Takotsubo Syndrome or Peripartum Cardiomyopathy? Depends on Who You Are Talking to
Abstract
1. Introduction
1.1. Peripartum Cardiomyopathy
1.2. Takotsubo Syndrome
- “Transient hypokinesis, akinesis, or dyskinesis of the left ventricular mid-segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial coronary distribution; a stressful trigger is often, but not always present.
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
- New electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin.
- Absence of:
- Phaeochromocytoma
- Myocarditis”
- “Patients show transient left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal TTS).
- An emotional, physical, or combined trigger can precede the takotsubo syndrome event, but this is not obligatory.
- Neurologic disorders (e.g., subarachnoid haemorrhage, stroke/transient ischaemic attack, or seizures) as well as pheochromocytoma may serve as triggers for takotsubo syndrome.
- New ECG abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes.
- Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common.
- Significant coronary artery disease is not a contradiction in takotsubo syndrome.
- Patients have no evidence of infectious myocarditis.
- Postmenopausal women are predominantly affected.” [14]
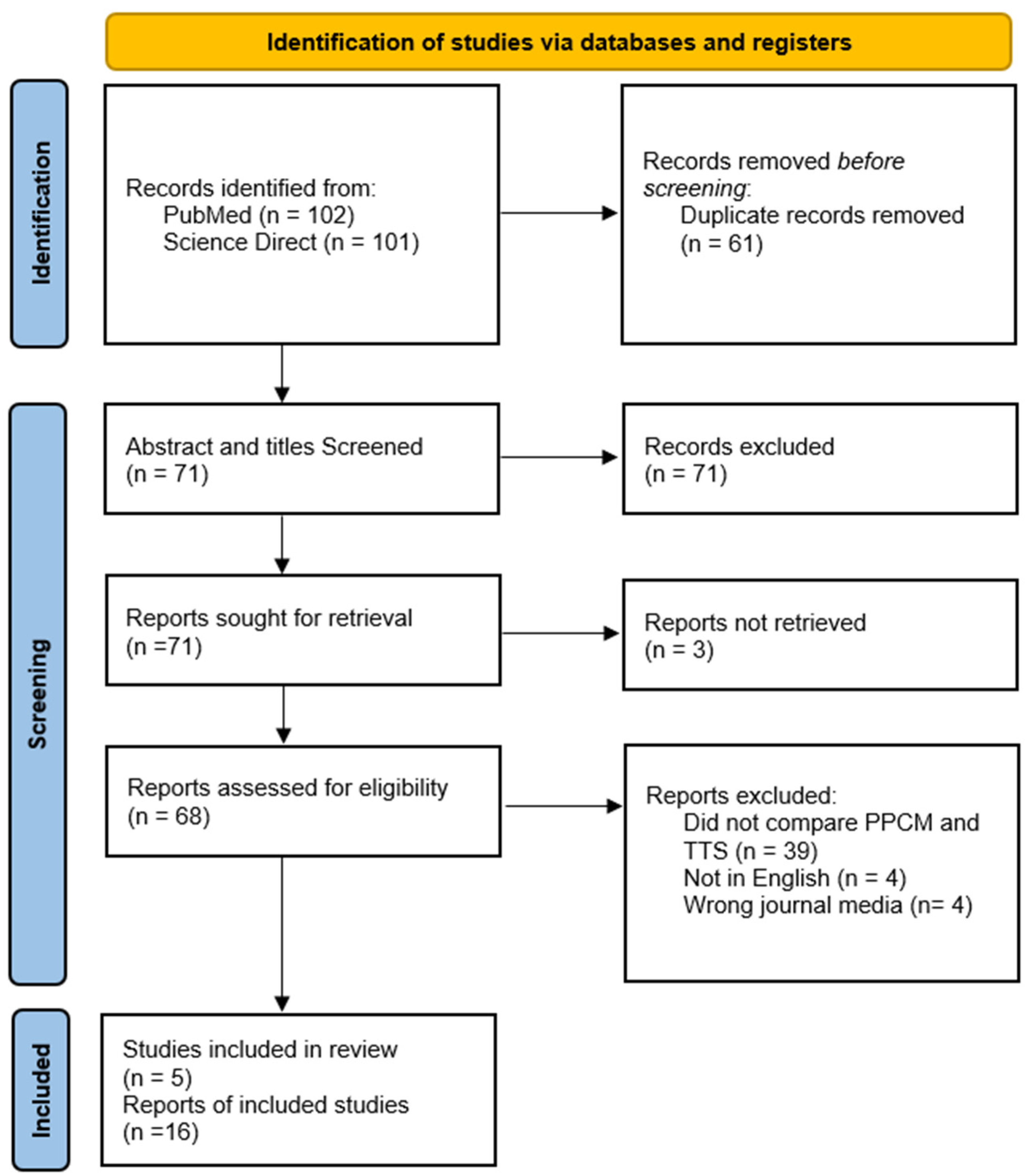
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Search Strategy
3. Results
4. Discussion
Is This All One Syndrome?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayan, B.; Nelson-Piercy, C. Medical problems in pregnancy. Clin. Med. 2017, 17, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Thorne, I.; Nelson-Piercy, C.; Knight, M. Recognised training routes are needed to sustain new maternal medicine networks. Clin. Med. 2018, 18, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Bunch, K.; Tuffnell, D.; Jayakody, H.; Shakespeare, J.; Kotnis, R.; Kenyon, S.; Kurinczuk, J. (Eds.) Saving Lives, Improving Mothers’ Care—Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014–2016; National Perinatal Epidemiology Unit: University of Oxford: Oxford, UK, 2018; Available online: https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202018%20-%20Web%20Version.pdf (accessed on 3 July 2024).
- Jakes, A.D.; Watt-Coote, I.; Coleman, M.; Nelson-Piercy, C. Obstetric medical care and training in the United Kingdom. Obstet. Med. 2017, 10, 40–42. [Google Scholar] [CrossRef]
- NHS England. Maternal Medicine Networks: Service Specification; NHS Englang: Leeds, UK, 14 October 2021. [Google Scholar]
- Demakis, J.G.; Rahimtoola, S.H.; Sutton, G.C.; Meadows, W.R.; Szanto, P.B.; Tobin, J.R.; Gunnar, R.M. Natural Course of Peripartum Cardiomyopathy. Circulation 1971, 44, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- Bhandary, A.; Rambhatla, T.; Coplan, N.; Kronzon, I. Peripartum cardiomyopathy: A contemporary review. J. Clin. Prev. Cardiol. 2018, 7, 54. [Google Scholar] [CrossRef]
- Itoh, T.; Toda, N.; Yoshizawa, M.; Osaki, T.; Maegawa, Y.; Yoshizawa, R.; Ishikawa, Y.; Nishiyama, O.; Nakajima, S.; Nakamura, M.; et al. Impact of the Great East Japan Earthquake and Tsunami on the Incidence of Takotsubo Syndrome Using a Multicenter, Long-Term Regional Registry. Circ. J. 2021, 85, 1834–1839. [Google Scholar] [CrossRef]
- Sato, M.; Fujita, S.; Saito, A.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Ishiguro, J.; Okabe, M.; Nakamura, Y.; Nagai, T.; et al. Increased Incidence of Transient Left Ventricular Apical Ballooning (So-Called Takotsubo’ Cardiomyopathy) After the Mid-Niigata Prefecture Earthquake. Circ. J. 2006, 70, 947–953. [Google Scholar] [CrossRef]
- Komamura, K. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J. Cardiol. 2014, 6, 602. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Scantlebury, D.C.; Prasad, A. Diagnosis of Takotsubo Cardiomyopathy. Circ. J. 2014, 78, 2129–2139. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheppard, M.N. Takotsubo Syndrome—Stress-induced Heart Failure Syndrome. Eur. Cardiol. 2015, 10, 83–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brenner, R.; Weilenmann, D.; Maeder, M.T.; Jörg, L.; Bluzaite, I.; Rickli, H.; De Pasquale, G.; Ammann, P. Clinical Characteristics, Sex Hormones, and Long-Term Follow-Up in Swiss Postmenopausal Women Presenting with Takotsubo Cardiomyopathy. Clin. Cardiol. 2012, 35, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kim, S.R.; Park, S.-J.; Seo, J.-H.; Kim, E.K.; Yang, J.H.; Chang, S.-A.; Choi, J.-O.; Lee, S.-C.; Park, S.W. Clinical characteristics and long-term outcomes of peripartum takotsubo cardiomyopathy and peripartum cardiomyopathy. ESC Heart Fail. 2020, 7, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features Outcomes Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef]
- Jackson, A.M.; Macartney, M.; Brooksbank, K.; Brown, C.; Dawson, D.; Francis, M.; Japp, A.; Lennie, V.; Leslie, S.J.; Martin, T.; et al. A 20-year population study of peripartum cardiomyopathy. Eur. Heart J. 2023, 44, 5128–5141. [Google Scholar] [CrossRef]
- Koziol, K.J.; Aronow, W.S.; Giuliani, E.; Nunziata, A. Peripartum cardiomyopathy: Current understanding of pathophysiology, diagnostic workup, management, and outcomes. Curr. Probl. Cardiol. 2023, 48, 101716. [Google Scholar] [CrossRef]
- Assad, J.; Femia, G.; Pender, P.; Badie, T.; Rajaratnam, R. Takotsubo Syndrome: A Review of Presentation, Diagnosis and Management. Clin. Med. Insights Cardiol. 2022, 16, 11795468211065782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A.; Ravi, R.; Sivakumar, R.K.; Chidambaram, V.; Majella, M.G.; Sinha, S.; Adamo, L.; Lau, E.S.; Al’Aref, S.J.; Asnani, A.; et al. Prolactin Inhibition in Peripartum Cardiomyopathy: Systematic Review and Meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101461. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; De Innocentiis, C.; Verrengia, E.; Ceriello, L.; Mantini, C.; Pietrangelo, C.; Irsuti, F.; Gabriele, S.; D’Alleva, A.; Khanji, M.Y.; et al. The Role of Multimodality Cardiovascular Imaging in Peripartum Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 4. [Google Scholar] [CrossRef]
- Izumo, M.; Akashi, Y.J. Role of echocardiography for takotsubo cardiomyopathy: Clinical and prognostic implications. Cardiovasc. Diagn. Ther. 2018, 8, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.C.; Elkayam, U.; Rajagopalan, N.; Modi, K.; Briller, J.E.; Drazner, M.H.; Wells, G.L.; McManara, D.M. Electrocardiographic findings in peripartum cardiomyopathy. Clin. Cardiol. 2019, 42, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, L.; Butt, N.; Ahmad, S.A.; Kayani, W.T.; Sangong, A.; Patel, V.; Khalid, N. Electrocardiographic changes in Takotsubo cardiomyopathy. J. Electrocardiology 2021, 65, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Röntgen, P.; Vogel-Claussen, J.; Schwab, J.; Westenfeld, R.; Ehlermann, P.; Berliner, D.; Podewski, E.; Hilfiker-Kleiner, D.; Bauersachs, J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: A cardiovascular magnetic resonance study. ESC Heart Fail. 2015, 2, 139–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isaak, A.; Ayub, T.H.; Merz, W.M.; Faron, A.; Endler, C.; Sprinkart, A.M.; Pieper, C.C.; Kuetting, D.; Dabir, D.; Attenberger, U.; et al. Peripartum Cardiomyopathy: Diagnostic and Prognostic Value of Cardiac Magnetic Resonance in the Acute Stage. Diagnostics 2022, 12, 378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schelbert, E.B.; Elkayam, U.; Cooper, L.T.; Givertz, M.M.; Alexis, J.D.; Briller, J.; Felker, G.M.; Chaparro, S.; Kealey, A.; Pisarcik, J.; et al. Myocardial Damage Detected by Late Gadolinium Enhancement Cardiac Magnetic Resonance Is Uncommon in Peripartum Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e005472. [Google Scholar] [CrossRef]
- Arora, N.P.; Mahajan, N.; Mohamad, T.; Kottam, A.; Afonso, L.C.; Danrad, R.; Li, T. Cardiac Magnetic Resonance Imaging in Peripartum Cardiomyopathy. Am. J. Med. Sci. 2014, 347, 112–117. [Google Scholar] [CrossRef]
- Fernández-Pérez, G.C.; Aguilar-Arjona, J.A.; de la Fuente, G.T.; Samartín, M.; Ghioldi, A.; Arias, J.C.; Sánchez-González, J. Takotsubo Cardiomyopathy: Assessment with Cardiac MRI. Am. J. Roentgenol. 2010, 195, W139–W145. [Google Scholar] [CrossRef] [PubMed]
- Matsisushita, K.; Lachmet-Thébaud, L.; Marchandot, B.; Trimaille, A.; Sato, C.; Dagrenat, C.; Greciano, S.; De Poli, F.; Leddet, P.; Peillex, M.; et al. Incomplete Recovery from Takotsubo Syndrome Is a Major Determinant of Cardiovascular Mortality. Circ. J. 2021, 85, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Fett, J.D.; Sannon, H.; Thélisma, E.; Sprunger, T.; Suresh, V. Recovery from severe heart failure following peripartum cardiomyopathy. Int. J. Gynecol. Obstet. 2009, 104, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Komiyama, T.; Kobayashi, H.; Ikari, Y. Gender Differences in Takotsubo Syndrome. Biology 2022, 11, 653. [Google Scholar] [CrossRef]
- Kim, J.I.; Yerasi, C.; Azzouqa, A.; Koiffman, E.; Weissman, G.; Wang, Z.; Moran, J.; Torguson, R.; Satler, L.F.; Pichard, A.D.; et al. Patient characteristics in variable left ventricular recovery from Takotsubo syndrome. Cardiovasc. Revascularization Med. 2018, 19, 247–250. [Google Scholar] [CrossRef]
- Jurisic, S.; Gili, S.; Cammann, V.L.; Kato, K.; Szawan, K.A.; D’Ascenzo, F.; Jaguszewski, M.; Bossone, E.; Citro, R.; Sarcon, A.; et al. Clinical Predictors and Prognostic Impact of Recovery of Wall Motion Abnormalities in Takotsubo Syndrome: Results from the International Takotsubo Registry. J. Am. Heart Assoc. 2019, 8, e011194. [Google Scholar] [CrossRef]
- Singh, K.; Carson, K.; Shah, R.; Sawhney, G.; Singh, B.; Parsaik, A.; Gilutz, H.; Usmani, Z.; Horowitz, J. Meta-Analysis of Clinical Correlates of Acute Mortality in Takotsubo Cardiomyopathy. Am. J. Cardiol. 2014, 113, 1420–1428. [Google Scholar] [CrossRef]
- Horowitz, J.D.; Nguyen, T.H. Differentiating Tako-Tsubo cardiomyopathy from myocardial infarction. Eur. Soc. Cardiol. 2014, 13, N7. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yang, W.I.; Moon, J.Y.; Shim, M.; Yang, P.S.; Kang, S.H.; Kim, S.H.; Kim, W.J.; Sung, J.H.; Kim, I.J.; Lim, S.W.; et al. Clinical features differentiating Takotsubo cardiomyopathy in the peripartum period from peripartum cardiomyopathy. Heart Vessel. 2020, 35, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Oindi, F.M.; Sequeira, E.; Sequeira, H.R.; Mutiso, S.K. Takotsubo cardiomyopathy in pregnancy: A case report and literature review. BMC Pregnancy Childbirth 2019, 19, 89. [Google Scholar] [CrossRef]
- Karaye, K.M.; Ishaq, N.A.; Sa’idu, H.; Balarabe, S.A.; Talle, M.A.; Isa, M.S.; Adamu, U.G.; Umar, H.; Okolie, H.I.; Shehu, M.N.; et al. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: Results from the PEACE Registry. ESC Heart Fail. 2020, 7, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Petrie, M.C.; van der Meer, P.; Mebazaa, A.; Hilfiker-Kleiner, D.; Jackson, A.M.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: An ESC EORP registry. Eur. Heart J. 2020, 41, 3787–3797. [Google Scholar] [CrossRef]
- Christley, Y.; Duffy, T.; Everall, I.P.; Martin, C.R. The Neuropsychiatric and Neuropsychological Features of Chronic Fatigue Syndrome: Revisiting the Enigma. Curr. Psychiatry Rep. 2013, 15, 353. [Google Scholar] [CrossRef] [PubMed]
- Mckay, P.G.; Walker, H.; Martin, C.R.; Fleming, M. Exploratory study into the relationship between the symptoms of chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME) and fibromyalgia (FM) using a quasiexperimental design. BMJ Open 2021, 11, e041947. [Google Scholar] [CrossRef] [PubMed]
| Peripartum Cardiomyopathy (PPCM) | Takotsubo Syndrome (TTS) | |
|---|---|---|
| Demographics | Pregnant women in the last trimester up to five months postpartum [8] | >89% of cases occur in women. Usually, postmenopausal [15,18] |
| Symptoms | Shortness of breath on exertion, chest pain, paroxysmal nocturnal dyspnoea, orthopnoea collapse and terminal arrhythmia [19,20,21] | Shortness of breath on exertion, chest pain, paroxysmal nocturnal dyspnoea, orthopnoea, collapse and terminal arrhythmia [22] |
| Presentation | Can present acutely however late presentation and delayed diagnoses are common due to symptoms can be attributed to postpartum or late pregnancy symptoms [19,21,23] | Tend to present acutely, usually sudden onset Can occur in patients in the context of acute illness such as sepsis [22,24] |
| Echocardiograph features | Temporal dependant Global hypokinesia Left ventricular and right ventricular dilatation and/or dysfunction, functional mitral and/or tricuspid regurgitation, pulmonary hypertension, and left atrial or bi-atrial enlargement. Systolic dysfunction Intracardiac thrombus [19,25] | Temporal dependant Symmetrical regional abnormalities involving the midventricular segments of the anterior, inferior, and lateral walls Left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities [14,26] Intracardiac thrombus [14,22] |
| Electrocardiograph features | Normal ECG, Sinus tachycardia Pathologic Q-waves, ST depression, T-wave abnormalities, 2nd- or 3rd-degree atrioventricular block, complete left or right bundle branch block, atrial fibrillation or flutter, and frequent atrial or ventricular ectopy [7,27] | Hyperacute: ST-segment elevation, ST-segment depression, and QTc prolongation Late features: T-wave inversion [12,22,28] |
| Cardiac Magnetic Resonance Imaging features | Acute presentation: High-signal T2 suggestive of oedema [25,29,30] Regional wall motion abnormalities [29] Late-Gadolinium enhancement sometimes seen—non-specific distribution. [29,31,32] Late Gadolinium enhancement confers worse recovery [31] | Acute: High-signal T2 suggestive of oedema [33,34], late Gadolinium enhancement suggestive of fibrosis is usually absent in the acute stage but can be present [12,22]. Late Gadolinium enhancement suggests more severe disease and less recovery. [22,34] |
| Aetiology | Prolactin mediated [19] Inflammation Two hit mechanism, genetic pre-disposition, and precipitating event Mostly unknown | Neuroendocrine storm (adrenaline, noradrenaline) [24] inflammation Reduced oestrogen levels Mostly unknown |
| Average Time for recovery | Highly variable [35] LVEF recovery time: 34% in 6 months 47% in 1 year 71% in 5 years [20] Mortality rate 1.6% to 27.6% [7] | Mean LVEF recovery at 60 days [18] Partial recovery rate 16–30%, persistent reduced LVEF associated with multiple co-morbidities [34,36,37] Late (>10 days) recovery 53% [38] Early (<10 days) recovery 47% [38] Mortality rate 4.5–5.6% [18,39] |
| Biochemical markers | BNP, Troponin, CRP microRNA-146a, cathepsin D, and interferon-gamma [7] | BNP, Troponin, CRP [12] |
| Yang, W-I et al. (2019) [43] | Kim, D-Y et al. (2020) [17] | |
|---|---|---|
| Study design | Retrospective observational single centre | Retrospective observational single centre |
| Number of patients | 37 21 (PPCM) 16 (TTS) | 31 21 (PPCM) 10 (TTS) |
| PPCM definition | LVEF < 45%, 3rd trimester of pregnancy, 6 months postpartum, left ventricular global hypokinesia | LVEF < 45%, 3rd trimester of pregnancy, 6 months postpartum, left ventricular global hypokinesia |
| TTS definition | Regional wall abnormalities, LVEF < 45% | Transient regional wall motion abnormalities (RWMAs) that extended beyond a single epicardial vascular distribution during the last month of pregnancy or within 5 months after delivery, with either electrocardiographic abnormalities or modest cardiac troponin elevation |
| Similarities between cohorts | Clinical characteristics, Biochemical markers | No statistically significant difference in the mode of delivery Similar rise in biomarkers |
| Differences between cohorts | Greater parity in TTS Earlier onset of symptoms in TTS Higher LVEF with quicker recovery Complete resolution of EF for all TTS patients at 1 month | Greater near-miss death events in TTS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falola, A.O.; Razvi, N.; Gada, R.; Thompson, D.R.; Martin, C.R. Takotsubo Syndrome or Peripartum Cardiomyopathy? Depends on Who You Are Talking to. Behav. Sci. 2024, 14, 777. https://doi.org/10.3390/bs14090777
Falola AO, Razvi N, Gada R, Thompson DR, Martin CR. Takotsubo Syndrome or Peripartum Cardiomyopathy? Depends on Who You Are Talking to. Behavioral Sciences. 2024; 14(9):777. https://doi.org/10.3390/bs14090777
Chicago/Turabian StyleFalola, Abigail O., Naveed Razvi, Ruta Gada, David R. Thompson, and Colin R. Martin. 2024. "Takotsubo Syndrome or Peripartum Cardiomyopathy? Depends on Who You Are Talking to" Behavioral Sciences 14, no. 9: 777. https://doi.org/10.3390/bs14090777
APA StyleFalola, A. O., Razvi, N., Gada, R., Thompson, D. R., & Martin, C. R. (2024). Takotsubo Syndrome or Peripartum Cardiomyopathy? Depends on Who You Are Talking to. Behavioral Sciences, 14(9), 777. https://doi.org/10.3390/bs14090777








