigge, Jared A. Nielsen, Jason R. Cooperrider, Annahir N. Cariello, Brittany G. Travers, Jeffrey S. Anderson, and et al. 2013. "Fusiform Correlates of Facial Memory in Autism" Behavioral Sciences 3, no. 3: 348-371. https://doi.org/10.3390/bs3030348
APA StyleTrontel, H. G., Duffield, T. C., Bigler, E. D., Froehlich, A., Prigge, M. B. D., Nielsen, J. A., Cooperrider, J. R., Cariello, A. N., Travers, B. G., Anderson, J. S., Zielinski, B. A., Alexander, A., Lange, N., & Lainhart, J. E. (2013). Fusiform Correlates of Facial Memory in Autism. Behavioral Sciences, 3(3), 348-371. https://doi.org/10.3390/bs3030348




