Exploring Interindividual Variability in Resilience to Stress: Social Support, Coping Styles, and Diurnal Cortisol in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
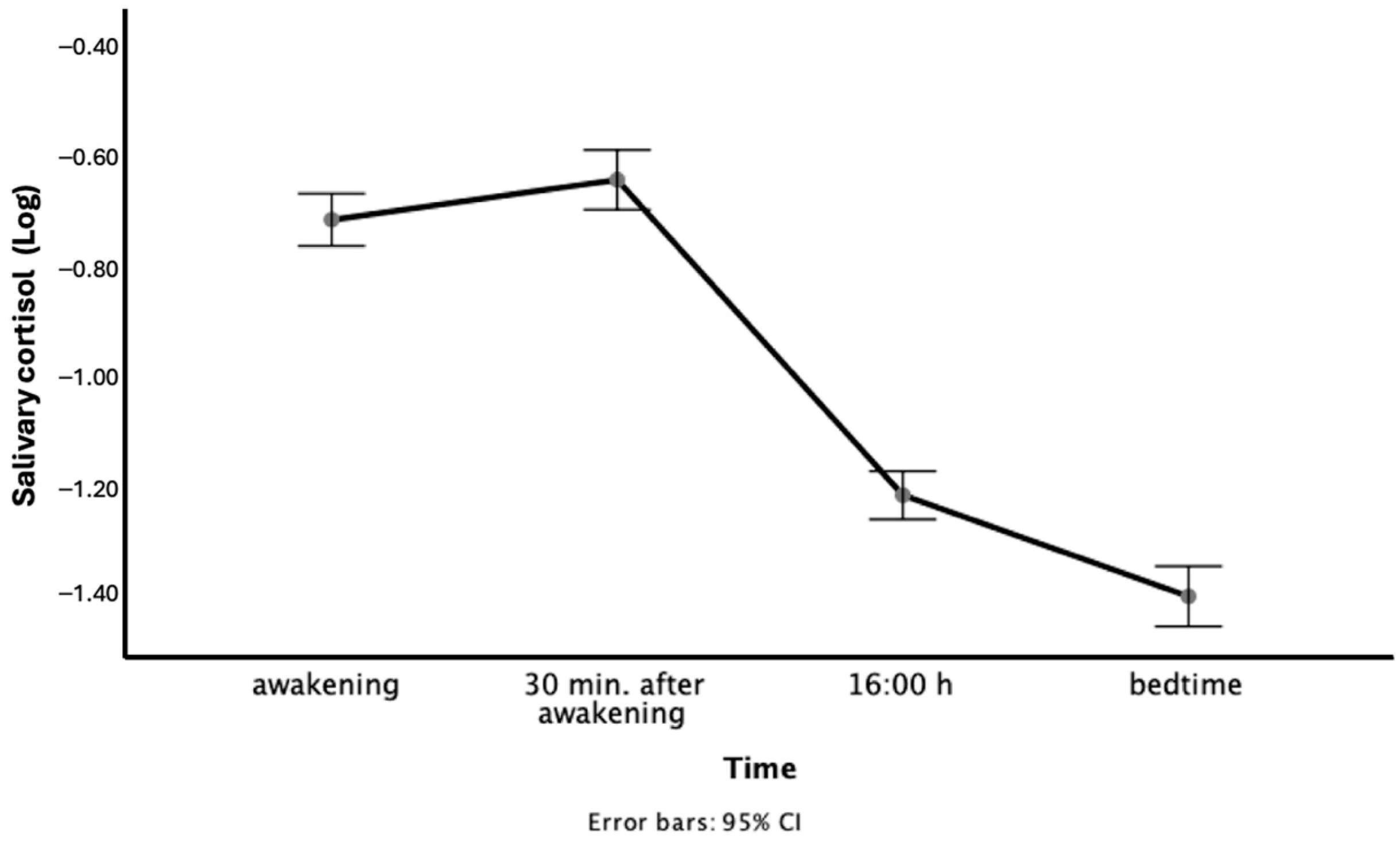
2.3.1. Salivary Cortisol
2.3.2. Individual Characteristics
2.3.3. Indicators of Subjective Stress
2.3.4. Indicators of Social Support Network and Coping Mechanisms
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPA | Hypothalamic-pituitary-adrenal |
| AUC | Cortisol area under the curve |
| CAR | Cortisol awakening response |
| PCS | Physical Component Summary |
| PSS | Perceived Stress Scale |
| PCI | Poactive coping inventory |
| SSQ-6 | Social support questionnaire |
Appendix A
| Variables | B | p | 95% CI | ß |
|---|---|---|---|---|
| Presence of psychological distress | ||||
| Health-related quality of life | 0.004 | 0.026 | 0.000, 0.007 | 0.297 |
| Emotional support seeking | 0.059 | 0.094 | −0.002, 0.115 | 0.246 |
| Strategic planning coping | 0.013 | 0.093 | 0.020, 0.167 | 0.438 |
| Reflective coping | −0.147 | 0.005 | −0.248, −0.046 | −0.488 |
| Absence of psychological distress | ||||
| Age | 0.003 | 0.080 | 0.000, 0.007 | 0.185 |
| Health-related quality of life | 0.003 | 0.026 | 0.000, 0.006 | 0.236 |
| Instrumental support seeking | −0.070 | 0.032 | −0.134, −0.006 | −0.240 |
| Preventive coping | 0.107 | 0.012 | 0.024, 0.189 | 0.284 |
References
- Adam, E. K., Hawkley, L. C., Kudielka, B. M., & Cacioppo, J. T. (2006). Day-to-day dynamics of experience—Cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Science of the USA, 103(45), 17058–17063. [Google Scholar] [CrossRef] [PubMed]
- Agbedia, O. O., Varma, V. R., Seplaki, C. L., Seeman, T. E., Fried, L. P., Li, L., Harris, G. C., Rebok, G. W., Xue, Q. L., Tan, E. J., Tanner, E., Parisi, J. M., McGill, S., & Carlson, M. C. (2011). Blunted diurnal decline of cortisol among older adults with low socioeconomic status. Annals of the New York Academy of Sciences, 1231, 56–64. [Google Scholar] [CrossRef][Green Version]
- Birditt, K. S., Nevitt, M. R., & Almeida, D. M. (2015). Daily interpersonal coping strategies: Implications for self-reported well-being and cortisol. Journal of Social and Personal Relationships, 32(5), 687–706. [Google Scholar] [CrossRef] [PubMed]
- Bjorklof, G. H., Engedal, K., Selbaek, G., Kouwenhoven, S. E., & Helvik, A. S. (2013). Coping and depression in old age: A literature review. Dementia and Geriatric Cognitive Disorders, 35(3–4), 121–154. [Google Scholar] [CrossRef] [PubMed]
- Bom, J., Bakx, P., Schut, F., & van Doorslaer, E. (2019). The impact of informal caregiving for older adults on the health of various types of caregivers: A systematic review. Gerontologist, 59(5), e629–e642. [Google Scholar] [CrossRef]
- Charmandari, E., Tsigos, C., & Chrousos, G. (2005). Endocrinology of the stress response. Annual Review of Physiology, 67, 259–284. [Google Scholar] [CrossRef]
- Clow, A., Thorn, L., Evans, P., & Hucklebridge, F. (2004). The awakening cortisol response: Methodological issues and significance. Stress, 7(1), 29–37. [Google Scholar] [CrossRef]
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [Google Scholar] [CrossRef]
- Cosco, T. D., Howse, K., & Brayne, C. (2017). Healthy ageing, resilience and wellbeing. Epidemiology and Psychiatric Sciences, 26(6), 579–583. [Google Scholar] [CrossRef]
- Courtin, E., & Knapp, M. (2017). Social isolation, loneliness and health in old age: A scoping review. Health & Social Care in the Community, 25(3), 799–812. [Google Scholar] [CrossRef]
- Djukanovic, I., Carlsson, J., & Arestedt, K. (2017). Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65–80 years old? A psychometric evaluation study. Health Qual Life Outcomes, 15(1), 193. [Google Scholar] [CrossRef]
- Dmitrieva, N. O., Almeida, D. M., Dmitrieva, J., Loken, E., & Pieper, C. F. (2013). A day-centered approach to modeling cortisol: Diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendocrinology, 38(10), 2354–2365. [Google Scholar] [CrossRef]
- Donovan, N. J., & Blazer, D. (2020). Social isolation and loneliness in older adults: Review and commentary of a national academies report. American Journal of Geriatric Psychiatry, 28(12), 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Duric, V., Clayton, S., Leong, M. L., & Yuan, L. L. (2016). Comorbidity factors and brain mechanisms linking chronic stress and systemic illness. Neural Plasticity, 2016, 5460732. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, A., Jiang, J., Katz, M. J., Sliwinski, M. J., Zimmerman, M. E., & Lipton, R. B. (2014). Validation of the perceived stress scale in a community sample of older adults. International Journal of Geriatric Psychiatry, 29(6), 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, K. F., & Shippee, T. P. (2009). Aging and cumulative inequality: How does inequality get under the skin? Gerontologist, 49(3), 333–343. [Google Scholar] [CrossRef]
- Fries, E., Dettenborn, L., & Kirschbaum, C. (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. [Google Scholar] [CrossRef]
- Gaffey, A. E., Bergeman, C. S., Clark, L. A., & Wirth, M. M. (2016). Aging and the HPA axis: Stress and resilience in older adults. Neuroscience & Biobehavioral Reviews, 68, 928–945. [Google Scholar] [CrossRef]
- Gale, C. R., Allerhand, M., Sayer, A. A., Cooper, C., Dennison, E. M., Starr, J. M., Ben-Shlomo, Y., Gallacher, J. E., Kuh, D., Deary, I. J., & The HALCyon Study Team. (2010). The structure of the Hospital Anxiety and Depression Scale in four cohorts of community-based, healthy older people: The HALCyon program. International Psychogeriatrics, 22(4), 559–571. [Google Scholar] [CrossRef]
- Gandek, B., Ware, J. E., Aaronson, N. K., Apolone, G., Bjorner, J. B., Brazier, J. E., Bullinger, M., Kaasa, S., Leplege, A., Prieto, L., & Sullivan, M. (1998). Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. Journal of Clinical Epidemiology, 51(11), 1171–1178. [Google Scholar] [CrossRef]
- Garrido, P. (2011). Aging and stress: Past hypotheses, present approaches and perspectives. Aging & Disease, 2(1), 80–99. [Google Scholar]
- Geronimus, A. T. (2001). Understanding and eliminating racial inequalities in women’s health in the United States: The role of the weathering conceptual framework. Journal of the American Medical Women’s Association, 56(4), 133–136. [Google Scholar] [PubMed]
- Gerritsen, L., Geerlings, M. I., Beekman, A. T., Deeg, D. J., Penninx, B. W., & Comijs, H. C. (2010). Early and late life events and salivary cortisol in older persons. Psychological Medicine, 40(9), 1569–1578. [Google Scholar] [CrossRef]
- Goncharova, N. D. (2020). The HPA Axis under stress and aging: Individual vulnerability is associated with behavioral patterns and exposure time. Bioessays, 42(9), e2000007. [Google Scholar] [CrossRef] [PubMed]
- Granger, D. A., Hibel, L. C., Fortunato, C. K., & Kapelewski, C. H. (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. [Google Scholar] [CrossRef]
- Greenglass, E., Schwarzer, R., Jakubiec, D., Fiksenbaum, L., & Taubert, S. (1999, July 12–14). The proactive coping inventory (PCI): A multidimensional research instrument. 20th International Conference of the Stress and Anxiety Research Society, Cracow, Poland. [Google Scholar]
- Grolli, R. E., Mingoti, M. E. D., Bertollo, A. G., Luzardo, A. R., Quevedo, J., Reus, G. Z., & Ignacio, Z. M. (2021). Impact of COVID-19 in the mental health in elderly: Psychological and biological updates. Molecular Neurobiology, 58(5), 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Hammerfald, K., Eberle, C., Grau, M., Kinsperger, A., Zimmermann, A., Ehlert, U., & Gaab, J. (2006). Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects—A randomized controlled trial. Psychoneuroendocrinology, 31(3), 333–339. [Google Scholar] [CrossRef]
- Hek, K., Direk, N., Newson, R. S., Hofman, A., Hoogendijk, W. J., Mulder, C. L., & Tiemeier, H. (2013). Anxiety disorders and salivary cortisol levels in older adults: A population-based study. Psychoneuroendocrinology, 38(2), 300–305. [Google Scholar] [CrossRef]
- Hsiao, F. H., Yang, T. T., Ho, R. T., Jow, G. M., Ng, S. M., Chan, C. L., Lai, Y. M., Chen, Y. T., & Wang, K. C. (2010). The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology, 35(4), 503–515. [Google Scholar] [CrossRef]
- Issalillah, F., & Aisyah, N. (2022). The elderly and the determinants of stress. Journal of Social Science Studies (JOS3), 2(1), 9–12. [Google Scholar] [CrossRef]
- Kabia, F. M., Rhebergen, D., van Exel, E., Stek, M. L., & Comijs, H. C. (2016). The predictive value of cortisol levels on 2-year course of depression in older persons. Psychoneuroendocrinology, 63, 320–326. [Google Scholar] [CrossRef]
- Kelly, S. J., & Ismail, M. (2015). Stress and type 2 diabetes: A review of how stress contributes to the development of type 2 diabetes. Annual Review of Public Health, 36, 441–462. [Google Scholar] [CrossRef]
- Klabunde, C. N., Warren, J. L., & Legler, J. M. (2002). Assessing comorbidity using claims data: An overview. Medical Care Journal, 40(Suppl. S8), IV-26–IV-35. [Google Scholar] [CrossRef]
- Kudielka, B. M., Gierens, A., Hellhammer, D. H., Wust, S., & Schlotz, W. (2012). Salivary cortisol in ambulatory assessment—Some dos, some don’ts, and some open questions. Psychosomatic Medicine, 74(4), 418–431. [Google Scholar] [CrossRef] [PubMed]
- Lord, S., Després, C., & Ramadier, T. (2011). When mobility makes sense: A qualitative and longitudinal study of the daily mobility of the elderly. Journal of Environmental Psychology, 31(1), 52–61. [Google Scholar] [CrossRef]
- Lupien, S. J., & Lepage, M. (2001). Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research, 127(1–2), 137–158. [Google Scholar] [CrossRef] [PubMed]
- Manly, C. A., & Wells, R. S. (2015). Reporting the use of multiple imputation for missing data in higher education research. Reaearch in Higher Education, 56, 397–409. [Google Scholar] [CrossRef]
- Moos, R. H., Brennan, P. L., Schutte, K. K., & Moos, B. S. (2006). Older adults’ coping with negative life events: Common processes of managing health, interpersonal, and financial/work stressors. International Journal of Aging and Human Development, 62(1), 39–59. [Google Scholar] [CrossRef]
- Odeniyi, I. A., Fasanmade, O. A., Ogbera, A. O., & Ohwovoriole, A. E. (2015). Body mass index and its effect on serum cortisol level. Nigerian Journal of Clinical Practice, 18(2), 194–197. [Google Scholar] [CrossRef]
- O’Donnell, K., Badrick, E., Kumari, M., & Steptoe, A. (2008). Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology, 33(5), 601–611. [Google Scholar] [CrossRef]
- Park, G. R., & An, J. S. (2016). The relationships between loss experiences and depression of the men and women elderly: Focused on the moderating effects of stress coping styles. Journal of Family Relations, 20(4), 105–130. [Google Scholar]
- Pauly, T., Drewelies, J., Kolodziejczak, K., Katzorreck, M., Lucke, A. J., Schilling, O. K., Kunzmann, U., Wahl, H. W., Ditzen, B., Ram, N., Gerstorf, D., & Hoppmann, C. A. (2021). Positive and negative affect are associated with salivary cortisol in the everyday life of older adults: A quantitative synthesis of four aging studies. Psychoneuroendocrinology, 133, 105403. [Google Scholar] [CrossRef] [PubMed]
- Penning, M. J., & Wu, Z. (2016). Caregiver stress and mental health: Impact of caregiving relationship and gender. Gerontologist, 56(6), 1102–1113. [Google Scholar] [CrossRef]
- Piazza, J. R., Dmitrieva, N. O., Charles, S. T., Almeida, D. M., & Orona, G. A. (2018). Diurnal cortisol profiles, inflammation, and functional limitations in aging: Findings from the MIDUS study. Health Psychology, 37(9), 839–849. [Google Scholar] [CrossRef]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. [Google Scholar] [CrossRef] [PubMed]
- Rao, R., & Androulakis, I. P. (2019). The physiological significance of the circadian dynamics of the HPA axis: Interplay between circadian rhythms, allostasis and stress resilience. Hormones and Behavior, 110, 77–89. [Google Scholar] [CrossRef]
- Rascle, N., Bruchon-Schweitzer, M., & Sarason, I. G. (2005). Short form of sarason’s social support questionnaire: French adaptation and validation. Psychological Reports, 97(1), 195–202. [Google Scholar] [CrossRef]
- Roberge, P., Dore, I., Menear, M., Chartrand, E., Ciampi, A., Duhoux, A., & Fournier, L. (2013). A psychometric evaluation of the French Canadian version of the Hospital Anxiety and Depression Scale in a large primary care population. Journal of Affective Disorder, 147(1–3), 171. [Google Scholar] [CrossRef]
- Sarason, I. G., Sarason, B. R., Shearin, E. N., & Pierce, G. R. (1987). A brief measure of social support: Practical and theoretical implications. Journal of Social and Personal Relationships, 4, 497–510. [Google Scholar] [CrossRef]
- Schoorlemmer, R. M., Peeters, G. M., van Schoor, N. M., & Lips, P. (2009). Relationships between cortisol level, mortality and chronic diseases in older persons. Clinical Endocrinology, 71(6), 779–786. [Google Scholar] [CrossRef]
- Sharifirad, G., Ghaffari, M., Zanjani, S., & Hassanzadeh, A. (2013). The effectiveness of educational intervention based on PRECEDE model on the level of stress among the elderly at elderly clubs. Journal of Education and Health Promotion, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T., Lupien, S. J., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wust, S., Dockray, S., Smyth, N., Evans, P., Kirschbaum, C., Miller, R., Wetherell, M. A., Finke, J. B., Klucken, T., & Clow, A. (2022). Evaluation and update of the expert consensus guidelines for the assessment of the cortisol awakening response (CAR). Psychoneuroendocrinology, 146, 105946. [Google Scholar] [CrossRef]
- Steptoe, A., & Kivimaki, M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9(6), 360–370. [Google Scholar] [CrossRef]
- Troy, A. S., Willroth, E. C., Shallcross, A. J., Giuliani, N. R., Gross, J. J., & Mauss, I. B. (2023). Psychological resilience: An affect-regulation framework. Annual Review of Psychology, 74, 547–576. [Google Scholar] [CrossRef]
- Vammen, M. A., Mikkelsen, S., Hansen, A. M., Grynderup, M. B., Andersen, J. H., Bonde, J. P., Buttenschon, H. N., Kolstad, H. A., Kaergaard, A., Kaerlev, L., Mors, O., Rugulies, R., & Thomsen, J. F. (2014). Salivary cortisol and depression in public sector employees: Cross-sectional and short term follow-up findings. Psychoneuroendocrinology, 41, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S. A., Hoogendijk, W. J., DeRijk, R. H., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. (2013). Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology, 38(9), 1494–1502. [Google Scholar] [CrossRef]
- White, I. R., Royston, P., & Wood, A. M. (2011). Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medecine, 30(4), 377–399. [Google Scholar] [CrossRef]
- Wolf, O. T., & Kudielka, B. M. (2008). Stress, health and ageing: A focus on postmenopausal women. International Menopause Society, 14(3), 129–133. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. (2015). World report on ageing and health. Available online: https://apps.who.int/iris/handle/10665/186463 (accessed on 8 March 2023).
- Wright, C. E., Kunz-Ebrecht, S. R., Iliffe, S., Foese, O., & Steptoe, A. (2005). Physiological correlates of cognitive functioning in an elderly population. Psychoneuroendocrinology, 30(9), 826–838. [Google Scholar] [CrossRef]
- Wrosch, C., Miller, G. E., Lupien, S., & Pruessner, J. C. (2008). Diurnal cortisol secretion and 2-year changes in older adults’ physical symptoms: The moderating roles of negative affect and sleep. Health Psychology, 27(6), 685–693. [Google Scholar] [CrossRef]
- Yiallouris, A., Tsioutis, C., Agapidaki, E., Zafeiri, M., Agouridis, A. P., Ntourakis, D., & Johnson, E. O. (2019). Adrenal aging and its implications on stress responsiveness in humans. Frontiers in Endocrinology, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. [Google Scholar] [CrossRef] [PubMed]
| Variables | CAR | AUC | Peak-to-Bed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | 95% CI | ß | B | p | 95% CI | ß | B | p | 95% CI | ß | |
| Control | ||||||||||||
| Body mass index | 0.001 | 0.804 | −0.004, 0.005 | 0.024 | 0.026 | 0.123 | −0.007, 0.058 | 0.144 | 0.000 | 0.592 | 0.000, 0.000 | 0.052 |
| Medication use | −0.026 | 0.291 | −0.075, 0.023 | −0.105 | −0.351 | 0.084 | −0.750, 0.048 | −0.168 | −0.003 | 0.191 | −0.008, 0.002 | −0.132 |
| Related to aging | ||||||||||||
| Age | 0.002 | 0.356 | −0.002, 0.005 | 0.095 | 0.034 | 0.022 | 0.005, 0.064 | 0.230 | 0.000 | 0.334 | 0.000, 0.000 | 0.100 |
| Sex a | 0.054 | 0.158 | −0.021, 0.129 | 0.141 | 0.219 | 0.482 | −0.395, 0.832 | 0.068 | 0.001 | 0.851 | −0.006, 0.008 | 0.019 |
| Income | −0.007 | 0.451 | −0.027, −0.012 | −0.084 | 0.031 | 0.697 | −0.128, 0.191 | 0.042 | 0.000 | 0.865 | −0.002, 0.002 | 0.019 |
| Education | 0.003 | 0.385 | −0.004, 0.011 | 0.091 | 0.017 | 0.606 | −0.047, 0.080 | 0.052 | 0.000 | 0.690 | −0.001, 0.001 | 0.042 |
| Living situation b | 0.012 | 0.673 | −0.042, 0.066 | 0.046 | −0.222 | 0.321 | −0.662, 0.219 | −0.104 | −0.001 | 0.579 | −0.006, 0.004 | −0.060 |
| Health-related QOL | 0.004 | 0.003 | 0.001, 0.006 | 0.307 | 0.029 | 0.006 | −0.006, 0.008 | 0.273 | 0.000 | 0.017 | 0.000, 0.000 | 0.248 |
| Comorbidities | 0.003 | 0.525 | −0.009, 0.015 | 0.054 | −0.011 | 0.817 | −0.105, 0.083 | −0.023 | 0.000 | 0.808 | −0.001, 0.001 | −0.025 |
| Subjective stress | ||||||||||||
| Major life events | 0.004 | 0.465 | −0.012, 0.020 | 0.044 | 0.168 | 0.012 | 0.038, 0.299 | 0.232 | 0.001 | 0.373 | −0.001, 0.002 | 0.084 |
| Perceived stress | 0.011 | 0.632 | −0.033, 0.054 | 0.053 | 0.045 | 0.801 | −0.311, 0.402 | 0.027 | −0.002 | 0.304 | −0.006, 0.002 | −0.115 |
| Coping strategies | ||||||||||||
| Proactive | 0.008 | 0.861 | −0.081, 0.097 | 0.021 | −0.595 | 0.108 | −10.323, 0.132 | −0.191 | −0.008 | 0.051 | −0.017, 0.000 | −0.242 |
| Strategic planning | 0.053 | 0.070 | −0.004, 0.111 | 0.215 | 0.011 | 0.963 | −0.461, 0.483 | 0.005 | 0.001 | 0.761 | −0.005, 0.006 | 0.036 |
| Reflexive | −0.090 | 0.093 | −0.194, 0.015 | −0.250 | −0.297 | 0.492 | −10.152, 0.557 | −0.099 | −0.004 | 0.386 | −0.014, 005 | −0.129 |
| Preventive | 0.039 | 0.331 | −0.040, 0.118 | 0.117 | 0.386 | 0.237 | −0.257, 10.029 | 0.139 | 0.005 | 0.190 | −0.002, 0.012 | 0.160 |
| Avoidance | −0.022 | 0.388 | −0.073, 0.029 | −0.089 | −0.398 | 0.060 | −10.901, 0.060 | −0.189 | −0.003 | 0.257 | −0.007, 0.002 | −0.118 |
| Social support | ||||||||||||
| Availability | −0.001 | 0.734 | −0.003, 0.002 | −0.038 | −0.009 | 0.453 | −0.034, 0.015 | −0.082 | 0.000 | 0.894 | 0.000, 0.000 | −0.015 |
| Satisfaction | 0.007 | 0.553 | −0.016, 0.029 | 0.060 | 0.055 | 0.547 | −0.126, 0.236 | 0.059 | 0.000 | 0.698 | −0.002, 0.002 | 0.039 |
| ESS | 0.055 | 0.064 | −0.003, 0.113 | 0.222 | 0.105 | 0.661 | −0.369, 0.580 | 0.051 | 0.003 | 0.272 | −0.002, 0.008 | 0.132 |
| ISS | −0.038 | 0.267 | −0.106, 0.030 | −0.147 | −0.115 | 0.682 | −0.669, 0.439 | −0.053 | −0.001 | 0.682 | −0.008, 0.005 | −0.055 |
| Variables | B | p | 95% CI | ß |
|---|---|---|---|---|
| CAR | ||||
| Health-related quality of life | 0.003 | 0.007 | 0.001, 0.005 | 0.229 |
| Emotional support seeking | 0.035 | 0.094 | −0.006, 0.076 | 0.141 |
| Avoidance coping | −0.035 | 0.093 | −0.077, 0.006 | −0.142 |
| AUC | ||||
| Medication use | −0.342 | 0.055 | −0.642, 0.008 | −0.163 |
| Age | 0.031 | 0.015 | 0.006, 0.056 | 0.208 |
| Health-related quality of life | 0.026 | 0.004 | 0.009, 0.044 | 0.246 |
| Major life event(s) | 0.164 | 0.008 | 0.043, 0.285 | 0.226 |
| Proactive coping | −0.526 | 0.051 | −10.055, 0.002 | −0.169 |
| Avoidance coping | −0.398 | 0.027 | −0.749, −0.047 | −0.189 |
| Peak-to-bed | ||||
| Medication use | −0.004 | 0.054 | −0.008, 0.000 | −0.162 |
| Health-related quality of life | 0.000 | 0.024 | 0.000, 0.000 | 0.193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richer, M.-J.; Grenier, S.; Plusquellec, P. Exploring Interindividual Variability in Resilience to Stress: Social Support, Coping Styles, and Diurnal Cortisol in Older Adults. Behav. Sci. 2025, 15, 631. https://doi.org/10.3390/bs15050631
Richer M-J, Grenier S, Plusquellec P. Exploring Interindividual Variability in Resilience to Stress: Social Support, Coping Styles, and Diurnal Cortisol in Older Adults. Behavioral Sciences. 2025; 15(5):631. https://doi.org/10.3390/bs15050631
Chicago/Turabian StyleRicher, Marie-Josée, Sébastien Grenier, and Pierrich Plusquellec. 2025. "Exploring Interindividual Variability in Resilience to Stress: Social Support, Coping Styles, and Diurnal Cortisol in Older Adults" Behavioral Sciences 15, no. 5: 631. https://doi.org/10.3390/bs15050631
APA StyleRicher, M.-J., Grenier, S., & Plusquellec, P. (2025). Exploring Interindividual Variability in Resilience to Stress: Social Support, Coping Styles, and Diurnal Cortisol in Older Adults. Behavioral Sciences, 15(5), 631. https://doi.org/10.3390/bs15050631





