Combined Effects of Nasal Ketamine and Trauma-Focused Psychotherapy in Treatment-Resistant Post-Traumatic Stress Disorder: A Pilot Case Series
Abstract
1. Introduction
2. Methods
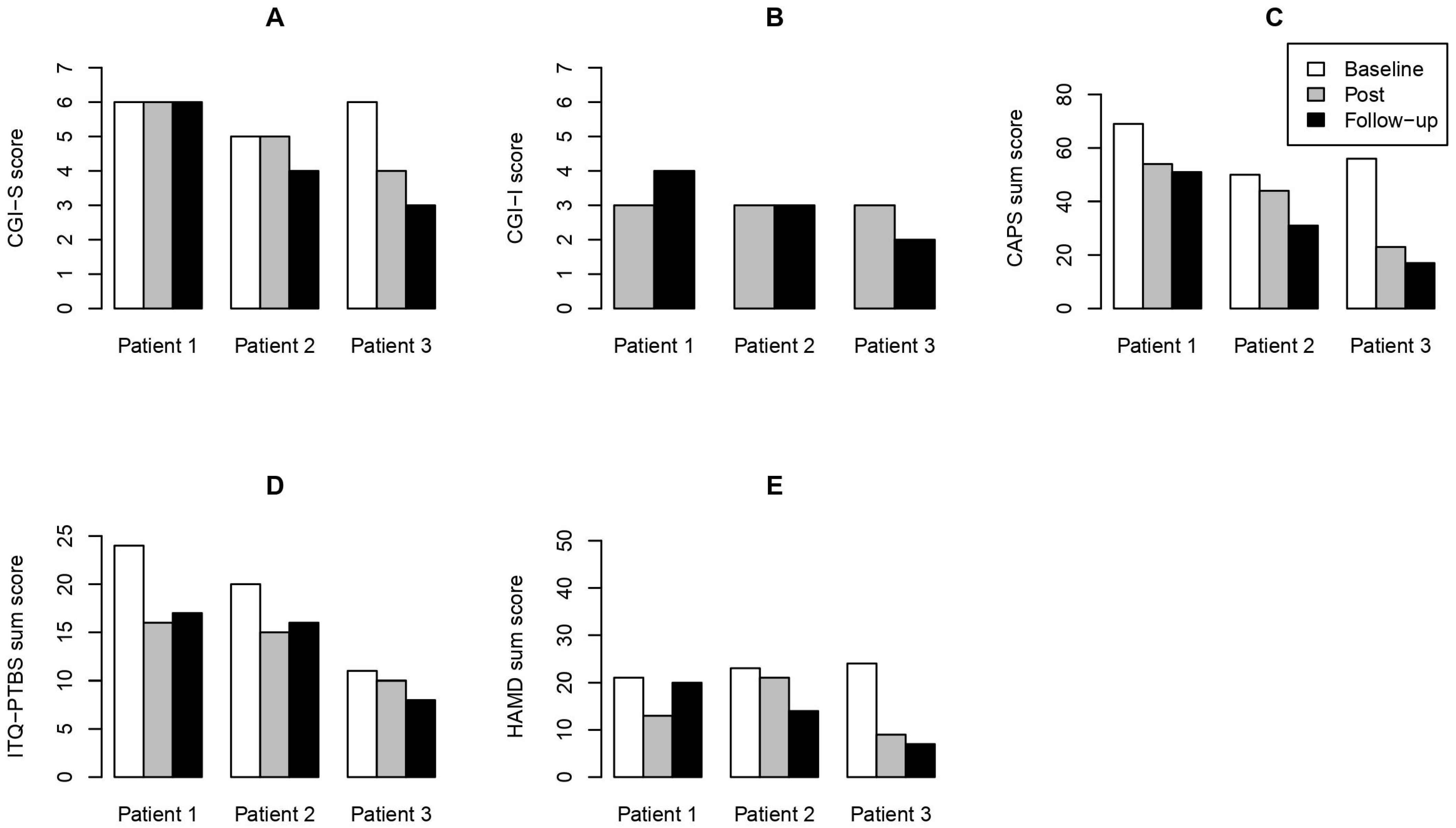
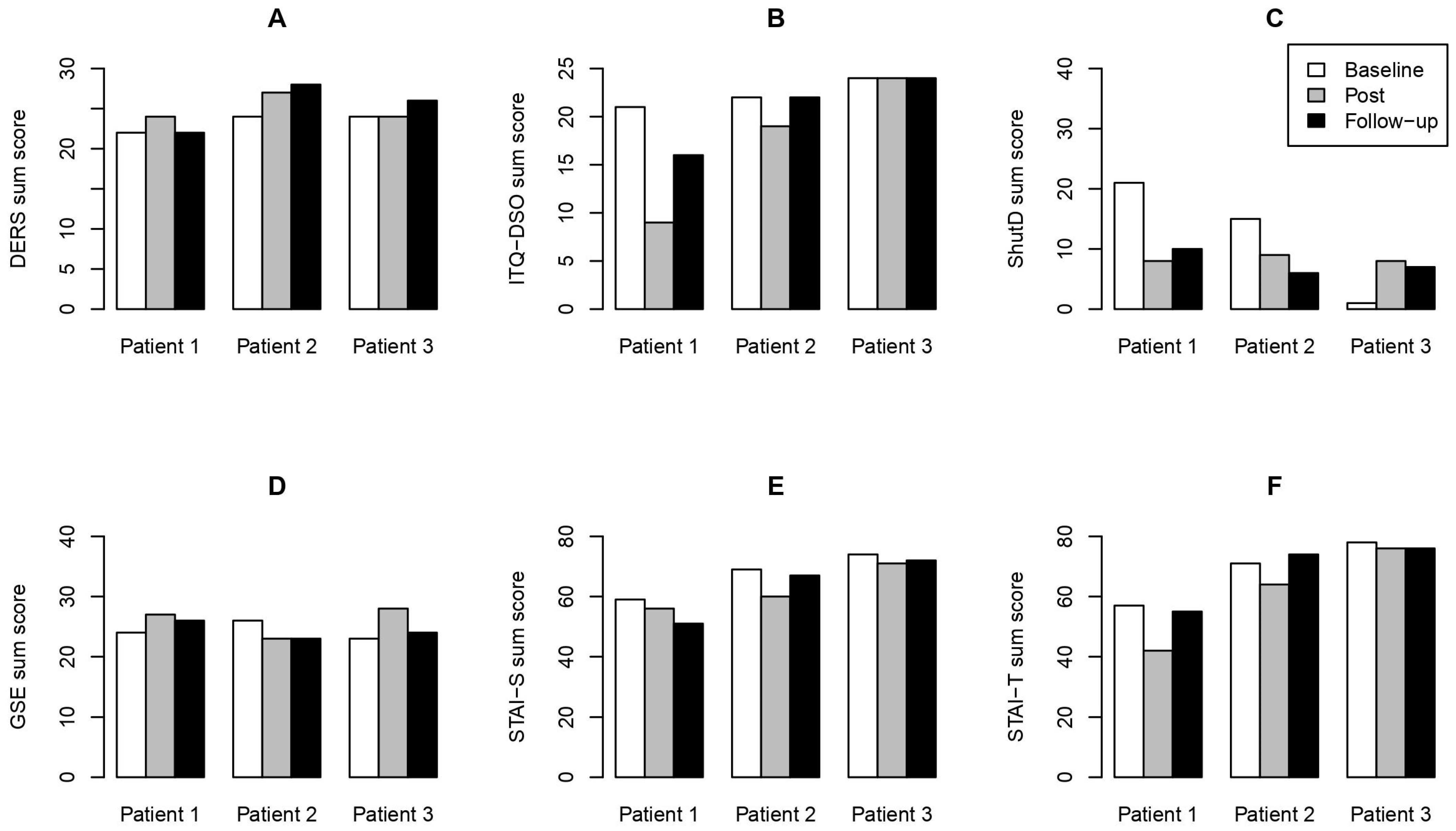
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Petukhova, M.V.; Sampson, N.A.; Aguilar-Gaxiola, S.; Alonso, J.; Andrade, L.H.; Bromet, E.J.; de Girolamo, G.; Haro, J.M.; Hinkov, H.; et al. Association of DSM-IV Posttraumatic Stress Disorder with Traumatic Experience Type and History in the World Health Organization World Mental Health Surveys. JAMA Psychiatry 2017, 74, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Post-Traumatic Stress Disorder: NICE Guideline. 2018. Available online: https://www.nice.org.uk/guidance/ng116 (accessed on 20 December 2023).
- Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am. Psychol. 2019, 74, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Phelps, A.J.; Lethbridge, R.; Brennan, S.; Bryant, R.A.; Burns, P.; Cooper, J.A.; Forbes, D.; Gardiner, J.; Gee, G.; Jones, K.; et al. Australian guidelines for the prevention and treatment of posttraumatic stress disorder: Updates in the third edition. Aust. N. Z. J. Psychiatry 2022, 56, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Gast, U.; Hofmann, A.; Knaevelsrud, C.; Lampe, A.; Liebermann, P.; Lotzin, A.; Maercker, A.; Rosner, R.; Wöller, W. (Eds.) S3-Leitlinie Posttraumatische Belastungsstörung; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-662-59782-8. [Google Scholar]
- Hoskins, M.D.; Bridges, J.; Sinnerton, R.; Nakamura, A.; Underwood, J.F.G.; Slater, A.; Lee, M.R.D.; Clarke, L.; Lewis, C.; Roberts, N.P.; et al. Pharmacological therapy for post-traumatic stress disorder: A systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur. J. Psychotraumatol. 2021, 12, 1802920. [Google Scholar] [CrossRef]
- Merz, J.; Schwarzer, G.; Gerger, H. Comparative efficacy and acceptability of pharmacological, psychotherapeutic, and combination treatments in adults with posttraumatic stress disorder: A network meta-analysis. JAMA Psychiatry 2019, 76, 904–913. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Medicines. Available online: https://www.ema.europa.eu/en/medicines (accessed on 19 May 2024).
- Drug Approvals and Databases. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 19 May 2024).
- Swissmedic. Arzneimittelinformationen. 2019. Available online: https://www.swissmedic.ch/swissmedic/de/home/services/arzneimittelinformationen.html (accessed on 19 May 2024).
- Guidetti, C.; Feeney, A.; Hock, R.S.; Iovieno, N.; Hernández Ortiz, J.M.; Fava, M.; Papakostas, G.I. Antidepressants in the acute treatment of post-traumatic stress disorder in adults: A systematic review and meta-analysis. Int. Clin. Psychopharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Stewart, A.; Smolenski, D.J.; Bush, N.E.; Cyr, B.; Beech, E.H.; Skopp, N.A.; Belsher, B.E. Posttraumatic Stress Disorder Treatment Dropout Among Military and Veteran Populations: A Systematic Review and Meta-Analysis. J. Trauma. Stress 2021, 34, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Roberts, N.P.; Gibson, S.; Bisson, J.I. Dropout from psychological therapies for post-traumatic stress disorder (PTSD) in adults: Systematic review and meta-analysis. Eur. J. Psychotraumatol. 2020, 11, 1709709. [Google Scholar] [CrossRef] [PubMed]
- Barawi, K.S.; Lewis, C.; Simon, N.; Bisson, J.I. A systematic review of factors associated with outcome of psychological treatments for post-traumatic stress disorder. Eur. J. Psychotraumatol. 2020, 11, 1774240. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.P.; Lotzin, A.; Schäfer, I. A systematic review and meta-analysis of psychological interventions for comorbid post-traumatic stress disorder and substance use disorder. Eur. J. Psychotraumatol. 2022, 13, 2041831. [Google Scholar] [CrossRef]
- Lavender, E.; Hirasawa-Fujita, M.; Domino, E.F. Ketamine’s dose related multiple mechanisms of actions: Dissociative anesthetic to rapid antidepressant. Behav. Brain Res. 2020, 390, 112631. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Esketamine is approved in Europe for treating resistant major depressive disorder. BMJ 2019, 367, l7069. [Google Scholar] [CrossRef]
- Feder, A.; Costi, S.; Rutter, S.B.; Collins, A.B.; Govindarajulu, U.; Jha, M.K.; Horn, S.R.; Kautz, M.; Corniquel, M.; Collins, K.A.; et al. A Randomized Controlled Trial of Repeated Ketamine Administration for Chronic Posttraumatic Stress Disorder. Am. J. Psychiatry 2021, 178, 193–202. [Google Scholar] [CrossRef]
- Feder, A.; Parides, M.K.; Murrough, J.W.; Perez, A.M.; Morgan, J.E.; Saxena, S.; Kirkwood, K.; Aan Het Rot, M.; Lapidus, K.A.; Wan, L.-B. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry 2014, 71, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Dadabayev, A.R.; Joshi, S.A.; Reda, M.H.; Lake, T.; Hausman, M.S.; Domino, E.; Liberzon, I. Low Dose Ketamine Infusion for Comorbid Posttraumatic Stress Disorder and Chronic Pain: A Randomized Double-Blind Clinical Trial. Chronic Stress 2020, 4, 247054702098167. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Wainer, I.W.; Moaddel, R.; Torjman, M.C.; Goldberg, M.; Sabia, M.; Parikh, T.; Pumariega, A.J. Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) psychotherapy prolong the therapeutic effects of single ketamine infusion on post-traumatic stress disorder and comorbid depression: A pilot randomized, placebo-controlled, crossover clinical trial. Asia Pac. J. Clin. Trials Nerv. Syst. Dis. 2017, 2, 80. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Roache, J.D.; Gueorguieva, R.; Averill, L.A.; Young-McCaughan, S.; Shiroma, P.R.; Purohit, P.; Brundige, A.; Murff, W.; Ahn, K.-H.; et al. Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: A double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology 2022, 47, 1574–1581. [Google Scholar] [CrossRef]
- Dames, S.; Kryskow, P.; Watler, C. A Cohort-Based Case Report: The Impact of Ketamine-Assisted Therapy Embedded in a Community of Practice Framework for Healthcare Providers with PTSD and Depression. Front. Psychiatry 2021, 12, 803279. [Google Scholar] [CrossRef]
- Halstead, M.; Reed, S.; Krause, R.; Williams, M.T. Ketamine-Assisted Psychotherapy for PTSD Related to Racial Discrimination. Clin. Case Stud. 2021, 20, 310–330. [Google Scholar] [CrossRef]
- Keizer, B.M.; Roache, J.D.; Jones, J.R.; Kalpinski, R.J.; Porcerelli, J.H.; Krystal, J.H. Continuous ketamine infusion for pain as an opportunity for psychotherapy for PTSD: A case series of ketamine-enhanced psychotherapy for PTSD and pain (KEP-P2). Psychother. Psychosom. 2020, 89, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Mangini, P.; Xin, Y. Ketamine-assisted psychotherapy for trauma-exposed patients in an outpatient setting: A clinical chart review study. J. Psychedelic Stud. 2021, 5, 94–102. [Google Scholar] [CrossRef]
- Shiroma, P.R.; Thuras, P.; Wels, J.; Erbes, C.; Kehle-Forbes, S.; Polusny, M. A proof-of-concept study of subanesthetic intravenous ketamine combined with prolonged exposure therapy among veterans with posttraumatic stress disorder. J. Clin. Psychiatry 2020, 81, 10118. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Mitrev, L.; Moaddell, R.; Wainer, I.W. d-Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, K.A.B.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Parides, M.K.; Soleimani, L.; Feder, A.; Iosifescu, D.V.; Charney, D.S.; Murrough, J.W. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biol. Psychiatry 2014, 76, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Ragnhildstveit, A.; Roscoe, J.; Bass, L.C.; Averill, C.L.; Abdallah, C.G.; Averill, L.A. The potential of ketamine for posttraumatic stress disorder: A review of clinical evidence. Ther. Adv. Psychopharmacol. 2023, 13, 204512532311541. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/. (accessed on 22 June 2024).
- Maercker, A.; Cloitre, M.; Bachem, R.; Schlumpf, Y.R.; Khoury, B.; Hitchcock, C.; Bohus, M. Complex post-traumatic stress disorder. Lancet 2022, 400, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Coventry, P.A.; Meader, N.; Melton, H.; Temple, M.; Dale, H.; Wright, K.; Cloitre, M.; Karatzias, T.; Bisson, J.; Roberts, N.P.; et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis. PLoS Med. 2020, 17, e1003262. [Google Scholar] [CrossRef] [PubMed]
- Karatzias, T.; Murphy, P.; Cloitre, M.; Bisson, J.; Roberts, N.; Shevlin, M.; Hyland, P.; Maercker, A.; Ben-Ezra, M.; Coventry, P.; et al. Psychological interventions for ICD-11 complex PTSD symptoms: Systematic review and meta-analysis. Psychol. Med. 2019, 49, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Fortress, A.M.; Smith, I.M.; Pang, K.C.H. Ketamine facilitates extinction of avoidance behavior and enhances synaptic plasticity in a rat model of anxiety vulnerability: Implications for the pathophysiology and treatment of anxiety disorders. Neuropharmacology 2018, 137, 372–381. [Google Scholar] [CrossRef]
- Whittaker, E.; Dadabayev, A.R.; Joshi, S.A.; Glue, P. Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211056743. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Zhou, W.-W.; Ji, Y.-J.; Li, Y.; Zhao, N.; Chen, H.-X.; Xue, R.; Mei, X.-G.; Zhang, Y.-Z.; Wang, H.-L.; et al. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology 2015, 232, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.-J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Cloitre, M.; Shevlin, M.; Brewin, C.R.; Bisson, J.I.; Roberts, N.P.; Maercker, A.; Karatzias, T.; Hyland, P. The International Trauma Questionnaire: Development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr. Scand. 2018, 138, 536–546. [Google Scholar] [CrossRef]
- Gratz, K.L.; Roemer, L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Guy, W. Clinical global impressions scale. Psychiatry 1976. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Schalinski, I.; Schauer, M.; Elbert, T. The shutdown dissociation scale (Shut-D). Eur. J. Psychotraumatology 2015, 6, 25652. [Google Scholar] [CrossRef] [PubMed]
- Schalinski, I.; Schauer, M.; Elbert, T. Shut-D—Shutdown-Dissoziationsskala. 2016. Available online: https://psycharchives.org/en/item/5c519cd7-ebb2-4a22-850f-b3d1ca25bddf (accessed on 20 December 2023).
- Spielberger, C.; Gorsuch, R.; Lushene, R.; Vagg, P.; Jacobs, G. Manual for the State-Trait Anxiety Inventory (Form Y): Self-Evaluation Questionnairepress; Consulting Psychologists: Palo Alto, CA, USA, 1983; pp. 1–36. [Google Scholar]
- Tipton, R.M.; Worthington, E.L. The measurement of generalized self-efficacy: A study of construct validity. J. Personal. Assess. 1984, 48, 545–548. [Google Scholar]
- PTSD: National Center for PTSD. Available online: www.ptsd.va.gov (accessed on 20 December 2023).
- Weathers, F.W.; Bovin, M.J.; Lee, D.J.; Sloan, D.M.; Schnurr, P.P.; Kaloupek, D.G.; Keane, T.M.; Marx, B.P. Clinician-administered ptsd scale for DSM-5. Psychol. Assess. 2015. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 18–65 years | Known aneurysmal vascular disease |
| Chronic PTSD (>6 months) | Known history of intracerebral hemorrhage |
| Two or more unsuccessful previous PTSD-specific treatments | Recent (within the last six weeks) cardiovascular event |
| Known hypersensitivity to ketamine or esketamine | |
| Untreated hypertension | |
| Untreated liver, kidney, or lung disease | |
| Untreated hyperthyroidism | |
| History of traumatic brain injury of at least moderate severity | |
| History of (hypo-)manic or psychotic episodes | |
| Current use of opioids, benzodiazepines, or opioid antagonists | |
| Meeting criteria for a substance use disorder in the past 6 months | |
| Use of ketamine without medical prescription within the last two years | |
| Women of childbearing potential without effective contraception | |
| Current pregnancy | |
| If psychotropic medications are being taken: no stable medication including dosage within the last two months |
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years) | 41 | 26 | 59 |
| Sex | f | f | f |
| Diagnoses according to DSM-5 |
|
|
|
| Physical diseases | Mild mitral insufficiency | Migraine | None |
| Resting blood pressure (mmHg) | 123/66 | 101/80 | 140/85 |
| Number of stressful events personally witnessed (according to LEC-5) | 8 | 3 | 3 |
| CTQ score at baseline | 77 | 70 | 67 |
| CGI-S score at baseline | 6 | 5 | 6 |
| CAPS score at baseline | 69 | 50 | 56 |
| Concomitant psychotropic medication (per day) |
|
|
|
| Dosage of ketamine at each session (0.5 mg/kg) | 35 mg | 25 mg | 35 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohde, J.; Hickmann, E.; Buchmann, M.; Kronenberg, G.; Vetter, S.; Seifritz, E.; Kleim, B.; Olbrich, S. Combined Effects of Nasal Ketamine and Trauma-Focused Psychotherapy in Treatment-Resistant Post-Traumatic Stress Disorder: A Pilot Case Series. Behav. Sci. 2024, 14, 717. https://doi.org/10.3390/bs14080717
Rohde J, Hickmann E, Buchmann M, Kronenberg G, Vetter S, Seifritz E, Kleim B, Olbrich S. Combined Effects of Nasal Ketamine and Trauma-Focused Psychotherapy in Treatment-Resistant Post-Traumatic Stress Disorder: A Pilot Case Series. Behavioral Sciences. 2024; 14(8):717. https://doi.org/10.3390/bs14080717
Chicago/Turabian StyleRohde, Judith, Elena Hickmann, Marco Buchmann, Golo Kronenberg, Stefan Vetter, Erich Seifritz, Birgit Kleim, and Sebastian Olbrich. 2024. "Combined Effects of Nasal Ketamine and Trauma-Focused Psychotherapy in Treatment-Resistant Post-Traumatic Stress Disorder: A Pilot Case Series" Behavioral Sciences 14, no. 8: 717. https://doi.org/10.3390/bs14080717
APA StyleRohde, J., Hickmann, E., Buchmann, M., Kronenberg, G., Vetter, S., Seifritz, E., Kleim, B., & Olbrich, S. (2024). Combined Effects of Nasal Ketamine and Trauma-Focused Psychotherapy in Treatment-Resistant Post-Traumatic Stress Disorder: A Pilot Case Series. Behavioral Sciences, 14(8), 717. https://doi.org/10.3390/bs14080717






