Physical Activity, Cognitive Function, and Learning Processes: The Role of Environmental Context
Abstract
1. Introduction
1.1. Cognitive and Educational Implications of Physical Activity in Urban Environments
1.2. Research Rationale and Hypotheses
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Measures
2.4.1. Digit Span Test
2.4.2. Continuous Performance Test (CPT)
2.4.3. Stroop Test
2.4.4. Perceived Stress Scale (PSS)
2.4.5. Heart Rate Variability (HRV)
2.5. Physical Activity Intervention Program
- Weeks 1–4: Adaptation and Building Endurance
- Warm-Up (10 min):
- ○
- Brisk walking or light jogging: Performed for 5 min, followed by dynamic stretching (approximately 5 min) to prepare muscles and improve joint mobility.
- Aerobic Exercises (30 min):
- ○
- Brisk walking or light jogging: 15 min of brisk walking or light jogging.
- ○
- Stationary bike or treadmill for the control group, and equivalent walking or cycling activities performed outdoors in parks for the experimental group: 15 min of cycling or walking on a treadmill or cycle ergometer for the control group.
- Strength Exercises (15 min):
- ○
- Squats (3 sets of 12 repetitions).
- ○
- Lunges (3 sets of 12 repetitions per leg).
- ○
- Push-ups (3 sets of 10–15 repetitions).
- ○
- Planks (3 sets of 20 s each).
- Cool-Down (5 min):
- ○
- Static stretching to relax muscles and improve flexibility.
- Weeks 5–8: Increased Intensity and Greater Resistance Work
- Warm-Up (10 min):
- ○
- Light running or cycling: 5 min of light running or low-intensity cycling, followed by dynamic stretching.
- Aerobic Exercises (35 min):
- ○
- Moderate running (or jogging): 20 min of moderate running for the control group; the experimental group alternated between running or walking in various urban environments, such as parks or busy streets, to examine the impact of different environmental stimuli.
- ○
- Intermittent effort: 5 min of jogging alternating with 1 min of intense running, followed by 1 min of walking, for the control group.
- Strength Exercises (15 min):
- ○
- Bodyweight squats (4 sets of 12 repetitions).
- ○
- Lunges in motion (4 sets of 12 repetitions per leg).
- ○
- Push-ups (4 sets of 12 repetitions).
- ○
- Planks (4 sets of 30 s each).
- Cool-Down (5 min):
- ○
- Static stretching (5 min to improve flexibility and reduce the risk of injury).
- Weeks 9–12: Optimization and Enhancement
- Warm-Up (10 min):
- ○
- Moderate running or cycling with increasing intensity: 5 min of light running, followed by dynamic stretching (5 min) to prepare muscles and joints.
- Aerobic Exercises (40 min):
- ○
- Running at moderate to high intensity (control group)/alternating between parks and busy streets (experimental group): 20 min of progressively intense running.
- ○
- Circuit of aerobic and anaerobic exercises: 10 min of interval training, with 1 min of high-intensity running, followed by 1 min of walking or active rest.
- ○
- High-intensity functional exercise: 10 min of functional training, including exercises like mountain climbers, jumping jacks, and burpees.
- Strength Exercises (15 min):
- ○
- Squats with load (5 sets of 12 repetitions).
- ○
- Weighted lunges (5 sets of 12 repetitions per leg).
- ○
- Push-ups with variations (narrow or wide hand placement) (5 sets of 12 repetitions).
- ○
- Side planks (5 sets of 30 s per side).
- Cool-Down (5 min):
- ○
- Static stretching for the whole body to promote muscle relaxation and reduce the risk of injury.
2.6. Statistical Analysis
3. Results
3.1. Paired t-Test
3.2. ANOVA
3.3. Independent t-Test
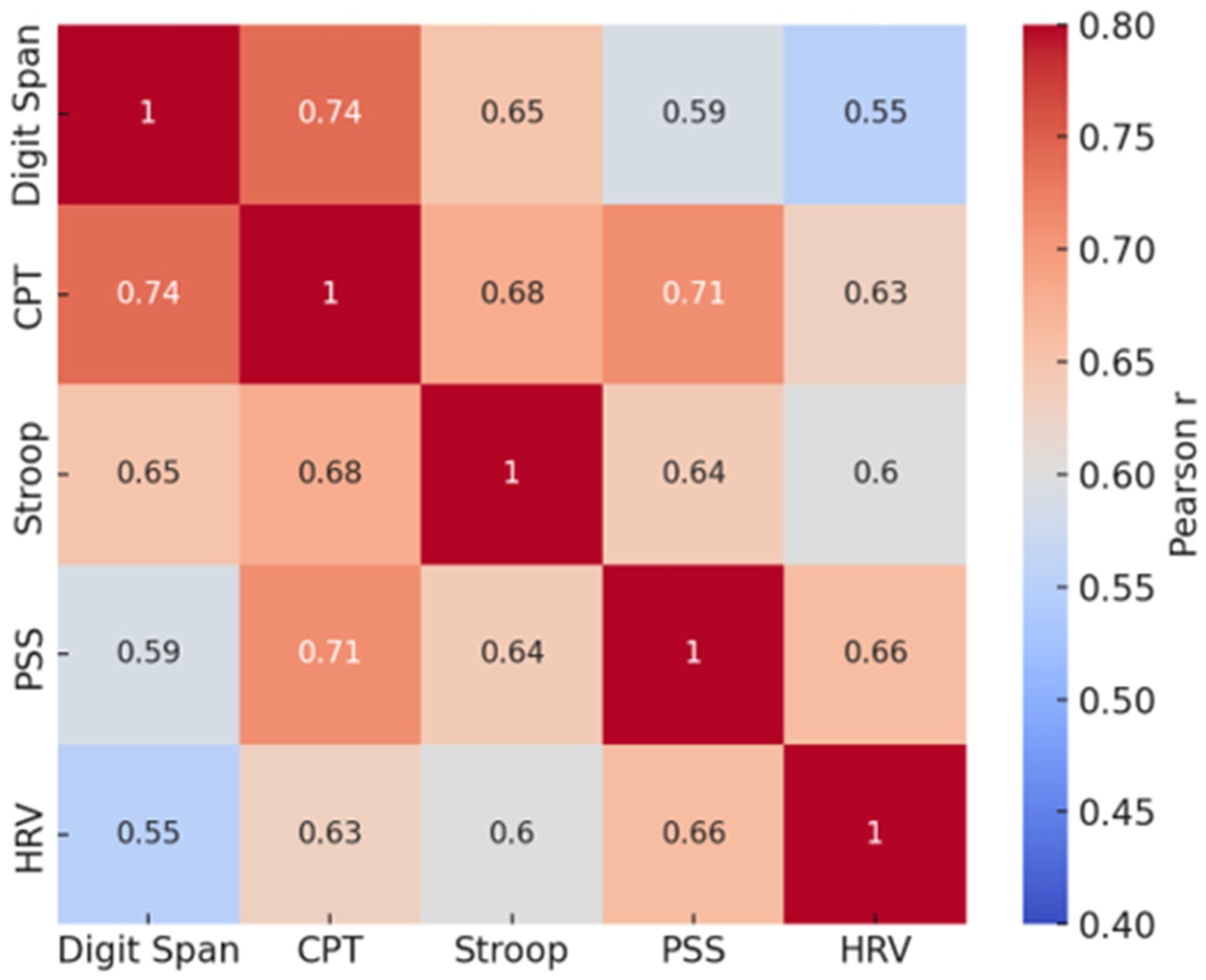
3.4. Pearson Correlations
4. Discussion
4.1. Cognitive, Psychological, and Physiological Outcomes
4.2. Limitations and Future Directions
4.3. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Álvarez-Bueno, C., Pesce, C., Cavero-Redondo, I., Sánchez-López, M., Martínez-Hortelano, J. A., & Martínez-Vizcaíno, V. (2017). The effect of physical activity interventions on children’s cognition and metacognition: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(9), 729–738. [Google Scholar] [CrossRef]
- Berman, M. G., Jonides, J., & Kaplan, S. (2008). The cognitive benefits of interacting with nature. Psychological Science, 19(12), 1207–1212. [Google Scholar] [CrossRef]
- Bettio, L. E. B., Thacker, J. S., Rodgers, S. P., Brocardo, P. S., Christie, B. R., & Gil-Mohapel, J. (2020). Interplay between hormones and exercise on hippocampal plasticity across the lifespan. Biochimica et Biophysica Acta. Molecular Basis of Disease, 1866(8), 165821. [Google Scholar] [CrossRef]
- Bhattacharya, P., Chatterjee, S., & Roy, D. (2023). Impact of exercise on brain neurochemicals: A comprehensive review. Sport Sciences for Health, 19(2), 405–452. [Google Scholar] [CrossRef]
- Bidzan-Bluma, I., & Lipowska, M. (2018). Physical activity and cognitive functioning of children: A systematic review. International Journal of Environmental Research and Public Health, 15(4), 800. [Google Scholar] [CrossRef] [PubMed]
- Byshevets, N., Andrieieva, O., Dutchak, M., Shynkaruk, O., Dmytriv, R., Zakharina, I., Serhiienko, K., & Hres, M. (2024). The influence of physical activity on stress-associated conditions in higher education students. Physical Education Theory and Methodology, 24(2), 245–253. [Google Scholar] [CrossRef]
- Canas, J. J., Fajardo, I., & Salmeron, L. (2006). Cognitive flexibility. International Encyclopedia of Ergonomics and Human Factors, 1(3), 297–301. [Google Scholar]
- Cañas, J., Quesada, J., Antolí, A., & Fajardo, I. (2003). Cognitive flexibility and adaptability to environmental changes in dynamic complex problem-solving tasks. Ergonomics, 46(5), 482–501. [Google Scholar] [CrossRef]
- Chandra, M., Rai, C. B., Kumari, N., Sandhu, V. K., Chandra, K., Krishna, M., Kota, S. H., Anand, K. S., & Oudin, A. (2022). Air pollution and cognitive impairment across the life course in humans: A systematic review with specific focus on income level of study area. International Journal of Environmental Research and Public Health, 19(3), 1405. [Google Scholar] [CrossRef]
- de Greeff, J. W., Bosker, R. J., Oosterlaan, J., Visscher, C., & Hartman, E. (2018). Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. Journal of Science and Medicine in Sport, 21(5), 501–507. [Google Scholar] [CrossRef]
- Devarajan, R., Prabhakaran, D., & Goenka, S. (2020). Built environment for physical activity—An urban barometer, surveillance, and monitoring. Obesity Reviews, 21(1), e12938. [Google Scholar] [CrossRef]
- Dhuli, K., Naureen, Z., Medori, M. C., Fioretti, F., Caruso, P., Perrone, M. A., Nodari, S., Manganotti, P., Xhufi, S., Bushati, M., Bozo, D., Connelly, S. T., Herbst, K. L., & Bertelli, M. (2022). Physical activity for health. Journal of Preventive Medicine and Hygiene, 63(2 Suppl. S3), E150–E159. [Google Scholar] [CrossRef]
- Di Palma, D., Tafuri, M. G., & Ogbondah, L. D. (2025). Sport as a tool for inclusion and sustainability in secondary school: A qualitative-quantitative analysis. Rivista di Studi sulla Sostenibilità, 10(1), 93–108. [Google Scholar] [CrossRef]
- Donnelly, J. E., Hillman, C. H., Castelli, D., Etnier, J. L., Lee, S., Tomporowski, P., Lambourne, K., & Szabo-Reed, A. N. (2016). Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Medicine and Science in Sports and Exercise, 48(6), 1197–1222. [Google Scholar] [CrossRef] [PubMed]
- Duzel, E., van Praag, H., & Sendtner, M. (2016). Can physical exercise in old age improve memory and hippocampal function? Brain, 139(3), 662–673. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K. I., Donofry, S. D., Sewell, K. R., Brown, B. M., & Stillman, C. M. (2022). Cognitive aging and the promise of physical activity. Annual Review of Clinical Psychology, 18(1), 417–442. [Google Scholar] [CrossRef]
- Estes, W. (2022). Handbook of learning and cognitive processes. Psychology Press. [Google Scholar]
- Fernández-Jiménez, C., Dumitrache, C. G., Rubio, L., & Ruiz-Montero, P. J. (2024). Self-perceptions of ageing and perceived health status: The mediating role of cognitive functioning and physical activity. Ageing & Society, 44(3), 622–641. [Google Scholar]
- Ferreira Vorkapic, C., Alves, H., Araujo, L., Joaquim Borba-Pinheiro, C., Coelho, R., Fonseca, E., Oliveira, A., & Dantas, E. H. (2021). Does physical activity improve cognition and academic performance in children? A systematic review of randomized controlled trials. Neuropsychobiology, 80(6), 454–482. [Google Scholar] [CrossRef]
- García-Hermoso, A., Ramírez-Vélez, R., Lubans, D. R., & Izquierdo, M. (2021). Effects of physical education interventions on cognition and academic performance outcomes in children and adolescents: A systematic review and meta-analysis. British Journal of Sports Medicine, 55(21), 1224–1232. [Google Scholar] [CrossRef]
- Gates, N., & Valenzuela, M. (2010). Cognitive exercise and its role in cognitive function in older adults. Current Psychiatry Reports, 12, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gavelin, H. M., Dong, C., Minkov, R., Bahar-Fuchs, A., Ellis, K. A., Lautenschlager, N. T., Mellow, M. L., Wade, A. T., E Smith, A., Finke, C., Krohn, S., & Lampit, A. (2021). Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Research Reviews, 66, 101232. [Google Scholar] [CrossRef]
- Gorman, S., Larcombe, A. N., & Christian, H. E. (2021). Exposomes and metabolic health through a physical activity lens: A narrative review. Journal of Endocrinology, 249(1), R25–R41. [Google Scholar] [CrossRef]
- Greco, G., Fischetti, F., Cataldi, S., & Latino, F. (2019). Effects of Shotokan Karate on resilience to bullying in adolescents. Journal of Human Sport and Exercise, 14(Proc. 4), S890–S899. [Google Scholar] [CrossRef]
- Hakun, J. G., Benson, L., Qiu, T., Elbich, D. B., Katz, M., Shaw, P. A., Sliwinski, M. J., & Mossavar-Rahmani, Y. (2025). Cognitive health benefits of everyday physical activity in a diverse sample of middle-aged adults. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 59(1), kaae059. [Google Scholar] [CrossRef]
- Hallal, P. C., Victora, C. G., Azevedo, M. R., & Wells, J. C. (2006). Adolescent physical activity and health: A systematic review. Sports Medicine, 36(12), 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Han, K. T. (2021). The effect of environmental factors and physical activity on emotions and attention while walking and jogging. Journal of Leisure Research, 52(5), 619–641. [Google Scholar] [CrossRef]
- Hannan, A. J. (2014). Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathology and Applied Neurobiology, 40(1), 13–25. [Google Scholar] [CrossRef] [PubMed]
- Harada, K., Lee, S., Park, H., Shimada, H., Makizako, H., Doi, T., Yoshida, D., Tsutsumimoto, K., Anan, Y., Uemura, K., & Suzuki, T. (2016). Going outdoors and cognitive function among community-dwelling older adults: Moderating role of physical function. Geriatrics & Gerontology International, 16(1), 65–73. [Google Scholar]
- Hillman, C. H., Erickson, K. I., & Kramer, A. F. (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience, 9(1), 58–65. [Google Scholar] [CrossRef] [PubMed]
- Hueston, C. M., Cryan, J. F., & Nolan, Y. M. (2017). Stress and adolescent hippocampal neurogenesis: Diet and exercise as cognitive modulators. Translational Psychiatry, 7(4), e1081. [Google Scholar] [CrossRef]
- Iso-Markku, P., Aaltonen, S., Kujala, U. M., Halme, H. L., Phipps, D., Knittle, K., Vuoksimaa, E., & Waller, K. (2024). Physical activity and cognitive decline among older adults: A systematic review and meta-analysis. JAMA Network Open, 7(2), e2354285. [Google Scholar] [CrossRef]
- Johnson, R. B., & Christensen, L. (2017). Educational research: Quantitative, qualitative, and mixed approaches. SAGE Publications. ISBN 978-1-4522-4440-2. [Google Scholar]
- Juneja, N. (2021). Education in urban areas. In The Routledge handbook of education in India (pp. 26–41). Routledge. [Google Scholar]
- Kashihara, K., Maruyama, T., Murota, M., & Nakahara, Y. (2009). Positive effects of acute and moderate physical exercise on cognitive function. Journal of Physiological Anthropology, 28(4), 155–164. [Google Scholar] [CrossRef]
- Kekäläinen, T., Luchetti, M., Terracciano, A., Gamaldo, A. A., Mogle, J., Lovett, H. H., Brown, J., Rantalainen, T., Sliwinski, M. J., & Sutin, A. R. (2023). Physical activity and cognitive function: Moment-to-moment and day-to-day associations. The International Journal of Behavioral Nutrition and Physical Activity, 20(1), 137. [Google Scholar] [CrossRef]
- Kestens, K., Degeest, S., & Miatton, M. (2021). An auditory Stroop test to implement in cognitive hearing sciences: Development and normative data. International Journal of Psychological Research, 14(2), 37–51. [Google Scholar] [CrossRef]
- Kim, T. W., & Sung, Y. H. (2017). Regular exercise promotes memory function and enhances hippocampal neuroplasticity in experimental autoimmune encephalomyelitis mice. Neuroscience, 346, 173–181. [Google Scholar] [CrossRef]
- Kleinloog, J. P., Nijssen, K. M., Mensink, R. P., & Joris, P. J. (2022). Effects of physical exercise training on cerebral blood flow measurements: A systematic review of human intervention studies. International Journal of Sport Nutrition and Exercise Metabolism, 33(1), 47–59. [Google Scholar] [CrossRef]
- Latino, F., Cataldi, S., & Fischetti, F. (2021). Effects of an 8-week yoga-based physical exercise intervention on teachers’ burnout. Sustainability, 13(4), 2104. [Google Scholar] [CrossRef]
- Latomme, J., Calders, P., Van Waelvelde, H., Mariën, T., & De Craemer, M. (2022). The role of brain-derived neurotrophic factor (BDNF) in the relation between physical activity and executive functioning in children. Children, 9(5), 596. [Google Scholar] [CrossRef]
- Lee, J., An, D., Singnoy, C., & Kim, Y. (2024). Psychosocial and environmental correlates of physical activity in Korean adults. Perceptual and Motor Skills, 131(2), 537–550. [Google Scholar] [CrossRef] [PubMed]
- Li, A., Yau, S. Y., Machado, S., Wang, P., Yuan, T. F., & So, K. F. (2019). Enhancement of hippocampal plasticity by physical exercise as a polypill for stress and depression: A review. CNS & Neurological Disorders Drug Targets, 18(4), 294–306. [Google Scholar] [CrossRef]
- Li, X., & Li, C. (2025). Promoting healthy aging: Physical activity and its dual effects on physical health and cognitive function in Chinese older adults. Frontiers in Public Health, 13, 1561060. [Google Scholar] [CrossRef]
- Liang, J., Wang, H., Zeng, Y., Qu, Y., Liu, Q., Zhao, F., Duan, J., Jiang, Y., Li, S., Ying, J., Li, J., & Mu, D. (2021). Physical exercise promotes brain remodeling by regulating epigenetics, neuroplasticity and neurotrophins. Reviews in the Neurosciences, 32(6), 615–629. [Google Scholar] [CrossRef]
- Manohar, S., Chen, G. D., Ding, D., Liu, L., Wang, J., Chen, Y. C., Chen, L., & Salvi, R. (2022). Unexpected consequences of noise-induced hearing loss: Impaired hippocampal neurogenesis, memory, and stress. Frontiers in Integrative Neuroscience, 16, 871223. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, T., Vlaar, J., Naylor, P. J., Hanning, R. M., Le Mare, L., & Mâsse, L. C. (2020). Individual and environmental factors associated with participation in physical activity as adolescents transition to secondary school: A qualitative inquiry. International Journal of Environmental Research and Public Health, 17(20), 7646. [Google Scholar] [CrossRef]
- Mitchell, R. (2013). Is physical activity in natural environments better for mental health than physical activity in other environments? Social Science & Medicine, 91, 130–134. [Google Scholar] [CrossRef]
- Moore, T. M., Visoki, E., Argabright, S. T., Didomenico, G. E., Sotelo, I., Wortzel, J. D., Naeem, A., Gur, R. C., E Gur, R., Warrier, V., Guloksuz, S., & Barzilay, R. (2022). Modeling environment through a general exposome factor in two independent adolescent cohorts. Exposome, 2(1), osac010. [Google Scholar] [CrossRef]
- Morsanuto, S., Peluso Cassese, F., Tafuri, F., & Tafuri, D. (2023). Outdoor education, integrated soccer activities, and learning in children with autism spectrum disorder: A project aimed at achieving the sustainable development goals of the 2030 agenda. Sustainability, 15(18), 13456. [Google Scholar] [CrossRef]
- Nakagawa, T., Koan, I., Chen, C., Matsubara, T., Hagiwara, K., Lei, H., Hirotsu, M., Yamagata, H., & Nakagawa, S. (2020). Regular moderate-to vigorous-intensity physical activity rather than walking is associated with enhanced cognitive functions and mental health in young adults. International Journal of Environmental Research and Public Health, 17(2), 614. [Google Scholar] [CrossRef] [PubMed]
- Nurzynska, D., Di Meglio, F., Romano, V., Miraglia, R., Sacco, A. M., Latino, F., Bancone, C., Della Corte, A., Maiello, C., Amarelli, C., Montagnani, S., & Castaldo, C. (2013). Cardiac primitive cells become committed to a cardiac fate in adult human heart with chronic ischemic disease but fail to acquire mature phenotype: Genetic and phenotypic study. Basic Research in Cardiology, 108(1), 320. [Google Scholar] [CrossRef] [PubMed]
- Pesce, C., Ballester, R., & Benzing, V. (2021). Giving physical activity and cognition research ‘some soul’: Focus on children and adolescents. European Journal of Human Movement, 47, 1–7. [Google Scholar]
- Pfledderer, C. D., Burns, R. D., Byun, W., Carson, R. L., Welk, G. J., & Brusseau, T. A. (2021). School-based physical activity interventions in rural and urban/suburban communities: A systematic review and meta-analysis. Obesity Reviews, 22(9), e13265. [Google Scholar] [CrossRef]
- Pham, T., Lau, Z. J., Chen, S. A., & Makowski, D. (2021). Heart rate variability in psychology: A review of HRV indices and an analysis tutorial. Sensors, 21(12), 3998. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. A., Brown, D. J., Shopland, N., Burton, A., & Mahmud, M. (2022). Explainable multimodal machine learning for engagement analysis by continuous performance test. In International conference on human-computer interaction (pp. 386–399). Springer International Publishing. [Google Scholar]
- Robinson, H., Dave, N., Barzilay, R., Wagner, A., Kells, N., & Keller, A. S. (2025). The effect of the “exposome” on developmental brain health and cognitive outcomes. Neuropsychopharmacology, 51, 169–184. [Google Scholar] [CrossRef]
- Sallis, J. F., Cerin, E., Conway, T. L., Adams, M. A., Frank, L. D., Pratt, M., Salvo, D., Schipperijn, J., Smith, G., Cain, K. L., Davey, R., Kerr, J., Lai, P., Mitáš, J., Reis, R., Sarmiento, O. L., Schofield, G., Troelsen, J., Van Dyck, D., … Owen, N. (2016). Physical activity in relation to urban environments in 14 cities worldwide: A cross-sectional study. The Lancet, 387(10034), 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Scamardella, F., Casillo, V., & Cusano, P. (2020a). Engagement and tennis: The applicability of occupational psychology to the world of sport. Journal of Human Sport and Exercise, 15(Proc2), 173–176. [Google Scholar]
- Scamardella, F., Russo, N., & Napolitano, F. (2020b). The phenomenon of load management. Journal of Physical Education and Sport, 20, 2306–2309. [Google Scholar]
- Sewell, K. R., Erickson, K. I., Rainey-Smith, S. R., Peiffer, J. J., Sohrabi, H. R., & Brown, B. M. (2021). Relationships between physical activity, sleep and cognitive function: A narrative review. Neuroscience and Biobehavioral Reviews, 130, 369–378. [Google Scholar] [CrossRef]
- Sibbick, E., Boat, R., Sarkar, M., Groom, M., & Cooper, S. B. (2022). Acute effects of physical activity on cognitive function in children and adolescents with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Mental Health and Physical Activity, 23, 100469. [Google Scholar] [CrossRef]
- Singh, B., Bennett, H., Miatke, A., Dumuid, D., Curtis, R., Ferguson, T., Brinsley, J., Szeto, K., Petersen, J. M., Gough, C., Eglitis, E., Simpson, C. E., Ekegren, C. L., Smith, A. E., Erickson, K. I., & Maher, C. (2025). Effectiveness of exercise for improving cognition, memory and executive function: A systematic umbrella review and meta-meta-analysis. British Journal of Sports Medicine, 59(12), 866–876. [Google Scholar] [CrossRef]
- Srinivas, N. S., Vimalan, V., Padmanabhan, P., & Gulyás, B. (2021). An overview on cognitive function enhancement through physical exercises. Brain Sciences, 11(10), 1289. [Google Scholar] [CrossRef]
- Strehli, I., Burns, R. D., Bai, Y., Ziegenfuss, D. H., Block, M. E., & Brusseau, T. A. (2021). Mind–body physical activity interventions and stress-related physiological markers in educational settings: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health, 18(1), 224. [Google Scholar] [CrossRef]
- Tafuri, F., & Latino, F. (2024). School medical service: Strategies to promote psycho-physiological well-being. Pediatric Reports, 16(1), 214–231. [Google Scholar] [CrossRef]
- Tao, B., Lu, T., Chen, H., & Yan, J. (2025). The effects of moderate-to high-intensity physical exercise on emotion regulation and subsequent cognitive control in highly psychologically stressed college students. Healthcare, 13(17), 2100. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M., Leyhr, D., & Sudeck, G. (2024). Physical activity improves stress load, recovery, and academic performance-related parameters among university students: A longitudinal study on daily level. BMC Public Health, 24(1), 598. [Google Scholar] [CrossRef] [PubMed]
- Tian, S., Liang, Z., Qiu, F., & Wang, X. (2023). Physical activity on executive function in sedentary individuals: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE, 18(12), e0294251. [Google Scholar] [CrossRef]
- Vella-Brodrick, D. A., & Gilowska, K. (2022). Effects of nature (greenspace) on cognitive functioning in school children and adolescents: A systematic review. Educational Psychology Review, 34(3), 1217–1254. [Google Scholar] [CrossRef]
- Vineis, P., Robinson, O., Chadeau-Hyam, M., Dehghan, A., Mudway, I., & Dagnino, S. (2020). What is new in the exposome? Environment International, 143, 105887. [Google Scholar] [CrossRef]
- Vrijheid, M. (2014). The exposome: A new paradigm to study the impact of environment on health. Thorax, 69(9), 876–878. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E. I., Smith, L., Northey, J., Rattray, B., & Cherbuin, N. (2020). Towards an understanding of the physical activity-BDNF-cognition triumvirate: A review of associations and dosage. Ageing Research Reviews, 60, 101044. [Google Scholar] [CrossRef]
- Walters, G., Dring, K. J., Williams, R. A., Needham, R., & Cooper, S. B. (2025). Outdoor physical activity is more beneficial than indoor physical activity for cognition in young people. Physiology & Behavior, 295, 114888. [Google Scholar] [CrossRef]
- Wang, H., Yang, Y., Xu, J., Niu, L., Zhang, Y., Shi, J., & Shen, L. (2023). Meta-analysis on the effects of moderate-intensity exercise intervention on executive functioning in children. PLoS ONE, 18(2), e0279846. [Google Scholar] [CrossRef]
- Wang, Z., Aaltonen, S., Teeuwen, R., Milias, V., Peuters, C., Raimbault, B., Palviainen, T., Lumpe, E., Dick, D., Salvatore, J. E., Foraster, M., Dadvand, P., Júlvez, J., Psyllidis, A., van Kamp, I., & Kaprio, J. (2025). The urban physical environment and leisure-time physical activity in early midlife: A FinnTwin12 study. Health & Place, 94, 103495. [Google Scholar] [CrossRef]
- Warburton, D. E. R., & Bredin, S. S. D. (2017). Health benefits of physical activity: A systematic review of current systematic reviews. Current Opinion in Cardiology, 32(5), 541–556. [Google Scholar] [CrossRef]
- Wild, C. P. (2005). Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiology Biomarkers & Prevention, 14(8), 1847–1850. [Google Scholar]
- Woods, D. L., Kishiyama, M. M., Yund, E. W., Herron, T. J., Edwards, B., Poliva, O., Hink, R. F., & Reed, B. (2011). Improving digit span assessment of short-term verbal memory. Journal of Clinical and Experimental Neuropsychology, 33(1), 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z., Hotterbeex, P., Marent, P. J., Cerin, E., Thomis, M., & van Uffelen, J. (2024). Physical activity, sedentary behaviour, and cognitive function among older adults: A bibliometric analysis from 2004 to 2024. Ageing Research Reviews, 97, 102283. [Google Scholar] [CrossRef] [PubMed]
- Yao, W., Zhang, X., & Gong, Q. (2021). The effect of exposure to the natural environment on stress reduction: A meta-analysis. Urban Forestry & Urban Greening, 57, 126932. [Google Scholar] [CrossRef]
- Yeh, H. P., Stone, J. A., Churchill, S. M., Wheat, J. S., Brymer, E., & Davids, K. (2016). Physical, psychological and emotional benefits of green physical activity: An ecological dynamics perspective. Sports Medicine, 46(7), 947–953. [Google Scholar] [CrossRef]
- Yılmaz Koğar, E., & Koğar, H. (2024). A systematic review and meta-analytic confirmatory factor analysis of the perceived stress scale (PSS-10 and PSS-14). Stress and Health, 40(1), e3285. [Google Scholar] [CrossRef]
- Zhao, Y. L., Hao, Y. N., Ge, Y. J., Zhang, Y., Huang, L. Y., Fu, Y., Zhang, D. D., Ou, Y. N., Cao, X. P., Feng, J. F., Cheng, W., Tan, L., & Yu, J. T. (2025). Variables associated with cognitive function: An exposome-wide and mendelian randomization analysis. Alzheimer’s Research & Therapy, 17(1), 13. [Google Scholar]
- Zijlema, W. L., Triguero-Mas, M., Smith, G., Cirach, M., Martinez, D., Dadvand, P., Gascon, M., Jones, M., Gidlow, C., Hurst, G., Masterson, D., Ellis, N., Berg, M. v. D., Maas, J., van Kamp, I., Hazel, P. v. D., Kruize, H., Nieuwenhuijsen, M. J., & Julvez, J. (2017). The relationship between natural outdoor environments and cognitive functioning and its mediators. Environmental Research, 155, 268–275. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Experimental Group (30 Participants) | Control Group (30 Participants) |
|---|---|---|
| Number of participants | 30 | 30 |
| Age (mean ± SD) | 16.5 ± 5.2 years | 16.3 ± 4.8 years |
| Gender (male/female) | 15 males, 15 females | 14 males, 16 females |
| Health status | Good health, no severe medical conditions | Good health, no severe medical conditions |
| Physical activity (weekly frequency) | 2–3 sessions of moderate physical activity weekly | 2–3 sessions of moderate physical activity weekly |
| Exclusion criteria | Neurological/psychiatric disorders, uncontrolled cardiovascular diseases | Neurological/psychiatric disorders, uncontrolled cardiovascular diseases |
| Type of exercise | Physical activity in variable urban environments (parks, busy streets, covered urban courtyard) | Physical activity in a controlled environment (indoor gym) |
| Variable | EG Pre (Mean ± SD) | EG Post (Mean ± SD) | t-Value (EG) | p-Value (EG) | CG Pre (Mean ± SD) | CG Post (Mean ± SD) | t-Value (CG) | p-Value (CG) | Post-Intervention Comparison (EG vs. CG) t-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Digit Span Test | 15 ± 2.6 | 18 ± 2.5 | 5.23 | 0.00002 | 15 ± 2.3 | 16 ± 2.0 | 1.45 | 0.175 | 3.18 | 0.003 |
| CPT | 43 ± 5.2 | 48 ± 5.5 | 4.75 | 0.0001 | 42 ± 5.0 | 44 ± 4.8 | 1.12 | 0.265 | 2.52 | 0.014 |
| Stroop Test | 18 ± 3.0 | 23 ± 3.1 | 6.12 | 0.00003 | 19 ± 2.8 | 21 ± 2.7 | 1.30 | 0.210 | 2.14 | 0.037 |
| PSS | 21 ± 2.1 | 16 ± 2.0 | −7.35 | 0.00001 | 19 ± 2.4 | 18 ± 2.3 | −0.85 | 0.402 | −3.62 | 0.001 |
| HRV | 34 ± 4.9 | 40 ± 5.0 | 4.90 | 0.00005 | 35 ± 4.5 | 36 ± 4.2 | 0.78 | 0.460 | 2.52 | 0.014 |
| Variable | F-Value | p-Value | Significant Change |
|---|---|---|---|
| Digit Span Test (Memory) | 8.34 | <0.05 | Yes |
| CPT (Attention) | 14.52 | <0.05 | Yes |
| Stroop Test (Flexibility) | 12.56 | <0.05 | Yes |
| PSS (Stress) | 6.12 | <0.05 | Yes |
| HRV (Heart Rate Variability) | 9.47 | <0.05 | Yes |
| Variable | t-Value | p-Value | Significant Change |
|---|---|---|---|
| Digit Span Test (Memory) | 4.32 | <0.05 | Yes |
| CPT (Attention) | 6.25 | <0.05 | Yes |
| Stroop Test (Flexibility) | 5.76 | <0.05 | Yes |
| PSS (Stress) | −5.67 | <0.05 | Yes |
| HRV (Heart Rate Variability) | 4.56 | <0.05 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latino, F.; Tafuri, G.; Amato, G.; Romano, G. Physical Activity, Cognitive Function, and Learning Processes: The Role of Environmental Context. Behav. Sci. 2025, 15, 1630. https://doi.org/10.3390/bs15121630
Latino F, Tafuri G, Amato G, Romano G. Physical Activity, Cognitive Function, and Learning Processes: The Role of Environmental Context. Behavioral Sciences. 2025; 15(12):1630. https://doi.org/10.3390/bs15121630
Chicago/Turabian StyleLatino, Francesca, Giovanni Tafuri, Giulia Amato, and Generoso Romano. 2025. "Physical Activity, Cognitive Function, and Learning Processes: The Role of Environmental Context" Behavioral Sciences 15, no. 12: 1630. https://doi.org/10.3390/bs15121630
APA StyleLatino, F., Tafuri, G., Amato, G., & Romano, G. (2025). Physical Activity, Cognitive Function, and Learning Processes: The Role of Environmental Context. Behavioral Sciences, 15(12), 1630. https://doi.org/10.3390/bs15121630





