Differential Modulation of Attention by Aversive Associative and Statistical Learning in Distinct Visual Search Modes
Abstract
1. Introduction
2. Experiment 1
2.1. Method
2.1.1. Participants
2.1.2. Apparatus
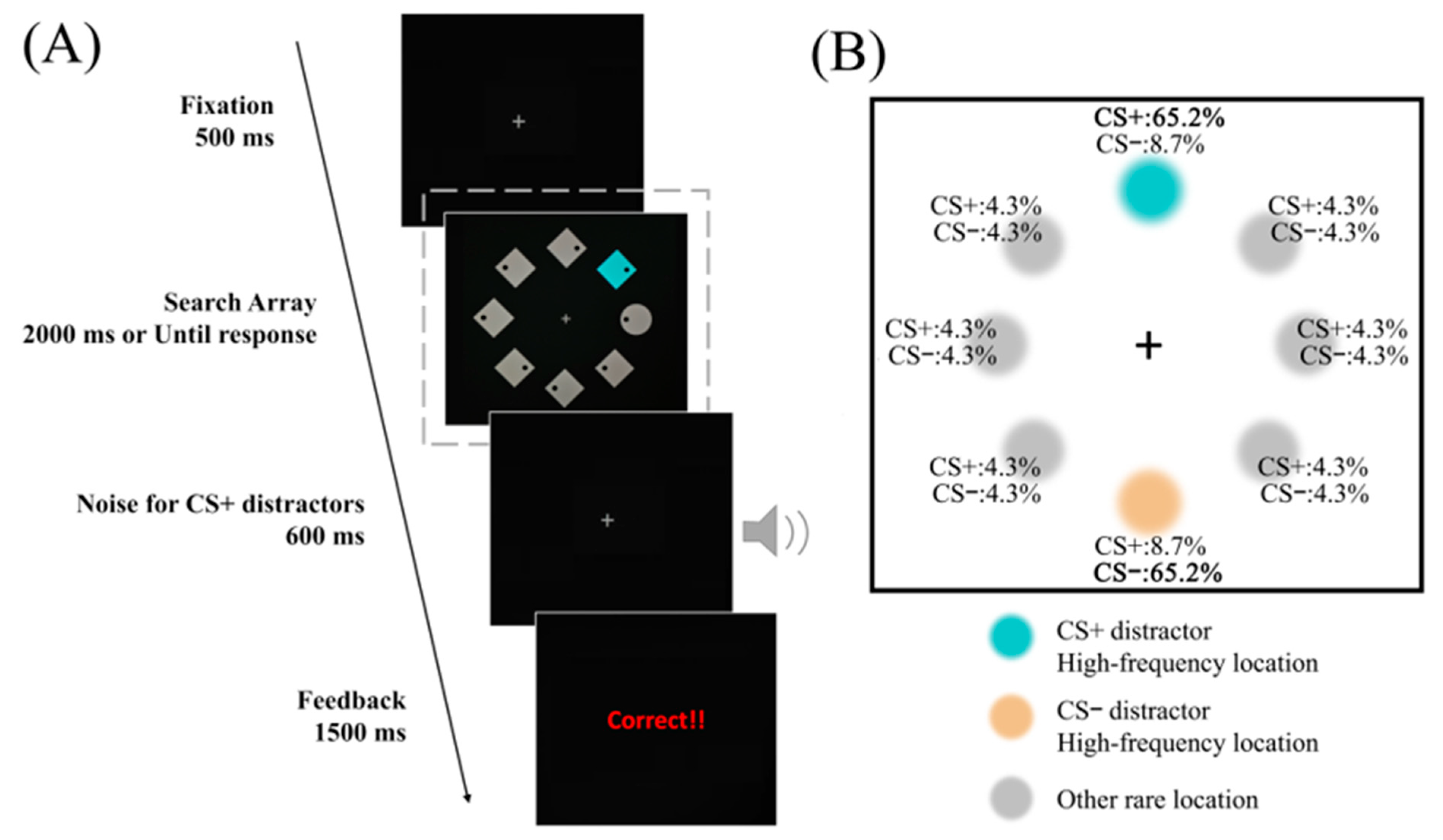
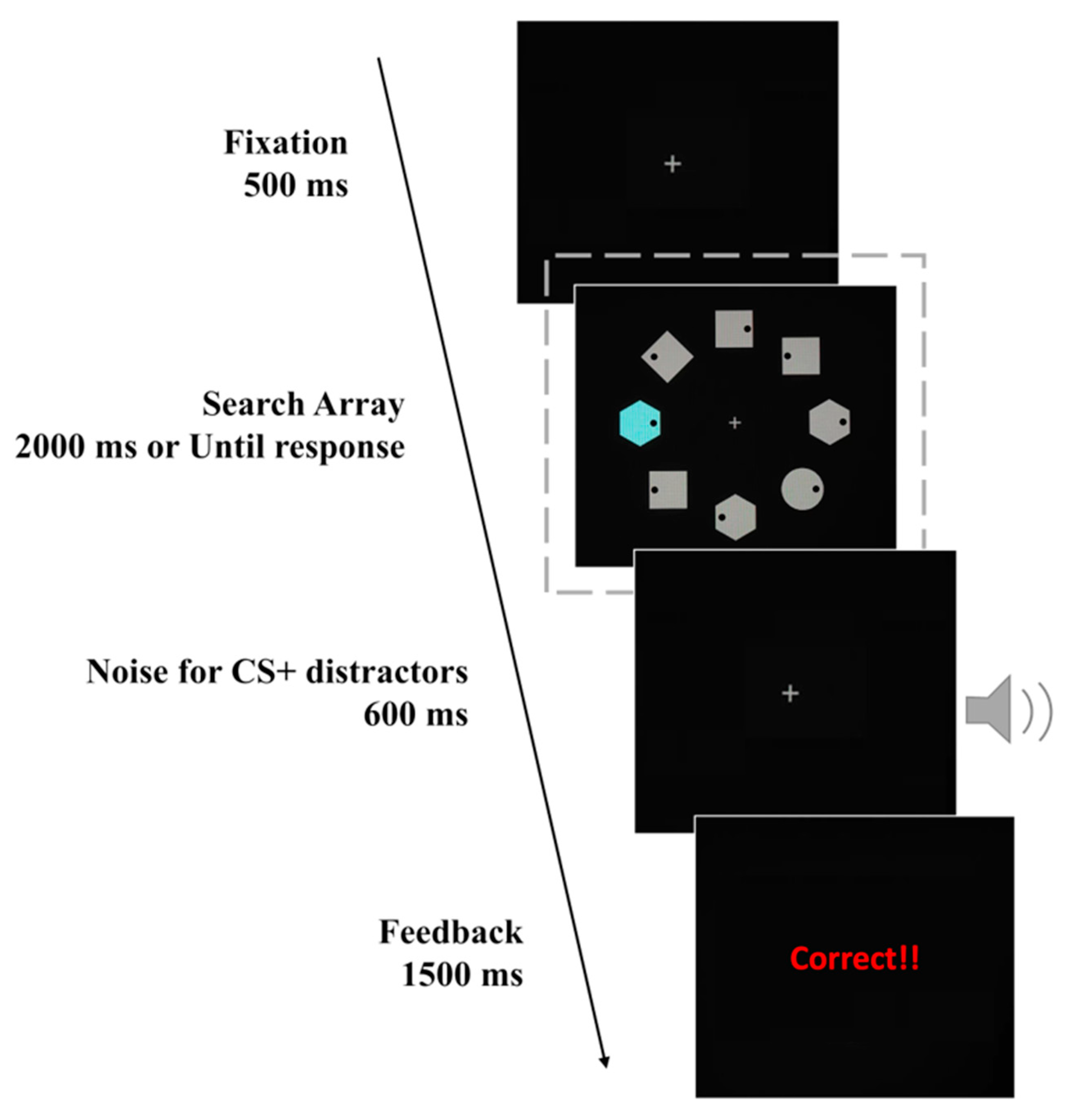
2.1.3. Stimuli and Procedure
2.1.4. Post-Experiment Measures
2.1.5. Data Analysis
2.2. Results
2.2.1. Manipulation Checks
2.2.2. Target Detection in Distractor—Present Conditions
2.2.3. Target Location Effects When Distractors Are Absent
2.2.4. Explicit Recognition of Statistical Regularities
2.3. Discussion
3. Experiment 2
3.1. Method
3.1.1. Participants
3.1.2. Stimuli and Procedure
3.2. Results
3.2.1. Manipulation Checks
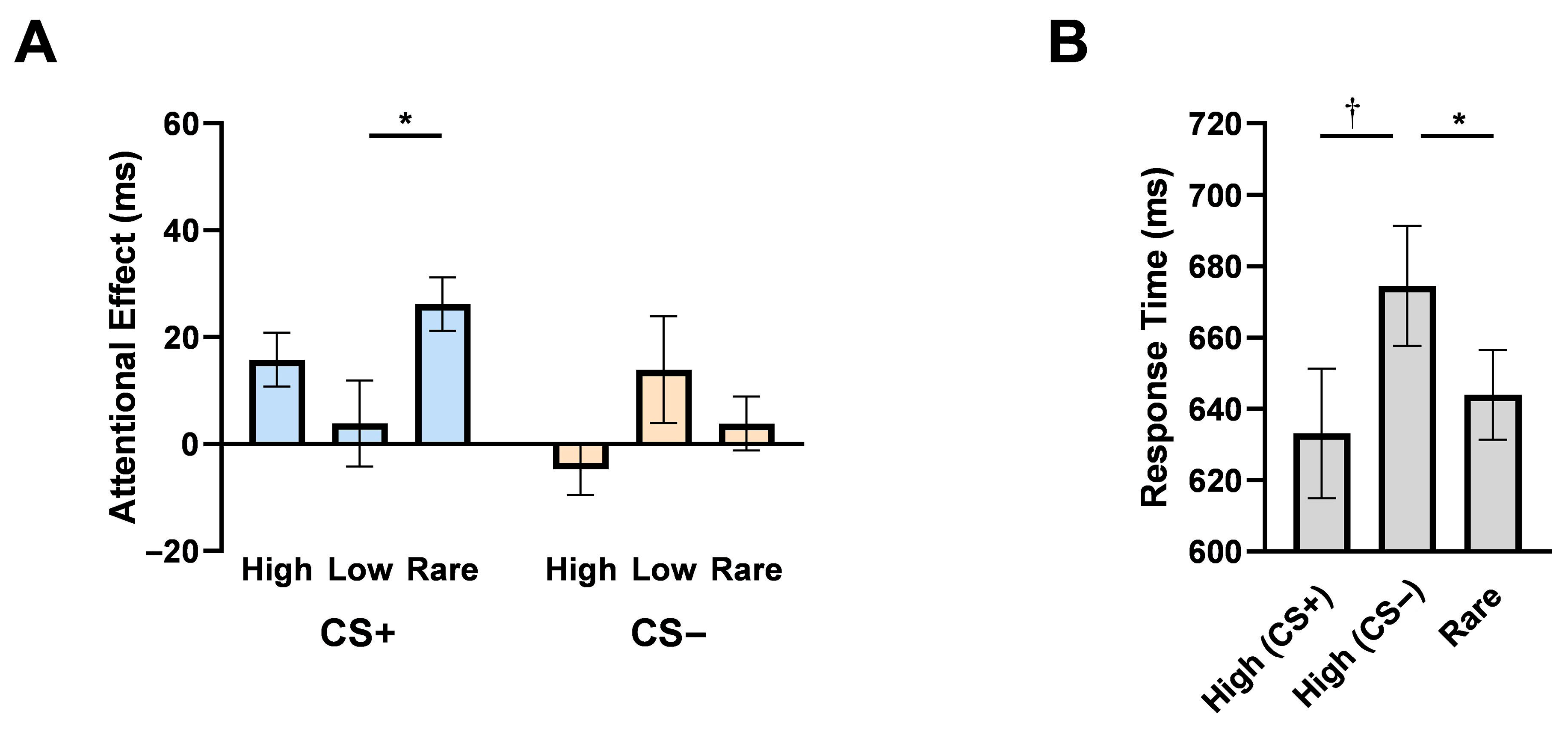
3.2.2. Target Detection in Distractor—Present Conditions
3.2.3. Target Location Effects When Distractors Are Absent
3.2.4. Explicit Recognition of Statistical Regularities
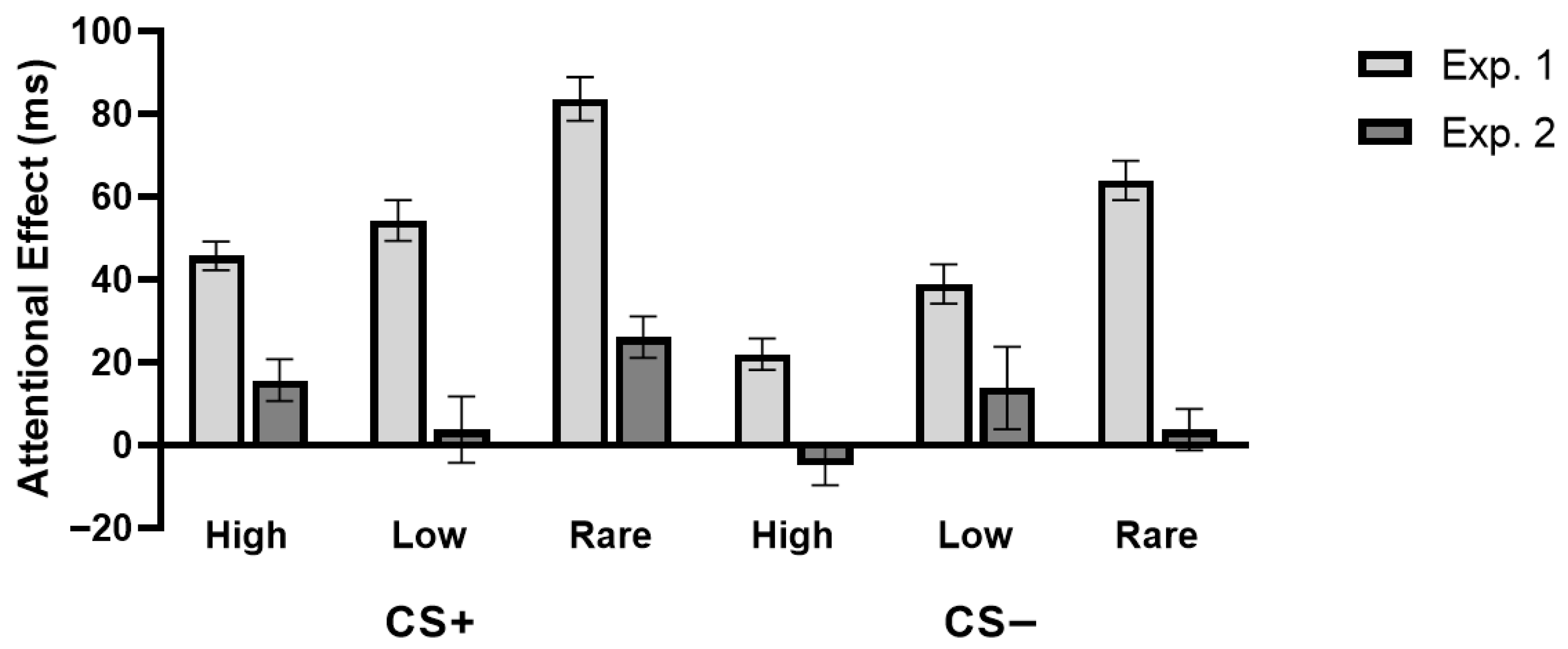
3.2.5. Comparison Between the Attentional Effects in Experiments 1 and 2
3.3. Discussion
4. General Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRT | Cathode-Ray Tube |
| RGB | Red, Green, Blue |
| ANOVA | Analysis of Variance |
| CS+ | Conditioned Stimulus, a stimulus presented with an aversive noise |
| CS− | The stimulus was presented without any aversive noise |
| Exp. 1 | Experiment 1 |
| Exp. 2 | Experiment 2 |
References
- Anderson, B. A., Kim, H., Kim, A. J., Liao, M. R., Mrkonja, L., Clement, A., & Grégoire, L. (2021). The past, present, and future of selection history. Neuroscience & Biobehavioral Reviews, 130, 326–350. [Google Scholar] [CrossRef]
- Anderson, B. A., Liao, M. R., & Grégoire, L. (2022). Pavlovian learning in the selection history-dependent control of overt spatial attention. Journal of Experimental Psychology: Human Perception and Performance, 48(8), 783–789. [Google Scholar] [CrossRef]
- Awh, E., Belopolsky, A. V., & Theeuwes, J. (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. [Google Scholar] [CrossRef]
- Bacon, W. F., & Egeth, H. E. (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55(5), 485–496. [Google Scholar] [CrossRef]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [Google Scholar] [CrossRef]
- Brosch, T., Sander, D., Pourtois, G., & Scherer, K. R. (2008). Beyond fear: Rapid spatial orienting toward positive emotional stimuli. Psychological Science, 19(4), 362–370. [Google Scholar] [CrossRef]
- Carrasco, M. (2011). Visual attention: The past 25 years. Vision Research, 51(13), 1484–1525. [Google Scholar] [CrossRef]
- Chelazzi, L., Eštočinová, J., Calletti, R., Lo Gerfo, E., Sani, I., Della Libera, C., & Santandrea, E. (2014). Altering spatial priority maps via reward-based learning. Journal of Neuroscience, 34(25), 8594–8604. [Google Scholar] [CrossRef]
- Chelazzi, L., Marini, F., Pascucci, D., & Turatto, M. (2019). Getting rid of visual distractors: The why, when, how, and where. Current Opinion in Psychology, 29, 135–147. [Google Scholar] [CrossRef]
- Chun, M. M., & Jiang, Y. (1998). Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology, 36(1), 28–71. [Google Scholar] [CrossRef]
- Chun, M. M., & Phelps, E. A. (1999). Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience, 2, 844–847. [Google Scholar] [CrossRef]
- Cohen, A., & Magen, H. (1999). Intra- and cross-dimensional visual search for single-feature targets. Perception and Psychophysics, 61(2), 291–307. [Google Scholar] [CrossRef][Green Version]
- Durran, S. J., Cairney, S. A., & Lewis, P. A. (2013). Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cerebral Cortex, 23(10), 2467–2478. [Google Scholar] [CrossRef]
- Failing, M., & Theeuwes, J. (2018). Selection history: How reward modulates selectivity of visual attention. Psychonomic Bulletin & Review, 25(2), 514–538. [Google Scholar] [CrossRef]
- Fan, Z., Aijun, W., & Ming, Z. (2021). The influence of feature-based statistical regularity of singletons on the attentional suppression effect. Acta Psychologica Sinica, 53(6), 555–564. [Google Scholar] [CrossRef]
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [Google Scholar] [CrossRef]
- Ferrante, O., Patacca, A., Di Caro, V., Della Libera, C., Santandrea, E., & Chelazzi, L. (2018). Altering spatial priority maps via statistical learning of target selection and distractor filtering. Cortex, 102, 67–95. [Google Scholar] [CrossRef]
- Gao, Y., & Theeuwes, J. (2022). Learning to suppress a location does not depend on knowing which location. Attention, Perception, & Psychophysics, 84(4), 1087–1097. [Google Scholar] [CrossRef]
- Gaspelin, N., Leonard, C. J., & Luck, S. J. (2015). Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychological Science, 26(11), 1740–1750. [Google Scholar] [CrossRef]
- Gaspelin, N., & Luck, S. J. (2018). The role of inhibition in avoiding distraction by salient stimuli. Trends in Cognitive Science, 22(1), 79–92. [Google Scholar] [CrossRef]
- Gdalyahu, A., Tring, E., Polack, P. O., Gruver, R., Golshani, P., Fanselow, M. S., Silva, A. J., & Trachtenberg, J. T. (2012). Associative fear learning enhances sparse network coding in primary sensory cortex. Neuron, 75, 121–132. [Google Scholar] [CrossRef]
- Geng, J. J., & Behrmann, M. (2005). Spatial probability as an attentional cue in visual search. Percept Psychophys, 67(7), 1252–1268. [Google Scholar] [CrossRef]
- Gerchen, F. M., & Kirsch, P. (2017). Combining task-related activation and connectivity analysis of fMRI data reveals complex modulation of brain networks. Human Brain Mapping, 38, 5726–5739. [Google Scholar] [CrossRef]
- Gutierrez, M., & Berggren, N. (2019). Anticipation of aversive threat potentiates task-irrelevant attentional capture. Cognition and Emotion, 34(5), 1036–1043. [Google Scholar] [CrossRef]
- Huang, C., Donk, M., & Theeuwes, J. (2022). Proactive enhancement and suppression elicited by statistical regularities in visual search. Journal of Experimental Psychology: Human Perception and Performance, 48, 443–457. [Google Scholar] [CrossRef]
- Huang, C., Vilotijevic, A., Theeuwes, J., & Donk, M. (2021). Proactive distractor suppression elicited by statistical regularities in visual search. Psychonomic Bulletin & Review, 28, 918–927. [Google Scholar] [CrossRef]
- Huang, L., & Pashler, H. (2005). Attention capacity and task difficulty in visual search. Cognition, 94(3), B101–B111. [Google Scholar] [CrossRef]
- Ivanov, Y., & Theeuwes, J. (2021). Distractor suppression leads to reduced flanker interference. Attention, Perception, & Psychophysics, 83(2), 624–636. [Google Scholar] [CrossRef]
- Jiang, Y. V. (2018). Habitual versus goal-driven attention. Cortex, 102, 107–120. [Google Scholar] [CrossRef]
- Kass, R. E., & Raftery, A. (1995). Bayes factors. Journal of the American Statistical Association, 90, 773–795. [Google Scholar] [CrossRef]
- Kerzel, D., Balbiani, C., Rosa, S., & Huynh Cong, S. (2022). Statistical learning in visual search reflects distractor rarity, not only attentional suppression. Psychonomic Bulletin & Review, 29(5), 1890–1897. [Google Scholar] [CrossRef]
- Kerzel, D., & Huynh Cong, S. (2024). Search mode, not the attentional window, determines the magnitude of attentional capture. Attention, Perception, & Psychophysics, 86(2), 457–470. [Google Scholar] [CrossRef]
- Kim, H., & Anderson, B. A. (2019). Dissociable components of experience-driven attention. Current Biology, 29(5), 841–845.e842. [Google Scholar] [CrossRef]
- Kim, H., & Anderson, B. A. (2020). How does the attention system learn from aversive outcomes? Emotion, 21(4), 898–903. [Google Scholar] [CrossRef]
- Kim, H., & Anderson, B. A. (2021). Combined influence of valence and statistical learning on the control of attention: Evidence for independent sources of bias. Cognition, 208, 104554. [Google Scholar] [CrossRef]
- Kong, S., Li, X., Wang, B., & Theeuwes, J. (2020). Proactively location-based suppression elicited by statistical learning. PLoS ONE, 15(6), e0233544. [Google Scholar] [CrossRef]
- Leber, A. B., & Egeth, H. E. (2006). Attention on autopilot: Past experience and attentional set. Visual Cognition, 14(4–8), 565–583. [Google Scholar] [CrossRef]
- LeDoux, J. E. (1996). The emotional brain: The mysterious underpinnings of emotional life. Simon & Schuster. Available online: https://psycnet.apa.org/record/1996-98824-000 (accessed on 2 June 2025).
- LeDoux, J. E. (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 2871–2878. [Google Scholar] [CrossRef]
- Le Pelley, M. E., Ung, R., Mine, C., Most, S. B., Watson, P., Pearson, D., & Theeuwes, J. (2022). Reward learning and statistical learning independently influence attentional priority of salient distractors in visual search. Attention, Perception, & Psychophysics, 84(5), 1446–1459. [Google Scholar] [CrossRef]
- Lim, S. L., Padmala, S., & Pessoa, L. (2009). Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proceedings of the National Academy of Sciences of the United States of America, 106, 16841–16846. [Google Scholar] [CrossRef]
- Luck, S. J., Gaspelin, N., Folk, C. L., Remington, R. W., & Theeuwes, J. (2021). Progress toward resolving the attentional capture debate. Visual Cognition, 29(1), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ma, X., & Abrams, R. A. (2023). Ignoring the unknown: Attentional suppression of unpredictable visual distraction. Journal of Experimental Psychology: Human Perception and Performance, 49(1), 1–6. [Google Scholar] [CrossRef]
- Masullo, M., Maffei, L., Iachini, T., Rapuano, M., Cioffi, F., Ruggiero, G., & Ruotolo, F. (2021). A questionnaire investigating the emotional salience of sounds. Applied Acoustics, 182, 108281. [Google Scholar] [CrossRef]
- McNab, F., Leroux, G., Strand, F., Thorell, L., Bergman, S., & Klingberg, T. (2008). Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia, 46(11), 2668–2682. [Google Scholar] [CrossRef]
- Minamoto, T., Osaka, M., & Osaka, N. (2010). Individual differences in working memory capacity and distractor processing: Possible contribution of top-down inhibitory control. Brain Research, 1335, 63–73. [Google Scholar] [CrossRef]
- Nickel, A. E., Hopkins, L. S., Minor, G. N., & Hannula, D. E. (2020). Attention capture by episodic long-term memory. Cognition, 201, 104312. [Google Scholar] [CrossRef]
- Nissens, T., Failing, M., & Theeuwes, J. (2016). People look at the object they fear: Oculomotor capture by stimuli that signal threat. Cognition and Emotion, 31(8), 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Ogden, A., Kim, H., & Anderson, B. A. (2023). Combined influence of valence and statistical learning on the control of attention II: Evidence from within-domain additivity. Attention, Perception, & Psychophysics, 85(2), 277–283. [Google Scholar] [CrossRef]
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [Google Scholar] [CrossRef]
- Rusz, D., Le Pelley, M. E., Kompier, M. A. J., Mait, L., & Bijleveld, E. (2020). Reward-driven distraction: A meta-analysis. Psychological Bulletin, 146, 872–899. [Google Scholar] [CrossRef]
- Schmidt, L. J., Belopolsky, A. V., & Theeuwes, J. (2015). Attentional capture by signals of threat. Cognition and Emotion, 29(4), 687–694. [Google Scholar] [CrossRef]
- Smith, S. D., Most, S. B., Newsome, L. A., & Zald, D. H. (2006). An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion, 6(3), 523–527. [Google Scholar] [CrossRef]
- Sokol-Hessner, P., Camerer, C. F., & Phelps, E. A. (2013). Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Social Cognitive and Affective Neuroscience, 8(2), 341–350. [Google Scholar] [CrossRef]
- Stankevich, B. A., & Geng, J. J. (2014). Reward associations and spatial probabilities produce additive effects on attentional selection. Attention, Perception, & Psychophysics, 76, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Stilwell, B. T., Egeth, H. E., & Gaspelin, N. (2024). Evidence against the low-salience account of attentional suppression. Journal of Experimental Psychology: Human Perception and Performance, 50(10), 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, J. (1992). Perceptual selectivity for colour and form. Perception & Psychophysics, 51, 599–606. [Google Scholar] [CrossRef]
- Theeuwes, J. (2019). Goal-driven, stimulus-driven, and history-driven selection. Current Opinion in Psychology, 29, 97–101. [Google Scholar] [CrossRef]
- Theeuwes, J. (2023). The attentional capture debate: When can we avoid salient distractors and when not? Journal of Cognition, 6(1), 35. [Google Scholar] [CrossRef]
- Theeuwes, J. (2025). Attentional capture and control. Annual Review of Psychology, 76(1), 251–273. [Google Scholar] [CrossRef]
- Theeuwes, J., Bogaert, L., & van Moorselaar, D. (2022). What to expect where and when: How statistical learning drives visual selection. Trends in Cognitive Sciences, 26(10), 860–872. [Google Scholar] [CrossRef]
- Turk-Browne, N. B., Jungé, J. A., & Scholl, B. J. (2005). The automaticity of visual statistical learning. Journal of Experimental Psychology: General, 134(4), 552–564. [Google Scholar] [CrossRef]
- Vadillo, M. A., Konstantinidis, E., & Shanks, D. R. (2016). Underpowered samples, false negatives, and unconscious learning. Psychonomic Bulletin & Review, 23, 87–102. [Google Scholar] [CrossRef]
- Vicente-Conesa, F., Giménez-Fernández, T., Luque, D., & Vadillo, M. A. (2023). Learning to suppress a distractor may not be unconscious. Attention, Perception & Psychophysics, 85, 796–813. [Google Scholar] [CrossRef]
- Wang, B., & Theeuwes, J. (2018). Statistical regularities modulate attentional capture independent of search strategy. Attention, Perception, & Psychophysics, 80, 1763–1774. [Google Scholar] [CrossRef]
- Wang, B., van Driel, J., Ort, E., & Theeuwes, J. (2019). Anticipatory distractor suppression elicited by statistical regularities in visual search. Journal of Cognitive Neuroscience, 31(10), 1535–1548. [Google Scholar] [CrossRef]
- Wang, Y., Luo, L., Chen, G., Luan, G., Wang, X., Wang, Q., & Fang, F. (2023). Rapid processing of invisible fearful faces in the human amygdala. Journal of Neuroscience, 43(8), 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Watson, P., Pearson, D., Wiers, R. W., & Le Pelley, M. E. (2019). Prioritizing pleasure and pain: Attentional capture by reward-related and punishment-related stimuli. Current Opinion in Behavioral Sciences, 26, 107–113. [Google Scholar] [CrossRef]
- Won, B.-Y., Kosoyan, M., & Geng, J. J. (2019). Evidence for second-order singleton suppression based on probabilistic expectations. Journal of Experimental Psychology: Human Perception and Performance, 45(1), 125–138. [Google Scholar] [CrossRef]
- Yang, W., Makita, K., Nakao, T., Kanayama, N., Machizawa, M. G., Sasaoka, T., Sugata, A., Kobayashi, R., Hiramoto, R., Yamawaki, S., Iwanaga, M., & Miyatani, M. (2018). Affective auditory stimulus database: An expanded version of the international affective digitized sounds (IADS-E). Behavior Research Methods, 50(4), 1415–1429. [Google Scholar] [CrossRef]
- Zhao, G., Wu, R., Wang, H., Chen, J., Li, S., Wang, Q., & Sun, H. J. (2024). Reward history and statistical learning independently impact attention search: An ERP study. Brian Science, 14(9), 874. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Guo, J.; Huang, C.; Wang, Y. Differential Modulation of Attention by Aversive Associative and Statistical Learning in Distinct Visual Search Modes. Behav. Sci. 2025, 15, 1274. https://doi.org/10.3390/bs15091274
Chen Y, Guo J, Huang C, Wang Y. Differential Modulation of Attention by Aversive Associative and Statistical Learning in Distinct Visual Search Modes. Behavioral Sciences. 2025; 15(9):1274. https://doi.org/10.3390/bs15091274
Chicago/Turabian StyleChen, Yue, Junzhen Guo, Chen Huang, and Yingying Wang. 2025. "Differential Modulation of Attention by Aversive Associative and Statistical Learning in Distinct Visual Search Modes" Behavioral Sciences 15, no. 9: 1274. https://doi.org/10.3390/bs15091274
APA StyleChen, Y., Guo, J., Huang, C., & Wang, Y. (2025). Differential Modulation of Attention by Aversive Associative and Statistical Learning in Distinct Visual Search Modes. Behavioral Sciences, 15(9), 1274. https://doi.org/10.3390/bs15091274





