It Is Movement All the Way Down: Broken Rhythms and Embodied Selfhood in Depersonalization
Abstract
1. Introduction
2. Living on Rhythms: Coupling Bodily Movements and Bodily Actions
3. Sensing and Attenuating the Self Through Movement
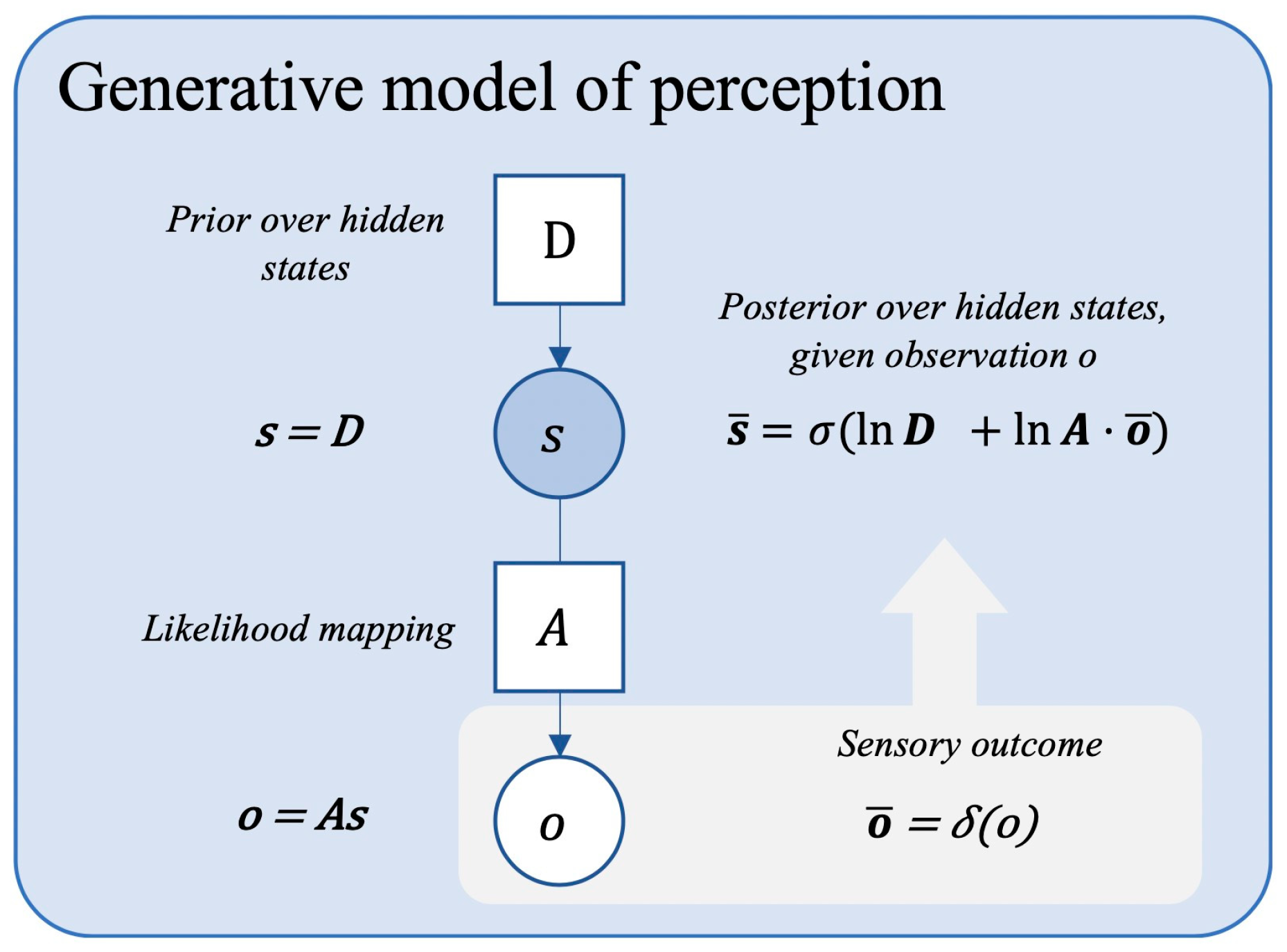
4. Perceiving the World Through Bodily Rhythms
5. When the Self Becomes Stuck: Altered Somatosensory Attenuation in Depersonalization
6. Broken Rhythms in the Self: Implications for Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | We would like to thank the Reviewer for pressing clarification on this point and providing useful feedback on how to better phrase the argument. |
| 2 | We would like to thank one Anonymous Reviewer for pressing clarification on this key point. |
References
- Adams, R. A., Stephan, K. E., Brown, H. R., Frith, C. D., & Friston, K. J. (2013). The computational anatomy of psychosis. Frontiers in Psychiatry, 4, 47. [Google Scholar] [CrossRef]
- Adler, J., Schabinger, N., Michal, M., Beutel, M. E., & Gillmeister, H. (2016). Is that me in the mirror? Depersonalisation modulates tactile mirroring mechanisms. Neuropsychologia, 85, 148–158. [Google Scholar] [CrossRef]
- Ainley, V., Apps, M. A. J., Fotopoulou, A., & Tsakiris, M. (2016). “Bodily precision”: A predictive coding account of individual differences in interoceptive accuracy. Philosophical Transactions of the Royal Society B, 371(1708), 20160003. [Google Scholar] [CrossRef]
- Ainley, V., Tajadura-Jiménez, A., Fotopoulou, A., & Tsakiris, M. (2012). Looking into myself: Changes in interoceptive sensitivity during mirror self-observation. Psychophysiology, 49(11), 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Al, E., Iliopoulos, F., Forschack, N., Nierhaus, T., Grund, M., Motyka, P., Gaebler, M., Nikulin, V. V., & Villringer, A. (2020). Heart–brain interactions shape somatosensory perception and evoked potentials. Proceedings of the National Academy of Sciences of the United States of America, 117(19), 10575–10584. [Google Scholar] [CrossRef] [PubMed]
- Allen, M., Levy, A., Parr, T., & Friston, K. J. (2022). In the body’s eye: The computational anatomy of interoceptive inference. PLoS Computational Biology, 18, e1010490. [Google Scholar] [CrossRef]
- Apps, M. A. J., & Tsakiris, M. (2014). The free-energy self: A predictive-coding account of self-recognition. Neuroscience & Biobehavioral Reviews, 41, 85–97. [Google Scholar] [CrossRef]
- Asimakidou, E., Job, X., & Kilteni, K. (2022). The positive dimension of schizotypy is associated with a reduced attenuation and precision of self-generated touch. Schizophr, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Azañón, E., Tame, L., Maravita, A., Linkenauger, S. A., Ferrè, E. R., Tajadura-Jiménez, A., & Longo, M. R. (2016). Multimodal contributions to body representation. Multisensory Research, 29(6–7), 635–661. [Google Scholar] [CrossRef]
- Azevedo, R. T., Garfinkel, S. N., Critchley, H. D., & Tsakiris, M. (2017). Cardiac afferent activity modulates the expression of racial stereotypes. Nature Communications, 8, 13854. [Google Scholar] [CrossRef]
- Azzalini, D., Rebollo, I., & Tallon-Baudry, C. (2019). Visceral signals shape brain dynamics and cognition. Trends in Cognitive Sciences, 23, 488–509. [Google Scholar] [CrossRef]
- Bastos, A. M., Lundqvist, M., Waite, A. S., Kopell, N., & Miller, E. K. (2020). Layer and rhythm specificity for predictive routing. Proceedings of the National Academy of Sciences of the United States of America, 117(49), 31459–31469. [Google Scholar] [CrossRef]
- Bays, P. M., Wolpert, D. M., & Flanagan, J. R. (2005). Perception of the consequences of self-action is temporally tuned and event-driven. Current Biology, 15, 1125–1128. [Google Scholar] [CrossRef]
- Bennett, M., Welsh, S., & Ciaunica, A. (2024). Why is anything conscious? arXiv, arXiv:2409.14545. [Google Scholar] [CrossRef]
- Betka, S., Canzoneri, E., Adler, D., Herbelin, B., Bello-Ruiz, J., Kannape, O. A., Similowski, T., & Blanke, O. (2020). Mechanisms of the breathing contribution to bodily self-consciousness in healthy humans: Lessons from machine-assisted breathing? Psychophysiology, 57(8), e13564. [Google Scholar] [CrossRef]
- Blakemore, S., Wolpert, D. M., & Frith, C. (1998). Central cancellation of self-produced tickle sensation. Nature Neuroscience, 1, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M., & Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature, 391(6669), 756. [Google Scholar] [CrossRef] [PubMed]
- Brianza, G., Tajadura-Jiménez, A., Maggioni, E., Pittera, D., Bianchi-Berthouze, N., & Obrist, M. (2019, September 2–6). As light as your scent: Effects of smell and sound on body-image perception [Conference paper]. INTERACT 2019: 17th IFIP TC 13 International Conference, Paphos, Cyprus. [Google Scholar]
- Brown, H., Adams, R. A., Parees, I., Edwards, M., & Friston, K. (2013). Active inference, sensory attenuation and illusions. Cognitive Processing, 14(4), 411–427. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J. S., Crouse, J. J., Shin, M., Tonini, E., Hindmarsh, G., de Haan, Z., Iorfino, F., Robillard, R., Naismith, S., Scott, E. M., & Hickie, I. B. (2025). Evidence for internal misalignment of circadian rhythms in youth with emerging mood disorders. Journal of Biological Rhythms, 7487304251349408. [Google Scholar] [CrossRef]
- Carrasco, M., Ling, S., & Read, S. (2004). Attention alters appearance. Nature Neuroscience, 7(3), 308–313. [Google Scholar] [CrossRef]
- Cavicchioli, M., Santoni, A., Chiappetta, F., Deodato, M., Di Dona, G., Scalabrini, A., Galli, F., & Ronconi, L. (2024). Psychological dissociation and temporal integration/segregation across the senses: An experimental study. Consciousness and Cognition, 124, 103731. [Google Scholar] [CrossRef]
- Chancel, M., & Ehrsson, H. H. (2023). Proprioceptive uncertainty promotes the rubber hand illusion. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 165, 70–85. [Google Scholar] [CrossRef]
- Ciaunica, A. (2016). Basic forms of pre-reflective self-consciousness: A developmental perspective. In S. Miguens, G. Preyer, & C. Morando (Eds.), Pre-reflective self-consciousness: Sartre and contemporary philosophy of mind (pp. 422–438). Routledge. [Google Scholar]
- Ciaunica, A. (2024). Selfless minds, unlimited bodies? Homeostatic bodily self-regulation in meditative experiences. OSF Preprints. [Google Scholar] [CrossRef]
- Ciaunica, A. (2025, May 13). The no body problem: Intelligence and selfhood in biological and artificial systems. PsyArXiv Preprints. [Google Scholar] [CrossRef]
- Ciaunica, A., & Charlton, J. (2018, October 1). When the self slips: What depersonalisation can say about the self. Aeon. Available online: https://aeon.co/essays/what-can-depersonalisation-disorder-say-about-the-self (accessed on 1 October 2024).
- Ciaunica, A., Charlton, J., & Farmer, H. (2020). When the window cracks: Transparency and the fractured self in depersonalisation. Phenomenology and the Cognitive Sciences, 20, 1–19. [Google Scholar] [CrossRef]
- Ciaunica, A., & Fotopoulou, A. (2017). The touched self: Psychological and philosophical perspectives on proximal intersubjectivity and the self. In C. Durt, T. Fuchs, & C. Tewes (Eds.), Embodiment, enaction, and culture—Investigating the constitution of the shared world (pp. 173–192). MIT Press. [Google Scholar]
- Ciaunica, A., Roepstorff, A., Fotopoulou, A. K., & Petreca, B. (2021). Whatever next and close to my self—The transparent senses and the “second skin”. Frontiers in Psychology, 12, 613587. [Google Scholar] [CrossRef] [PubMed]
- Ciaunica, A., Seth, A., Limanowski, J., Hesp, C., & Friston, K. J. (2022). I overthink-therefore I am not: An active inference account of altered sense of self and agency in depersonalisation disorder. Consciousness and Cognition, 101, 103320. [Google Scholar] [CrossRef]
- Clark, A. (2013). Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences, 36, 181–204. [Google Scholar] [CrossRef]
- Conant, R. C., & Ross Ashby, W. (1970). Every good regulator of a system must be a model of that system. International Journal of Systems Science, 1(2), 89–97. [Google Scholar] [CrossRef]
- Constant, A., Ramstead, M. J. D., Veissière, S. P. L., Campbell, J. O., & Friston, K. J. (2018). A variational approach to niche construction. Journal of the Royal Society, Interface, 15(141), 20170685. [Google Scholar] [CrossRef]
- Corcoran, A. W., Perrykkad, K., Feuerriegel, D., & Robinson, J. E. (2023/2025). Body as first teacher: The role of rhythmic visceral dynamics in early cognitive development. Perspectives on Psychological Science, 20(1), 45–75. [Google Scholar] [CrossRef]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–666. [Google Scholar] [CrossRef]
- Critchley, H. D., & Garfinkel, S. N. (2018). The influence of physiological signals on cognition. Current Opinion in Behavioral Sciences, 19, 13–18. [Google Scholar] [CrossRef]
- Damasio, A. (2000). The feeling of what happens: Body, emotion and the making of consciousness. Mariner Books. [Google Scholar]
- Dapor, C., Sperandio, I., & Meconi, F. (2023). Fading boundaries between the physical and the social world: Insights and novel techniques from the intersection of these two fields. Frontiers in Psychology, 13, 1028150. [Google Scholar] [CrossRef] [PubMed]
- De Bartolo, D., De Giorgi, C., Compagnucci, L., Betti, V., Antonucci, G., Morone, G., Paolucci, S., & Iosa, M. (2021). Effects of cognitive workload on heart and locomotor rhythms coupling. Neuroscience Letters, 762, 136140. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A. R., Coimbra, R. D. S., Thomas, E. M., Paz, M. C. R., Pellegrini, B., & Peyré-Tartaruga, L. A. (2021). The entrainment frequency of cardiolocomotor synchronization in long-distance race emerges spontaneously at the step frequency. Frontiers in Physiology, 11, 583030. [Google Scholar] [CrossRef]
- de Jaegher, H., & Di Paolo, E. (2007). Participatory sense-making: An enactive approach to social cognition. Phenomenology and the Cognitive Sciences, 6, 485–507. [Google Scholar] [CrossRef]
- Dewe, H., Watson, D. G., Kessler, K., & Braithwaite, J. J. (2018). The depersonalized brain: New evidence supporting a distinction between depersonalization and derealization from discrete patterns of autonomic suppression observed in a non-clinical sample. Consciousness and Cognition, 63, 29–46. [Google Scholar] [CrossRef]
- Edwards, M. J., Adams, R. A., Brown, H., Pareés, I., & Friston, K. J. (2012). A Bayesian account of ‘hysteria’. Brain: A Journal of Neurology, 135 Pt 11, 3495–3512. [Google Scholar] [CrossRef]
- Ehrsson, H. H., Holmes, N. P., & Passingham, R. E. (2005). Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. Journal of Neuroscience, 25(45), 10564–10573. [Google Scholar] [CrossRef]
- Engelen, T., Schuhmann, T., Sack, A. T., & Tallon-Baudry, C. (2024). The cardiac, respiratory and gastric rhythms independently modulate corticospinal excitability [Preprint]. bioRxiv. [Google Scholar] [CrossRef]
- Engelen, T., Solcà, M., & Tallon-Baudry, C. (2023). Interoceptive rhythms in the brain. Nature Neuroscience, 26(10), 1670–1684. [Google Scholar] [CrossRef]
- Faivre, N., Arzi, A., Lunghi, C., & Salomon, R. (2017). Consciousness is more than meets the eye: A call for a multisensory study of subjective experience. Neuroscience of Consciousness, 2017(1), nix003. [Google Scholar] [CrossRef]
- Feldman, H., & Friston, K. J. (2010). Attention, uncertainty, and free-energy. Frontiers in Human Neuroscience, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, E. R., & Haggard, P. (2016). The vestibular body: Vestibular contributions to bodily representations. Cognitive Neuropsychology, 33(1–2), 67–81. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P., & Frith, C. (2009). Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nature Reviews Neuroscience, 10, 48–58. [Google Scholar] [CrossRef]
- Fries, P. (2005). A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9, 474–480. [Google Scholar] [CrossRef]
- Fries, P. (2015). Rhythms for cognition: Communication through coherence. Neuron, 88(1), 220–235. [Google Scholar] [CrossRef]
- Friston, K. (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society B, 360, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. (2018). Am I self-conscious? (Or does self-organization entail self-consciousness?). Frontiers in Psychology, 9, 579. [Google Scholar] [CrossRef]
- Friston, K., FitzGerald, T., Rigoli, F., Schwartenbeck, P., & Pezzulo, G. (2017). Active inference: A process theory. Neural Computation, 29(1), 1–49. [Google Scholar] [CrossRef]
- Fuchs, T. (2005). Corporealized and disembodied minds: A phenomenological view of the body in melancholia and schizophrenia. Philosophy, Psychiatry, & Psychology, 12(2), 95–107. [Google Scholar]
- Gallagher, S. (2000). Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences, 4(1), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Pol, A., McConnell, R., & Kilner, J. M. (2020). Active sampling in visual search is coupled to the cardiac cycle. Cognition, 196, 104149. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Pol, A., Virdee, P., Villacampa, J., & Kilner, J. (2022). Active tactile discrimination is coupled with and modulated by the cardiac cycle. eLife, 11, e78126. [Google Scholar] [CrossRef]
- Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gatus, A., Jamieson, G., & Stevenson, B. (2022). Past and future explanations for depersonalization and derealization disorder: A role for predictive coding. Frontiers in Human Neuroscience, 16, 744487. [Google Scholar] [CrossRef]
- Gomez-Andres, A., Grau-Sánchez, J., Duarte, E., Rodriguez-Fornells, A., & Tajadura-Jiménez, A. (2020). Enriching footsteps sounds in gait rehabilitation in chronic stroke patients: A pilot study. Annals of the New York Academy of Sciences, 1467(1), 48–59. [Google Scholar] [CrossRef]
- Gu, J., Buidze, T., Zhao, K., Gläscher, J., & Fu, X. (2025). The neural network of sensory attenuation: A neuroimaging meta-analysis. Psychonomic Bulletin & Review, 32, 31–51. [Google Scholar] [CrossRef]
- Guralnik, O., Giesbrecht, T., Knutelska, M., Sirroff, B., & Simeon, D. (2007). Cognitive functioning in depersonalization disorder. The Journal of Nervous and Mental Disease, 195(12), 983–988. [Google Scholar] [CrossRef]
- Haggard, P. (2017). Sense of agency in the human brain. Nature Reviews Neuroscience, 18(4), 196–207. [Google Scholar] [CrossRef]
- Harris, D. J., Wilkinson, S., & Ellmers, T. J. (2023). From fear of falling to choking under pressure: A predictive processing perspective of disrupted motor control under anxiety. Neuroscience and Biobehavioral Reviews, 148, 105115. [Google Scholar] [CrossRef]
- Helmholtz, H. (1962). Concerning the perceptions in general. In Treatise on physiological optics. Dover. (Original work published 1866). [Google Scholar]
- Hesp, C., Smith, R., Parr, T., Allen, M., Friston, K. J., & Ramstead, M. J. D. (2021). Deeply felt affect: The emergence of valence in deep active inference. Neural Computation, 33(2), 398–446. [Google Scholar] [CrossRef] [PubMed]
- Hohwy, J. (2013). The predictive mind. Oxford University Press. [Google Scholar] [CrossRef]
- Hohwy, J. (2020). New directions in predictive processing. Mind & Language, 35(2), 209–223. [Google Scholar] [CrossRef]
- Horváth, J. (2015). Action-related auditory ERP attenuation: Paradigms and hypotheses. Brain Research, 1626, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G., & Waszak, F. (2011). ERP correlates of action effect prediction and visual sensory attenuation in voluntary action. NeuroImage, 56(3), 1632–1640. [Google Scholar] [CrossRef]
- Hunter, E. C., Sierra, M., & David, A. S. (2004). The epidemiology of depersonalisation and derealisation. A systematic review. Social Psychiatry and Psychiatric Epidemiology, 39(1), 9–18. [Google Scholar] [CrossRef]
- Jáuregui Renaud, K. (2015). Vestibular function and depersonalization/derealization symptoms. Multisensory Research, 28(5–6), 637–651. [Google Scholar] [CrossRef]
- Jensen, O., & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186. [Google Scholar] [CrossRef]
- Kaneno, Y., Pasqualotto, A., & Ashida, H. (2024). Influence of interoception and body movement on the rubber hand illusion. Frontiers in Psychology, 15, 1458726. [Google Scholar] [CrossRef]
- Kannape, O. A., & Blanke, O. (2012). Agency, gait and self-consciousness. International Journal of Psychophysiology, 83(2), 191–199. [Google Scholar] [CrossRef]
- Kannape, O. A., Schwabe, L., Tadi, T., & Blanke, O. (2010). The limits of agency in walking humans. Neuropsychologia, 48(6), 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K., Houborg, C., & Ehrsson, H. H. (2019). Rapid learning and unlearning of predicted sensory delays in self-generated touch. eLife, 8, e42888. [Google Scholar] [CrossRef]
- Kim, K. J., Ramiro Diaz, J., Iddings, J. A., & Filosa, J. A. (2016). Vasculo-neuronal coupling: Retrograde vascular communication to brain neurons. Journal of Neuroscience, 36, 12624–12639. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R. L., Nugent, S. T., Marlow, R. W., MacLeod, D. A., & Marble, A. E. (1989). Coupling of cardiac and locomotor rhythms. Journal of Applied Physiology, 66(1), 323–329. [Google Scholar] [CrossRef]
- Koreki, A., Funayama, M., Terasawa, Y., Onaya, M., & Mimura, M. (2021). Aberrant interoceptive accuracy in patients with schizophrenia performing a heartbeat counting task. Schizophrenia Bulletin Open, 2, sgaa067. [Google Scholar] [CrossRef]
- Kosuge, M., Honma, M., Masaoka, Y., Kosuge, S., Nakayama, M., Kamijo, S., Shikama, Y., & Izumizaki, M. (2023). Respiratory rhythm affects recalibration of body ownership. Scientific Reports, 13(1), 920. [Google Scholar] [CrossRef]
- Legrand, D. (2006). The bodily self: The sensori-motor roots of pre-reflective self-consciousness. Phenomenology and the Cognitive Sciences, 5(1), 89–118. [Google Scholar] [CrossRef]
- Lemche, E., Brammer, M. J., David, A. S., Surguladze, S. A., Phillips, M. L., Sierra, M., Williams, S. C. R., & Giampietro, V. P. (2013). Interoceptive-reflective regions differentiate alexithymia traits in depersonalization disorder. Psychiatry Research: Neuroimaging, 214(1), 66–72. [Google Scholar] [CrossRef] [PubMed]
- Leptourgos, P., & Corlett, P. R. (2020). Embodied predictions, agency, and psychosis. Frontiers in Big Data, 3, 27. [Google Scholar] [CrossRef]
- Ley-Flores, J., Alshami, E., Singh, A., Bevilacqua, F., Bianchi-Berthouze, N., Deroy, O., & Tajadura-Jiménez, A. (2022). Effects of pitch and musical sounds on body-representations when moving with sound. Scientific Reports, 12(1), 2676. [Google Scholar] [CrossRef]
- Ley-Flores, J., Turmo Vidal, L., Bianchi-Berthouze, N., Singh, A., Bevilacqua, F., & Tajadura-Jiménez, A. (2021, May 8–13). SoniBand: Understanding the effects of metaphorical movement sonifications on body perception and physical activity. CHI ’21: 2021 CHI Conference on Human Factors in Computing Systems, Yokohama, Japan. [Google Scholar] [CrossRef]
- Limanowski, J., & Blankenburg, F. (2013). Minimal self-models and the free-energy principle. Frontiers in Human Neuroscience, 7, 547. [Google Scholar] [CrossRef]
- Limanowski, J., & Friston, K. (2018). “Seeing the dark”: Grounding phenomenal transparency and opacity in precision estimation for active inference. Frontiers in Psychology, 9, 643. [Google Scholar] [CrossRef]
- Limanowski, J., & Friston, K. (2020). Attenuating oneself: An active inference perspective on “selfless” experiences. Philosophy and the Mind Sciences, 1(I), 1–16. [Google Scholar] [CrossRef]
- Liu, T., Abrams, J., & Carrasco, M. (2009). Voluntary attention enhances contrast appearance. Psychological Science, 20(3), 354–362. [Google Scholar] [CrossRef] [PubMed]
- Marina, N., Christie, I. N., Korsak, A., Doronin, M., Brazhe, A., Hosford, P. S., Wells, J. A., Sheikhbahaei, S., Humoud, I., Paton, J. F. R., Lythgoe, M. F., Semyanov, A., Kasparov, S., & Gourine, A. V. (2020). Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nature Communications, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Maturana, H. R., & Varela, F. J. (1980). Autopoiesis and cognition: The realization of the living. D. Reidel. [Google Scholar]
- Medford, N., Sierra, M., Stringaris, A., Giampietro, V., Brammer, M. J., & David, A. S. (2016). Emotional experience and awareness of self: Functional MRI studies of depersonalization disorder. Frontiers in Psychology, 7, 432. [Google Scholar] [CrossRef]
- Merleau-Ponty, M. (1962). Phenomenology of perception (C. Smith, Trans.). Routledge & Kegan Paul. Original work published 1945. [Google Scholar]
- Michal, M., Reuchlein, B., Adler, J., Reiner, I., Beutel, M. E., Vögele, C., Schächinger, H., & Schulz, A. (2014). Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS ONE, 9(2), e89823. [Google Scholar] [CrossRef]
- Millman, L. S. M., Hunter, E. C. M., Terhune, D. B., & Orgs, G. (2023). Online structured dance/movement therapy reduces bodily detachment in depersonalisation-derealization disorder. Complementary Therapies in Clinical Practice, 51, 101749. [Google Scholar] [CrossRef]
- Monti, A., Porciello, G., Tieri, G., & Aglioti, S. M. (2020). The “embreathment” illusion highlights the role of breathing in corporeal awareness. Journal of Neurophysiology, 123, 420–427. [Google Scholar] [CrossRef]
- Niizeki, K. (2005). Intramuscular pressure-induced inhibition of cardiac contraction: Implications for cardiac-locomotor synchronization. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 288(3), R645–R650. [Google Scholar] [CrossRef]
- Niizeki, K., Kawahara, K., & Miyamoto, Y. (1996). Cardiac, respiratory, and locomotor coordination during walking in humans. Folia Primatologica, 66(1–4), 226–239. [Google Scholar] [CrossRef]
- Niizeki, K., & Saitoh, T. (2014). Cardiolocomotor phase synchronization during rhythmic exercise. Journal of Physical Fitness and Sports Medicine, 3(1), 11–20. [Google Scholar] [CrossRef]
- Novak, V., Hu, K., Vyas, M., & Lipsitz, L. A. (2007). Cardiolocomotor coupling in young and elderly people. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 62(1), 86–92. [Google Scholar] [CrossRef]
- Owens, A. P., David, A. S., Low, D. A., Mathias, C. J., & Sierra-Siegert, M. (2015). Abnormal cardiovascular sympathetic and parasympathetic responses to physical and emotional stimuli in depersonalisation disorder. Frontiers in Neuroscience, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C. E., Davare, M., & Kilner, J. M. (2016). Physiological and perceptual sensory attenuation have different underlying neurophysiological correlates. Journal of Neuroscience, 36, 10803–10812. [Google Scholar] [CrossRef] [PubMed]
- Park, H. D., & Blanke, O. (2019). Coupling inner and outer body for self-consciousness. Trends in Cognitive Sciences, 23, 377–388. [Google Scholar] [CrossRef]
- Park, H. D., & Tallon-Baudry, C. (2014). The neural subjective frame: From bodily signals to perceptual consciousness. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 369(1641), 20130208. [Google Scholar] [CrossRef] [PubMed]
- Parr, T., & Friston, K. J. (2017). The active construction of the visual world. Neuropsychologia, 104, 92–101. [Google Scholar] [CrossRef]
- Pelentritou, A., Pfeiffer, C., Iten, M., Haenggi, M., Zubler, F., Schwartz, S., & De Lucia, M. (2025). Cardiac signals inform auditory regularity processing in the absence of consciousness. Proceedings of the National Academy of Sciences of the United States of America, 122(20), e2505454122. [Google Scholar] [CrossRef]
- Perkins, J. (2021). Life on autopilot: A guide to living with depersonalisation disorder. Jessica Kingsley. [Google Scholar]
- Poublan-Couzardot, A., Foncelle, A., Abdoun, O., Koun, E., Rossetti, Y., Pagnoni, G., & Lutz, A. (2025). Modulation of sensory attenuation by intensive meditation practice: An active inference perspective. Neuroscience of Consciousness, 1–16, (submitted). [Google Scholar]
- Qin, P., Wang, M., & Northoff, G. (2020). Linking bodily, environmental and mental states in the self: A three-level model based on a meta-analysis. Neuroscience & Biobehavioral Reviews, 115, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Quadt, L., Critchley, H. D., & Garfinkel, S. N. (2018). The neurobiology of interoception in health and disease. Annals of the New York Academy of Sciences, 1428(1), 112–128. [Google Scholar] [CrossRef] [PubMed]
- Quattrocki, E., & Friston, K. (2014). Autism, oxytocin and interoception. Neuroscience and Biobehavioral Reviews, 47, 410–430. [Google Scholar] [CrossRef] [PubMed]
- Quigley, K. S., Kanoski, S., Grill, W. M., Barrett, L. F., & Tsakiris, M. (2021). Functions of interoception: From energy regulation to experience of the self. Trends in Neurosciences, 44(1), 29–38. [Google Scholar] [CrossRef]
- Ramstead, M. J. D., Kirchhoff, M. D., Constant, A., & Friston, K. J. (2019). Multiscale integration: Beyond internalism and externalism. Synthese, 1–30. [Google Scholar]
- Rebollo, I., Devauchelle, A. D., Béranger, B., & Tallon-Baudry, C. (2018). Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. eLife, 7, e33321. [Google Scholar] [CrossRef]
- Ren, Q., Marshall, A. C., Liu, J., & Schütz-Bosbach, S. (2024). Listen to your heart: Trade-off between cardiac interoceptive processing and visual exteroceptive processing. NeuroImage, 299, 120808. [Google Scholar] [CrossRef]
- Richter, C. G., Babo-Rebelo, M., Schwartz, D., & Tallon-Baudry, C. (2017). Phase-amplitude coupling at the organism level: The amplitude of spontaneous alpha rhythm fluctuations varies with the phase of the infra-slow gastric basal rhythm. NeuroImage, 146, 951–958. [Google Scholar] [CrossRef]
- Rohde, M., Di Luca, M., & Ernst, M. O. (2011). The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE, 6(6), e21659. [Google Scholar] [CrossRef]
- Rosato, A., Larsson, M., Rullman, E., & Düal, S. A. (2024). Evidence of spontaneous cardiac-locomotor coupling during daily activities in healthy adults. Frontiers in Physiology, 15, 1394591. [Google Scholar] [CrossRef]
- Rouffet, D., Taylor, S., Sparrow, W. A., & Begg, R. (2008, December 15–18). Cardio-locomotor entrainment during walking in young healthy people: A preliminary study. 2008 International Conference on Intelligent Sensors, Sensor Networks and Information Processing (pp. 347–350), Sydney, NSW, Australia. [Google Scholar] [CrossRef]
- Saini, F., Ponzo, S., Silvestrin, F., Fotopoulou, A., & David, A. S. (2022). Depersonalization disorder as a systematic downregulation of interoceptive signals. Scientific Reports, 12(1), 22123. [Google Scholar] [CrossRef]
- Salami, A., Andreu-Perez, J., & Gillmeister, H. (2020). Symptoms of depersonalisation/derealisation disorder as measured by brain electrical activity: A systematic review. Neuroscience and biobehavioral reviews, 118, 524–537. [Google Scholar] [CrossRef]
- Saltafossi, M., Zaccaro, A., Perrucci, M. G., Ferri, F., & Costantini, M. (2023). The impact of cardiac phases on multisensory integration. Biological Psychology, 182, 108642. [Google Scholar] [CrossRef]
- Sandved-Smith, L., Hesp, C., Mattout, J., Friston, K., Lutz, A., & Ramstead, M. J. D. (2021). Towards a computational phenomenology of mental action: Modelling meta-awareness and attentional control with deep parametric active inference. Neuroscience of Consciousness, 2021(2), niab018. [Google Scholar] [CrossRef] [PubMed]
- Sargent, K., Martinez, E., Reed, A., Guha, A., Bartholomew, M., Diehl, C., Chang, C., Salama, S., Subotnik, K., Ventura, J., Nuechterlein, K., Miller, G., & Yee, C. (2025). Brain–body dysconnectivity: Deficient autonomic regulation of cortical function in first-episode schizophrenia. Psychological Medicine, 55, e1. [Google Scholar] [CrossRef] [PubMed]
- Schabinger, N., Gillmeister, H., Berti, S., Michal, M., Beutel, M. E., & Adler, J. (2018). Detached and distracted: ERP correlates of altered attentional function in depersonalisation. Biological Psychology, 134, 64–71. [Google Scholar] [CrossRef]
- Schulz, A., Köster, S., Beutel, M. E., Schächinger, H., Vögele, C., Rost, S., Rauh, M., & Michal, M. (2015). Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: Neurophysiological evidence for impaired cortical representation of bodily signals. Psychosomatic Medicine, 77(5), 506–516. [Google Scholar] [CrossRef] [PubMed]
- Sedeño, L., Couto, B., Melloni, M., Canales-Johnson, A., Yoris, A., Baez, S., Esteves, S., Velásquez, M., Barttfeld, P., Sigman, M., Kichic, R., Chialvo, D., Manes, F., Bekinschtein, T. A., & Ibanez, A. (2014). How do you feel when you can’t feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE, 9(6), e98769. [Google Scholar] [CrossRef]
- Sel, A., Azevedo, R. T., & Tsakiris, M. (2017). Heartfelt self: Cardio-visual integration affects self-face recognition and interoceptive cortical processing. Cerebral Cortex, 27, 5144–5155. [Google Scholar] [CrossRef]
- Seth, A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17, 565–573. [Google Scholar] [CrossRef]
- Seth, A. K., & Friston, K. J. (2016). Active interoceptive inference and the emotional brain. Philosophical Transactions of the Royal Society B, 371, 20160007. [Google Scholar] [CrossRef] [PubMed]
- Shergill, S. S., Samson, G., Bays, P. M., Frith, C. D., & Wolpert, D. M. (2005). Evidence for sensory prediction deficits in schizophrenia. The American Journal of Psychiatry, 162(12), 2384–2386. [Google Scholar] [CrossRef]
- Sierra, M., & Berrios, G. E. (1997). Depersonalization: A conceptual history. History of Psychiatry, 8 Pt 2(30), 213–229. [Google Scholar] [CrossRef]
- Sierra, M., & David, A. S. (2011). Depersonalization: A selective impairment of self-awareness. Consciousness and Cognition, 20(1), 99–108. [Google Scholar] [CrossRef]
- Sierra, M., Senior, C., Dalton, J., McDonough, M., Bond, A., Phillips, M. L., O’Dwyer, A. M., & David, A. S. (2002). Autonomic response in depersonalization disorder. Archives of General Psychiatry, 59(9), 833–838. [Google Scholar] [CrossRef]
- Simeon, D., & Abugel, J. (2006). Feeling unreal: Depersonalization disorder and the loss of the self. Oxford University Press. [Google Scholar]
- Simeon, D., Knutelska, M., Nelson, D., & Guralnik, O. (2003). Feeling unreal: A depersonalization disorder update of 117 cases. The Journal of Clinical Psychiatry, 64(9), 990–997. [Google Scholar] [CrossRef]
- Stanton, T. R., & Spence, C. (2020). The influence of auditory cues on bodily and movement perception. Frontiers in Psychology, 10, 3001. [Google Scholar] [CrossRef]
- Sterling, P. (2014). Homeostasis vs allostasis: Implications for brain function and mental disorders. JAMA Psychiatry, 71(10), 1192–1193. [Google Scholar] [CrossRef]
- Suzuki, K., Garfinkel, S. N., Critchley, H. D., & Seth, A. K. (2013). Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia, 51, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Tajadura-Jiménez, A., Basia, M., Deroy, O., Fairhurst, M. T., Marquardt, N., & Bianchi-Berthouze, N. (2015, April 18–23). As light as your footsteps: Altering walking sounds to change perceived body weight, emotional state and gait. 33rd Annual CHI Conference on Human Factors in Computing Systems (pp. 2943–2952), Seoul, Republic of Korea. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A., Cohen, H., & Bianchi-Berthouze, N. (2017). Bodily sensory inputs and anomalous bodily experiences in complex regional pain syndrome: Evaluation of the potential effects of sound feedback. Frontiers in Human Neuroscience, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Tajadura-Jiménez, A., Väljamäe, A., Toshima, I., Kimura, T., Tsakiris, M., & Kitagawa, N. (2012). Action sounds recalibrate perceived tactile distance. Current Biology, 22(13), R516–R517. [Google Scholar] [CrossRef]
- Takeuchi, S., Nishida, Y., & Mizushima, T. (2014). Effects of synchronization between cardiac and locomotor rhythms on oxygen pulse during walking. Journal of Sports Science & Medicine, 13(4), 881–887. [Google Scholar]
- Thompson, E. (2007). Mind in life: Biology, phenomenology, and the sciences of mind. Harvard University Press. [Google Scholar]
- Tort, A. B. L., Brankack, J., & Draguhn, A. (2018). Respiration-entrained brain rhythms are global but often overlooked. Trends in Neurosciences, 41, 186–197. [Google Scholar] [CrossRef]
- Tsakiris, M. (2017). The multisensory basis of the self: From body to identity to others. Quarterly Journal of Experimental Psychology, 70, 597–609. [Google Scholar] [CrossRef]
- Tsakiris, M., Carpenter, L., James, D., & Fotopoulou, A. (2010). Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Experimental Brain Research, 204(3), 343–352. [Google Scholar] [CrossRef] [PubMed]
- Varela, F., Lachaux, J.-P., Rodriguez, E., & Martinerie, J. (2001). The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience, 2, 229–239. [Google Scholar] [CrossRef]
- Varela, F. J., Thompson, E., & Rosch, E. (1991). The embodied mind: Cognitive science and human experience. MIT Press. [Google Scholar]
- Wakeham, D. J., Ivey, E., Saland, S. A., Lewis, J. S., Palmer, D., Morris, M., Bleich, J. L., Weyand, P. G., Brazile, T. L., Hearon, C. M., Jr., Sarma, S., MacNamara, J. P., Hieda, M., & Levine, B. D. (2023). Effects of synchronizing foot strike and cardiac phase on exercise hemodynamics in patients with cardiac resynchronization therapy: A within-subjects pilot study to fine-tune cardio-locomotor coupling for heart failure. Circulation, 148(25), 2008–2016. [Google Scholar] [CrossRef]
- Waszak, F., Cardoso-Leite, P., & Hughes, G. (2012). Action effect anticipation: Neurophysiological basis and functional consequences. Neuroscience & Biobehavioral Reviews, 36(2), 943–959. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, M. W. (2016). Characteristics and coupling of cardiac and locomotor rhythms during treadmill walking tasks [Ph.D. dissertation, University of North Carolina at Greensboro]. [Google Scholar]
- Wolpe, N., Ingram, J. N., Tsvetanov, K. A., Geerligs, L., Kievit, R. A., Henson, R. N., Wolpert, D. M., Cam, C. A. N., & Rowe, J. B. (2016). Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nature Communications, 7, 13034. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, N., & Tallon-Baudry, C. (2021). Coupling between the phase of a neural oscillation or bodily rhythm with behavior: Evaluation of different statistical procedures. NeuroImage, 236, 118050. [Google Scholar] [CrossRef]
- Yon, D., & Frith, C. D. (2021). Precision and the Bayesian brain. Current Biology, 31(17), R1026–R1032. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D. (2005). Subjectivity and selfhood: Investigating the first-person perspective. MIT Press. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, V.; Ciaunica, A. It Is Movement All the Way Down: Broken Rhythms and Embodied Selfhood in Depersonalization. Behav. Sci. 2025, 15, 1090. https://doi.org/10.3390/bs15081090
Alekseeva V, Ciaunica A. It Is Movement All the Way Down: Broken Rhythms and Embodied Selfhood in Depersonalization. Behavioral Sciences. 2025; 15(8):1090. https://doi.org/10.3390/bs15081090
Chicago/Turabian StyleAlekseeva, Veronika, and Anna Ciaunica. 2025. "It Is Movement All the Way Down: Broken Rhythms and Embodied Selfhood in Depersonalization" Behavioral Sciences 15, no. 8: 1090. https://doi.org/10.3390/bs15081090
APA StyleAlekseeva, V., & Ciaunica, A. (2025). It Is Movement All the Way Down: Broken Rhythms and Embodied Selfhood in Depersonalization. Behavioral Sciences, 15(8), 1090. https://doi.org/10.3390/bs15081090





