A Systematic Review of Educational Interventions for Informal Caregivers of People Living with Dementia in Low and Middle-Income Countries
Abstract
1. Introduction
- -
- To identify interventions with an educational component for informal dementia caregivers in LMICs;
- -
- To appraise the potential effects and quality of these intervention studies;
- -
- To make recommendations about future research regarding educational interventions for informal dementia caregivers in LMICs.
2. Method
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
- They reported on an intervention that included a focus or component described as “educational”, “psychoeducational” or similar;
- The intervention, including the educational component, was evaluated using formal research methodology (quantitative or qualitative);
- The study population comprised of informal caregivers of people living with a diagnosis of dementia in the community;
- The intervention was delivered in a country (or region of a country) categorised as being low- or middle-income [37].
- They were not peer-reviewed;
- They were reviews or protocols;
- The intervention was also or solely delivered to the person living with dementia (PLWD);
- The intervention was delivered in a high-income country or region;
- The paper was not written in or translated into English;
- The intervention was solely delivered to professional caregivers;
- The full text was not available.
2.3. Quality Appraisal
2.4. Intervention Evaluation
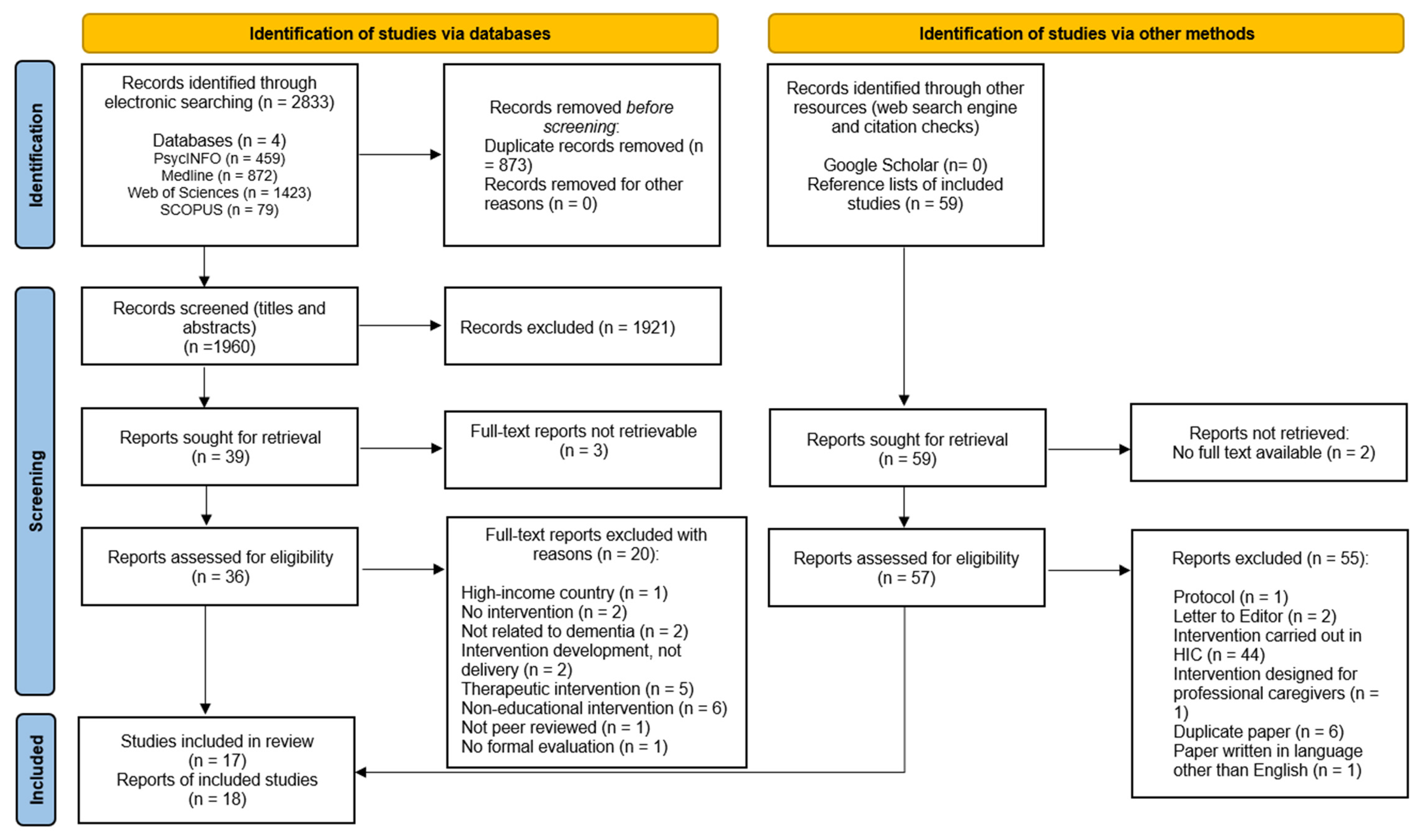
3. Results
3.1. Overview of Studies
3.2. Quality Appraisal
3.3. Interventions
3.3.1. Educational
3.3.2. Therapeutic
3.3.3. Support
3.3.4. Multicomponent
3.4. Intervention Delivery Characteristics
3.4.1. Group vs. Individual
3.4.2. Intervention Length and Dosage
3.4.3. Internet-Based vs. In-Person
3.5. Educational Component
4. Discussion
4.1. Summary
4.2. Nature of the Interventions
4.3. Education Delivery and Content
4.4. Clinical Implications and Future Research
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Alladi, S.; Hachinski, V. World dementia: One approach does not fit all. Neurology 2018, 91, 264–270. [Google Scholar] [CrossRef]
- Gallagher-Thompson, D.; Msw, Y.M.T.; Au, A.; Brodaty, H.; Charlesworth, G.; Gupta, R.; Lee, S.E.; Losada, A.; Shyu, Y.-I. International Perspectives on Nonpharmacological Best Practices for Dementia Family Caregivers: A Review. Clin. Gerontol. 2012, 35, 316–355. [Google Scholar] [CrossRef]
- Prince, M.; Knapp, M.; Guerchet, M.; McCrone, P.; Prina, M.; Comas-Herrera, A.; Wittenberg, R.; Adelaja, B.; Hu, B.; King, D.; et al. Dementia UK—Overview, 2nd ed.; Alzheimer’s Society: London, UK, 2014. [Google Scholar]
- Dominguez, J.; Jiloca, L.; Fowler, K.C.; De Guzman, M.F.; Dominguez-Awao, J.K.; Natividad, B.; Domingo, J.; Dominguez, J.D.; Reandelar, M.; Ligsay, A.; et al. Dementia Incidence, Burden and Cost of Care: A Filipino Community-Based Study. Front. Public Health 2021, 9, 628700. [Google Scholar] [CrossRef] [PubMed]
- Mattap, S.M.; Mohan, D.; McGrattan, A.M.; Allotey, P.; Stephan, B.C.; Reidpath, D.D.; Siervo, M.; Robinson, L.; Chaiyakunapruk, N. The economic burden of dementia in low- and middle-income countries (LMICs): A systematic review. BMJ Glob. Health 2022, 7, e007409. [Google Scholar] [CrossRef] [PubMed]
- Fam, J.; Mahendran, R.; Kua, E.H. Dementia care in low and middle-income countries. Curr. Opin. Psychiatry 2019, 32, 461–464. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.D.; He, G.; De Bellis, A. Family caregiver challenges in dementia care in a country with undeveloped dementia services. J. Adv. Nurs. 2014, 70, 1369–1380. [Google Scholar] [CrossRef]
- Corbett, A.; Stevens, J.; Aarsland, D.; Day, S.; Moniz-Cook, E.; Woods, R.; Brooker, D.; Ballard, C. Systematic review of services providing information and/or advice to people with dementia and/or their caregivers. Int. J. Geriatr. Psychiatry 2012, 27, 628–636. [Google Scholar] [CrossRef]
- European Union. Council Conclusions on Supporting People Living with Dementia: Improving Care Policies and Practices; Council of the European Union: Brussels, Belgium, 2015; No. 15055/15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52015XG1216(02)&from=EN/ (accessed on 7 January 2024).
- Brodaty, H.; Donkin, M. Family caregivers of people with dementia. Dialogues Clin. Neurosci. 2009, 11, 217–228. [Google Scholar] [CrossRef]
- Kahn, P.V.; Wishart, H.A.; Randolph, J.S.; Santulli, R.B. Caregiver Stigma and Burden in Memory Disorders: An Evaluation of the Effects of Caregiver Type and Gender. Curr. Gerontol. Geriatr. Res. 2016, 2016, 8316045. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; George, C.; Ferreira, N. Factors emerging from the “Zarit Burden Interview” and predictive variables in a UK sample of caregivers for people with dementia. Int. Psychogeriatr. 2018, 30, 1671–1678. [Google Scholar] [CrossRef]
- Black, B.S.; Johnston, D.; Rabins, P.V.; Morrison, A.; Lyketsos, C.; Samus, Q.M. Unmet Needs of Community-Residing Persons with Dementia and Their Informal Caregivers: Findings from the Maximizing Independence at Home Study. J. Am. Geriatr. Soc. 2013, 61, 2087–2095. [Google Scholar] [CrossRef]
- Adebiyi, A.O.; Fagbola, M.A.; Olakehinde, O.; Ogunniyi, A. Enacted and implied stigma for dementia in a community in south-west Nigeria. Psychogeriatrics 2016, 16, 268–273. [Google Scholar] [CrossRef]
- Mkhonto, F.; Hanssen, I. When people with dementia are perceived as witches. Consequences for patients and nurse education in South Africa. J. Clin. Nurs. 2018, 27, E169–E176. [Google Scholar] [CrossRef]
- Changoor, A. Tackling rising dementia burden in LMICS. Glob. Health Annu. Rev. 2019, 1. [Google Scholar]
- Nguyen, H.; Nguyen, T.; Tran, D.; Hinton, L. “It’s extremely hard but it’s not a burden”: A qualitative study of family caregiving for people living with dementia in Vietnam. PLoS ONE 2021, 16, e0259788. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Chien, W.-T.; Lee, I.Y.M. An experimental study on the effectiveness of a mutual support group for family caregivers of a relative with dementia in mainland China. Contemp. Nurse 2012, 40, 210–224. [Google Scholar] [CrossRef]
- Mushi, D.; Rongai, A.; Paddick, S.-M.; Dotchin, C.; Mtuya, C.; Walker, R. Social representation and practices related to dementia in Hai District of Tanzania. BMC Public Health 2014, 14, 260. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.D.; Li, X.; De Bellis, A.; Ullah, S. Caregiver distress and associated factors in dementia care in the community setting in China. Geriatr. Nurs. 2015, 36, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Abaasa, C.; Obua, C.; Wakida, E.K.; Rukundo, G.Z. A qualitative investigation of the psychosocial services utilised by care-givers of patients with Alzheimer’s disease and related dementias in southwestern Uganda. Ageing Soc. 2023, 43, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.A.; Khan, Y.H.; Khan, Z.M.; Najam, S. Dementia survey among Attendees of a Dementia Awareness Event in Karachi, Pakistan. Pak. J. Neurol. Sci. PJNS 2017, 12, 8. [Google Scholar]
- Cheng, S.-T.; Au, A.; Losada, A.; Thompson, L.W.; Gallagher-Thompson, D. Psychological Interventions for Dementia Caregivers: What We Have Achieved, What We Have Learned. Curr. Psychiatry Rep. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Frias, C.E.; Garcia-Pascual, M.; Montoro, M.; Ribas, N.; Risco, E.; Zabalegui, A. Effectiveness of a psychoeducational intervention for caregivers of People with Dementia with regard to burden, anxiety and depression: A systematic review. J. Adv. Nurs. 2020, 76, 787–802. [Google Scholar] [CrossRef]
- Gitlin, L.N.; Winter, L.; Dennis, M.P.; Hodgson, N.; Hauck, W.W. Targeting and Managing Behavioral Symptoms in Individuals with Dementia: A Randomized Trial of a Nonpharmacological Intervention. J. Am. Geriatr. Soc. 2010, 58, 1465–1474. [Google Scholar] [CrossRef]
- Kwok, T.; Wong, B.; Ip, I.; Chui, K.; Young, D.; Ho, F. Telephone-delivered psychoeducational intervention for Hong Kong Chinese dementia caregivers: A single-blinded randomized controlled trial. Clin. Interv. Aging 2013, 8, 1191–1197. [Google Scholar] [CrossRef]
- Christie, H.L.; Boots, L.M.M.; Tange, H.J.; Verhey, F.R.J.; de Vugt, M.E. Implementations of Evidence-Based eHealth Interventions for Caregivers of People with Dementia in Municipality Contexts (Myinlife and Partner in Balance): Evaluation Study. JMIR Aging 2021, 4, e21629. [Google Scholar] [CrossRef] [PubMed]
- Hinton, L.; Tran, D.; Nguyen, T.-N.; Ho, J.; Gitlin, L. Interventions to support family caregivers of people living with dementia in high, middle and low-income countries in Asia: A scoping review. BMJ Glob. Health 2019, 4, e001830. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Johnston, B.; Quinn, T.J. Measuring the success of interventions for caregivers: A focussed systematic review. Curr. Opin. Support. Palliat. Care 2019, 13, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hinton, L.; Nguyen, H.; Nguyen, H.T.; Harvey, D.J.; Nichols, L.; Martindale-Adams, J.; Nguyen, B.T.; Nguyen, A.N.; Nguyen, C.H.; Nguyen, T.T.H.; et al. Advancing family dementia caregiver interventions in low- and middle-income countries: A pilot cluster randomized controlled trial of Resources for Advancing Alzheimer’s Caregiver Health in Vietnam (REACH VN). Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12063. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.P.K.; Saw, A. An international systematic review of dementia caregiving interventions for Chinese families. Int. J. Geriatr. Psychiatry 2020, 35, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- James, T.; Mukadam, N.; Sommerlad, A.; Ceballos, S.G.; Livingston, G. Culturally tailored therapeutic interventions for people affected by dementia: A systematic review and new conceptual model. Lancet Health Longev. 2021, 2, e171–e179. [Google Scholar] [CrossRef] [PubMed]
- Stoner, C.R.; Lakshminarayanan, M.; Durgante, H.; Spector, A. Psychosocial interventions for dementia in low- and middle-income countries (LMICs): A systematic review of effectiveness and implementation readiness. Aging Ment. Health 2019, 25, 408–419. [Google Scholar] [CrossRef]
- Cochrane Effective Practice and Organisation of Care (EPOC). LMIC Filters. 2020. Available online: https://epoc.cochrane.org/lmic-filters (accessed on 7 November 2022).
- World Bank. Low and Middle Income. The World Bank Group. Available online: https://data.worldbank.org/country/XO (accessed on 24 October 2022).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Baruah, U.; Varghese, M.; Loganathan, S.; Mehta, K.M.; Gallagher-Thompson, D.; Zandi, D.; Dua, T.; Pot, A.M. Feasibility and preliminary effectiveness of an online training and support program for caregivers of people with dementia in India: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2021, 36, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Dewey, M.E.; D’Souza, J.; Dhume, R.; Motghare, D.D.; Shaji, K.S.; Menon, R.; Prince, M.; Patel, V. The Effectiveness of a Home Care Program for Supporting Caregivers of Persons with Dementia in Developing Countries: A Randomised Controlled Trial from Goa, India. PLoS ONE 2008, 3, e2333. [Google Scholar] [CrossRef]
- Fialho, P.P.A.; Köenig, A.M.; dos Santos, M.D.L.; Barbosa, M.T.; Caramelli, P. Positive effects of a cognitive-behavioral intervention program for family caregivers of demented elderly. Arq. Neuro-Psiquiatr. 2012, 70, 786–792. [Google Scholar] [CrossRef]
- Gavrilova, S.I.; Ferri, C.P.; Mikhaylova, N.; Sokolova, O.; Banerjee, S.; Prince, M. Helping carers to care—The 10/66 dementia research group’s randomized control trial of a caregiver intervention in Russia. Int. J. Geriatr. Psychiatry 2009, 24, 347–354. [Google Scholar] [CrossRef]
- Guerra, M.; Ferri, C.P.; Fonseca, M.; Banerjee, S.; Prince, M. Helping carers to care: The 10/66 dementia research group’s randomized control trial of a caregiver intervention in Peru. Braz. J. Psychiatry 2011, 33, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, G.; Hong, L. Impact of professionally facilitated peer support for family carers of people with dementia in a WeChat virtual community. J. Telemed. Telecare 2022, 28, 68–76. [Google Scholar] [CrossRef]
- Javadpour, A.; Ahmadzadeh, L.; Bahredar, M.J. An educative support group for female family caregivers: Impact on caregivers psychological distress and patient’s neuropsychiatry symptoms. Int. J. Geriatr. Psychiatry 2009, 24, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, N.; Beşer, N.; Zencir, M.; Şahiner, T.; Nesrin, E.; Ahmet, E.; Binali, Ç.; Çaǧdaş, E. Effects of a Comprehensive Educational Program on Quality of Life and Emotional Issues of Dementia Patient Caregivers. Geriatr. Nurs. 2005, 26, 378–386. [Google Scholar] [CrossRef]
- Magteppong, W.; Yamarat, K. The Effects of the Modified Transtheoretical Theory of Stress and Coping (TTSC) Program on Dementia Caregivers’ Knowledge, Burden, and Quality of Life. Int. J. Environ. Res. Public Health 2021, 18, 13231. [Google Scholar] [CrossRef]
- Pankong, O.; Pothiban, L.; Sucamvang, K.; Khampolsiri, T. A Randomized Controlled Trial of Enhancing Positive Aspects of Caregiving in Thai Dementia Caregivers for Dementia. Pac. Rim Int. J. Nurs. Res. 2018, 22, 131–143. [Google Scholar]
- Santos, R.L.; de Sousa, M.F.B.; Arcoverde, C.; Dourado, M.C.N. Efficacy of a psychoeducational group with caregivers of patients with dementia. Arch. Clin. Psychiatry 2013, 40, 162–164. [Google Scholar] [CrossRef]
- Senanarong, V.; Jamjumras, P.; Harmphadungkit, K.; Klubwongs, M.; Udomphanthurak, S.; Poungvarin, N.; Vannasaeng, S.; Cummings, J.L. A counseling intervention for caregivers: Effect on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry 2004, 19, 781–788. [Google Scholar] [CrossRef]
- Shata, Z.N.; Amin, M.R.; El-Kady, H.M.; Abu-Nazel, M.W. Efficacy of a multi-component psychosocial intervention program for caregivers of persons living with neurocognitive disorders, Alexandria, Egypt: A randomized controlled trial. Avicenna J. Med. 2017, 7, 54–63. [Google Scholar]
- Tawfik, N.M.; Sabry, N.A.; Darwish, H.; Mowafy, M.; Soliman, S.S. Psychoeducational Program for the Family Member Caregivers of People with Dementia to Reduce Perceived Burden and Increase Patient’s Quality of Life: A Randomized Controlled Trial. J. Prim. Care Community Health 2021, 12, 21501327211014088. [Google Scholar] [CrossRef]
- Tran, D.; Nguyen, H.; Pham, T.; Nguyen, A.T.; Nguyen, H.T.; Nguyen, N.B.; Harvey, D.; Hinton, L. Resources for Enhancing Alzheimer’s Caregiver Health in Vietnam (REACH VN): Exploratory Analyses of Outcomes of a Cluster Randomized Controlled Trial to Test the Feasibility and Preliminary Efficacy of a Family Dementia Caregiver Intervention in Vietnam. Am. J. Geriatr. Psychiatry 2022, 30, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Ab Razak, A. The effectiveness of a cultural-based support group for Malay dementia caregivers in Kelantan, Malaysia: A pre-post intervention study. Asean J. Psychiatry 2017, 18, 20–30. [Google Scholar]
- Zhang, S.Y.; Wu, F.; Tang, D.L.; Rong, X.S.; Guo, Q.H.; Fang, M.; Zhao, Q.H.; Zhao, Y.X. Pilot testing the caregiver self-management intervention for caregivers of relatives with dementia. Geriatr. Nurs. 2020, 41, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Nichols, L.O.; Martindale-Adams, J.; Burns, R.; Zuber, J.; Graney, M.J. REACH VA: Moving from Translation to System Implementation. Gerontologist 2016, 56, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, R.; McCurry, S.M.; Teri, L. STAR-Caregivers: A community-based approach for teaching family caregiv-ers to use behavioral strategies to reduce affective disturbances in persons with dementia. Alzheimer’s Care Q. 2005, 6, 146–153. [Google Scholar]
- Dickinson, C.; Dow, J.; Gibson, G.; Hayes, L.; Robalino, S.; Robinson, L. Psychosocial intervention for carers of people with dementia: What components are most effective and when? A systematic review of systematic reviews. Int. Psychogeriatr. 2017, 29, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Shaji, K.S.; Smitha, K.; Lal, K.P.; Prince, M.J. Caregivers of people with Alzheimer’s disease: A qualitative study from the Indian 10/66 Dementia Research Network. Int. J. Geriatr. Psychiatry 2003, 18, 1–6. [Google Scholar] [CrossRef]
- Parker, D.; Mills, S.; Abbey, J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: A systematic review. Int. J. Evid. -Based Health 2008, 6, 137–172. [Google Scholar] [CrossRef]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How We Design Feasibility Studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef]
- Walter, E.; Pinquart, M. How Effective Are Dementia Caregiver Interventions? An Updated Comprehensive Meta-Analysis. Gerontol. 2020, 60, e609–e619. [Google Scholar] [CrossRef]
- Baker, Z.G.; Nkimbeng, M.; Cuevas, P.E.G.; Quiñones, A.R.; Kang, H.K.; E Gaugler, J.; Hinton, L.; Gitlin, L.N.; Shippee, T.P. Simultaneously Developing Interventions for Low-/Middle-Income and High-Income Settings: Considerations and Opportunities. Gerontologist 2023, 63, 568–576. [Google Scholar] [CrossRef]
- Carter, G.; Monaghan, C.; Santin, O. What is known from the existing literature about peer support interventions for carers of individuals living with dementia: A scoping review. Health Soc. Care Community 2020, 28, 1134–1151. [Google Scholar] [CrossRef]
- Stoner, C.R.; Lakshminarayanan, M.; Mograbi, D.C.; Vaitheswaran, S.; Bertrand, E.; Brum, P.S.; Durgante, H.; Ferri, C.P.; Mkenda, S.; Walker, R.; et al. Development and acceptability of a brief, evidence-based Dementia Awareness for Caregivers course in low- and middle-income countries. Dementia 2022, 21, 598–617. [Google Scholar] [CrossRef]
| Authors/ Date | Location/ Participants (n)/Duration | Design | Intervention | Dosage | Outcome Measures | Significant Results | Quality Score | Comments |
|---|---|---|---|---|---|---|---|---|
| Baruah, Varghese, Loganathan, Mehta, Gallagher-Thompson, Zandi, Dua and Pot, 2021 [40] | India n = 151 3 months | Randomised control trial Control: educational e-book Sample: National advertising and recruitment | Online iSupport Program 23 lessons related (with interactive learning situations) to themes: - What is dementia? (1) - Being a caregiver (4) - Caring for me (3) - Providing everyday care (5) - Dealing with behaviour changes (10) + Relaxation activity after each lesson | Online access for 3 months N.B. Carers encouraged to attend 5+ lessons | Caregiver: - Zarit Burden Interview (ZBI) - Center for Epidemiological Studies Depression–10 item scale (CES-D10) - Approaches to Dementia Questionnaire (ADQ) - RIS Eldercare Self-efficacy scale. - Mastery scale PLWD: None. | Significant difference in ADQ-19 scores (p = 0.030) at post-treatment between treatment and control—treatment had increase in positive attitudes towards PLWD. | 20/27 | Feasibility measured through recruitment and retention statistics. |
| Dias, Dewey, D’Souza, Dhume, Motghare, Shaji, Menon, Prince and Patel, 2008 [41]* | Goa, India n = 81 6 months N.B.Visit frequency dependent on individual need. | Randomised control trial Control: Waiting list Sample: Recruited through adverts and health services | Home Care Program Stepped care and tailored model. Delivered by home care advisors- - Basic education about dementia - Education about common behaviour problems and management - Caregiver support (e.g., in ADLs) - Referrals when behaviour problems escalated and needed medication intervention. - Networking of families to allow for support groups. - Advice regarding existing government schemes for elders | Average 9.225 h (Mean home visits = 12.3, average time = 45 min) N.B. Nine additional peer support groups run | Caregiver: -Neuropsychiatric Inventory Questionnaire (NPI-Q) Distress subscale D) - Zarit Burden Interview (ZBI) - General Health Questionnaire (GHQ) PLWD: -Neuropsychiatric Inventory Questionnaire (NPI-Q) (Severity subscale—S) - Everyday Abilities Scale for India (EASI) | Significant reduction in GHQ and NPI-Q (D) in the intervention group compared with control. (p-values not provided) | 23/27 | Dyad in study |
| Fialho, Köenig, Santos, Barbosa and Caramelli, 2012 [42] | Brazil n = 40 8 weeks | Quasi-experimental No control group. No sample or recruitment details | Cognitive-behavioural intervention program (Based on Training of Social Skills (TSS)) - Education - Cognitive, emotional, and social skills training - Support/empathy - Social comparison and shared learning - Strategies to modify own behaviour - Reinforcing persistence and effort - Cognitive strategies - Diary/therapy schedule - Activity organisation and preparation | 16 h Weekly sessions (2 h) | Caregiver: - Zarit Burden Interview (ZBI) - Quality of Life scale for caregivers of people living with Alzheimer’s Disease (QoL-AD) - The list of stress symptoms (LSS) - Jalowiec Coping scale (JCS) - Trait Anxiety Scale (A-Trait) (from the State Trait anxiety Inventory (STAI)) - Major depressive episode module of the Mini International Neuropsychiatric Interview (NPI- Q MINI) 5.0. (DSM-IV). PLWD: -QoL-AD for PLWD (answered by family) - Mini mental state examination (MMSE), Disability assessment for dementia (DAD) and Neuropsychiatric Intervention Questionnaire (NPI-Q) (only pre-intervention) | Significant reduction in reported NPI-Q symptoms (p = 0.034) Significant reduction in trait anxiety scores (A scale—STAI) (p = 0.005) Significant improvement in PLWD QoL-AD (p = 0.040) | 17/27 | |
| Gavrilova, Ferri, Mikhaylova, Sokolova, Banerjee and Prince, 2009 [43] * | Russia n = 60 5 weeks | Randomised control trial (single blind parallel group) Control: Treatment as usual Sample recruited via medical centres | 10/66 “Helping Carers to Care” Intervention 1—Assessment (1 session) (carer knowledge of dementia and family care arrangements) 2—Basic education (2 sessions) (introduction to dementia, the progression, causes, local care/treatment) 3—Training on ‘problem’ behaviour (2 sessions) (e.g., personal hygiene, dressing, repeated questioning, aggression, wandering) | 2.5 h Weekly 30-min sessions | Caregiver: - Zarit Burden Interview (ZBI) - Self-reporting questionnaire (SRQ-20) - Caregiver quality of life (WHOQOL-BREF) -Neuropsychiatric Inventory Questionnaire (NPI-Q) PLWD: -Neuropsychiatric Inventory Questionnaire (NPI-Q) -DEMQOL | Significant reduction in burden (ZBI) for intervention group compared with control (p = 0.03) | 25/27 | |
| Guerra, Ferri, Fonseca, Banerjee and Prince, 2011 [44] * | Peru n = 58 5 weeks | Randomised control trial Control: Waiting list Sample: Local survey and memory clinic | 10/66 “Helping Carers to Care” Intervention 1—Assessment (1 session) (carer knowledge of dementia and family care arrangements) 2—Basic education (2 sessions) (introduction to dementia, the progression, causes, local care/treatment) 3—Training on ‘problem’ behaviour (2 sessions) (e.g., personal hygiene, dressing, repeated questioning, aggression, wandering) | 2.5 h Weekly 30-min sessions | Caregiver: - Zarit Burden Interview (ZBI) - Self-reporting questionnaire (SRQ-20) - Caregiver quality of life (WHOQOL-BREF) -Neuropsychiatric Inventory Questionnaire (NPI-Q) PLWD: -Neuropsychiatric Inventory Questionnaire (NPI-Q) -DEMQOL | Significant reduction in burden (ZBI) for intervention group compared with control (p < 0.001) | 25/27 | |
| Han, Guo and Hong, 2022 [45] | China n = 159 3–6 months N.B. Carers could enter the intervention at different time points between 0 and 3 months | Quasi-experimental—single group repeated measures. No control Snowballing sample via online forum and health clinics | WeChat virtual community—professional facilitated peer support 6 elements: 1—Peer emotional support 2—Lectures and consultation (13 topics—,e.g., dementia knowledge, care strategies, communicating) 3—Technique support 4—Reading 5—Maintaining a friendly environment 6—Participation and peer support | Online access for 3–6 months | Caregiver: - Self- Efficacy Questionnaire for Chinese Family Caregivers (SEQCFC) -Neuropsychiatric Inventory Questionnaire (NPIQ) - Perceived Stress Scale of Chinese version (PSS-C) - Center for Epidemiologic Studies Depression Scale (CES-D10) - Zarit Burden Interview (ZBI) - Learned Helplessness Scale PLWD: None. | Statistically significant decrease in stress (PSS-C) (p < 0.05), helplessness (p < 0.001) and depression (CES-D10) (p < 0.05) Statistically significant increase in self-efficacy (SEQCFC) (p < 0.05) | 17/27 | |
| Hinton, Nguyen, Nguyen, Harvey, Nichols, Martindale-Adams, Nguyen, Nguyen, Nguyen, Nguyen, Nguyen, Nguyen, Nguyen, Nguyen, Tiet, Nguyen, Nguyen, Nguyen, and Pham, 2020 [32] *** | Vietnam n = 60 2 to 3 months | Pilot cluster randomised control trial Control: Single 1:1 face to face educational session about dementia and written dementia resources Sample: Convenience through clustered local health services | REACH VN—manualised multicomponent intervention Home visits 4 core training sessions: 1—Problem solving 2—Mood management/cognitive restructuring 3—Stress management 4—Communication + 2 more session based on clinical judgment/caregiver needs | Estimated 8.6 to 13 h. Weekly 1-h home visits | Caregiver: - Zarit Burden Interview (ZBI) (4 item) - Patient Health Questionnaire (PHQ-4) - Alzheimer’s disease knowledge scale PLWD: None. | Significant decrease in burden in favour of intervention (ZBI) (p = 0.02) Significant decrease in PHQ-4 in intervention compared with control (p = 0.03) | 24/27 | Feasibility measured through recruitment and retention statistics. |
| Javadpour, Ahmadzadeh and Bahredar, 2009 [46] | Iran n = 29 8 weeks | Quasi-experimental repeated measures No control Random sample (no further details given) | Educative support group Each session contained: - 30-min educative talks providing information about dementia/challenging behaviours/problems faced by caregivers - 90-min interactive activities including discussions and sharing experiences | 16 h Weekly 2-h sessions | Caregiver: - Perceived Stress Scale-10 (PSS-10) - General Health Questionnaire (GHQ) (Farsi) - Neuropsychiatry Inventory (NPI) PLWD: - Neuropsychiatry Inventory (NPI) - Clinical Dementia Rating (CDR) | Significant decreases in PSS scores (p = 0.0001), GHQ scores (p = 0.0001), NPI scores (p = 0.001) | 13/27 | All female caregivers |
| Kuzu, Beser, Zencir, Sahiner, Nesrin, Ahmet, Binali and Cagdas, 2005 [47] | Turkey n = 32 4 weeks | Quasi-experimental repeated measures No control Sample: Recruited through hospitals, Alzheimer’s association and community through word-of-mouth ad local media | Comprehensive educational program reinforced by an individualised component (CEPRIC) 3 components: 1—General information session (dementia, behaviour disorders, home and daily life) 2—Individualised educational component (specific problems identified through questionnaire) 3—Educational booklet | Not specified | Caregiver: - Duke Scale - Beck depression scale (BDS) - Beck anxiety inventory (BAI) PLWD: -Mini mental state examination (MMSE) | Significant decreases in BDS (p = 0.008), BAI (p = 0.01) Significant decreases in Duke scale subscales of physical health concerns (p = 0.001) and general health concerns (p = 0.004) | 18/27 | Dyad in study Nursing diagnoses also given before and after intervention |
| Magteppong and Yamarat, 2021 [48] | Thailand n = 60 8 weeks (follow-up at 20 weeks) | Quasi-experimental (pre/post parallel groups interventions study) Control: Treatment as usual (handbook provided post-intervention) Sample: Purposive via local hospital records and day centre attendees | Modified Transtheoretical Theory --of Stress and Coping (TTSC) Program (multicomponent) Aims: increase caregiver knowledge, reduce burden and increase quality of life Week: 1—Group health education (handbook provided) 2—Home visit (Stress, appraisal and coping) 3 -7—Telephone follow-ups 8—Home visit (Stress, appraisal and coping) | 3.25—6.20 h Weekly contact- 1 × group meeting 2 × home visits 5 × telephone follow-ups | Caregiver: - Dementia Knowledge Assessment (DKA) - Thai burden interview for caregivers of patients with chronic illness - World Health Organisation’s Quality of life—Thai (WHO QoL) PLWD: None. | Significant increase in knowledge score for intervention compared with control at week 8 and 20 (p < 0.05) Significant difference in quality of life in favour of intervention compared with control at 8 and 20 weeks (p < 0.05) | 22/27 | |
| Pankong, Pothiban, Sucamvang and Khampolsiri, 2018 [49] | Thailand n = 72 8 weeks (follow-up at 12 and 20 weeks) | Randomised control trial Control: Treatment as usual Sample: Invited through local hospitals | Enhancing positive aspects of caregiving program 6 group sessions covering: 1—dementia knowledge/ADLs/behaviour management 2—meditation and spirituality 3—sharing experiences 4—role modelling/verbal reinforcements 1 individual session + dementia care booklet | 12 h 6 × 2-h sessions Additional phone call, length not specified | Caregiver: - Positive aspects of caring questionnaire (PACQ) - Thai general wellbeing schedule (TGWS) PLWD: None. | Significant increase PACQ in intervention compared with control at weeks 8, 12 and 20 (p < 0.0001) Significant increase in wellbeing (TGWS) scores over time (p < 0.001) but no significant difference between the groups | 22/27 | |
| Santos, Sousa, Arcoverde and Dourado, 2013 [50] | Brazil n = 18 6 months | Quasi-experimental (pre/post) No control No sampling details given | Psychoeducational group (based on STAR-Caregivers model) Sessions included discussions about experiences, expressing emotions and educational lectures about dementia (types, BPSD ** etc.) | 39 h Weekly 90-min sessions | Caregiver: - Caregivers version of quality of life in Alzheimer’s disease scale(QoL-AD) - Zarit Burden Interview (ZBI) - Beck depression inventory (BDI) - Beck Anxiety inventory (BAI) PLWD: -Clinical dementia rating (CDR) - Pfeffer Functional Activities Questionnaire (FAQ) -Cornell scale for depression in dementia (CSDD) -Neuropsychiatric Inventory Questionnaire (NPIQ) | Significant decrease in BDI scores between pre and post-assessments (p = 0.011) | 17/27 | |
| Senanarong, Jamjumras, Harmphadungkit, Klubwongs, Udomphanthurak, Poungvarin, Vannasaeng and Cummings, 2004 [51] | Thailand n = 50 6 months | Randomised parallel group intervention study. Control: Treatment as usual Sample: Recruited from hospital memory clinic | Counselling intervention for caregivers Content of group counselling and support sessions: - Sharing experiences - Information provided about techniques/coping - educational content (dementia prognosis and progression etc.) - Adaptions to environment - Identifying needs and understanding behaviours | 3.75 h 5 × 45-min sessions (every 6–8 weeks) | Caregiver: Neuropsychiatric Inventory Questionnaire (NPIQ) PLWD: - Thai mental state examination (TMSE) - Functional assessment questionnaire (FAQ) -Thai activities of daily living measure -Clinical dementia rating (CDR) | Significant decrease in NPI-Q scores in intervention group between baseline and month 6 (p = 0.045) but not between the groups | 22/27 | |
| Shata, Amin, El-Kady and Abu-Nazel, 2017 [52] | Egypt n = 120 8 weeks—(post-measures after 3 months) | Randomised control trial Control: Waiting list Sample: Convenience sample through hospital clinic | Multicomponent psychosocial intervention program 3 components: 1- Group psychoeducation (2 sessions) 2—Brief group CBT (6 sessions) 3—Group support sessions (parallel to all sessions) | 6–8 h Weekly 45–60-min sessions | Caregiver: - Knowledge questionnaire - Hamilton Depression Rating Scale (HDRS) (Arabic) - Taylor Manifest anxiety scale (TMAS) - Zarit Burden Interview (ZBI) PLWD: - Mini mental state examination (MMSE) | Significant decrease in anxiety (TMAS), depression (HDRS) and perceived burden (ZBI) for intervention compared with control at 8 weeks and 3 months (p < 0.001) Significant improvement in dementia knowledge in intervention group compared with control (p < 0.001) | 24/27 | |
| Tawfik, Sabry, Darwish, Mowafy and Soliman, 2021 [53] | Egypt n = 60 8 weeks | Randomised control trial Control: Treatment as usual Sample: Identified by researcher at Cairo University hospital outpatient unit | Psychoeducational Program Main objectives: 1—Giving information about different dementia behaviours (e.g., agitation, wandering) and tips to deal with them. 2—Caregiver support and de-stress techniques Sessions included role playing, brainstorming, group discussion and videos. | 8 h Weekly 1-h sessions | Caregiver: - Zarit Burden Interview (ZBI) - Arabic Quality of life in Alzheimer’s disease questionnaire for caregivers (QoL-AD) PLWD: Arabic Quality of life in Alzheimer’s disease questionnaire for patients (QoL-AD) | Significant improvement in ZBI scores for intervention group compared with control at post-measure (p < 0.001) | 23/27 | |
| Tran, Nguyen, Pham, Nguyen, Nguyen, Nguyen, Harvey, and Hinton, 2022 [54] *** | Vietnam n = 60 2 to 3 months | Pilot cluster randomised control trial Control: Single 1:1 face to face educational session about dementia and written dementia resources Sample: Convenience through clustered local health services | REACH VN—manualised multicomponent intervention Home visits 4 core training sessions: 1—Problem solving 2—Mood management/cognitive restructuring 3—Stress management 4—Communication + 2 more session based on clinical judgment/caregiver needs | Estimated 8.6 to 13 h. Weekly 1-h home visits | Caregiver: REACH risk priority assessment (from REACH VA manual) (Variables: general health, caregiver frustrations, stress symptoms, general stress, behaviours, bother with behaviours) PLWD: None. | Significant decrease in caregiver frustration variable in intervention group compared with control (p = 0.01) | 22/27 | |
| Zakaria and Ab Razak, 2017 [55] | Malaysia n = 16 12 weeks | Quasi-experimental pre/post-study No control Sample: Convenience recruitment through a local memory clinic | Cultural-based support group Facilitated by healthcare professionals. Each session had 2 parts: 1—Psychoeducation session. 2—Mutual sharing and problem-solving Theme examples: - Introduction to principles and role within support group - understanding dementia - practical caregiving skills - supports for caregivers - effective communication - safe and healthy environment | 12 h 2-h sessions every 2 weeks. | Caregiver: - Caregiver strain index (CSI) - Hospital anxiety and depression scale (HADS) - Caregiver quality of life (WHOQOL-BREF) PLWD: None. | Significant decrease in CSI scores from pre to post (p = 0.01) Significant improvement in specific domains of the WHOQOL-BREF from pre to post: physical (p = 0.01), psychological (p = 0.006) and environmental (p = 0.002) | 19/27 | |
| Zhang, Wu, Tang, Rong, Guo, Fang, Zhao, and Zhao, 2020 [56] | China n = 41 36 weeks | Quasi-experimental Control: individual telephone support Sample: Recruited from 2 hospitals | Caregiver self-management support intervention (C-SMS) Components: 1—Illustrated educational booklet (3 volumes—basic dementia care knowledge, symptom and problem identification and interventions, knowledge and skills for self-management) and a booklet of local contact details and support options) 2–6 bi-weekly support group sessions (12 weeks) 3–3 educational presentations during a 6-month follow-up period | 15–18 h 6 × 2-weekly 2.5–3-h group sessions + 3 presentations with length not specified (over 6-month follow-up period) | Caregiver: - Caregiver health related QoL (HRQoL) - Self-efficacy questionnaire for Chinese family caregivers (SEQCFC) PLWD: -Chinese version of the Disability Assessment in Dementia (DAD) -Neuropsychiatric Inventory-Questionnaire (NPI-Q) | Significant improvement in HRQoL in intervention compared with control (p = 0.017) Significant improvement in specific domains of self-efficacy for intervention compared with control: managing BPSD * (p = 0.013) and managing distress (p = 0.034) | 20/27 | Also measured physical outcomes—instances of caregiver metabolic syndrome Also measured retention and attrition statistics |
| Overall Study Content/Design | Study Authors | Educational Delivery Methods | Educational Content | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Didactic | Written | Discussion | Interactive | Individual | Dementia Knowledge | Delivering Care | BPSD | Self-Care | Local Resources | ||
| Educational | Javadpour et al., 2009 [46] | X | X | X | X | X | X | ||||
| Kuzu, et al., 2005 [47] | X | X | X | X | X | X | X | X | |||
| Santos et al., 2013 [50] | X | X | X | X | X | X | |||||
| Tawfik et al., 2021 [53] | X | X | X | X | X | ||||||
| Therapeutic | Fialho et al., 2012 [42] | X | X | X | X | X | |||||
| Senanarong et al., 2004 [51] | X | X | X | X | X | ||||||
| Support | Han et al., 2022 [45] | X | X | X | X | X | X | X | |||
| Zakaria and Ab Razak, 2017 [55] | X | X | X | X | X | ||||||
| Multi-component | Baruah et al., 2021 [40] | X | X | X | X | X | |||||
| Dias et al., 2008 [41] | X | X | X | X | X | X | |||||
| Gavrilova et al., 2009 [43]/Guerra et al., 2011 [44] | X | X | X | X | X | ||||||
| Hinton et al., 2020 [32]/Tran et al., 2022 [54] | X | X | X | X | X | X | |||||
| Magteppong and Yamarat, 2021 [48] | X | X | X | X | X | X | X | X | |||
| Pankong et al., 2018 [49] | X | X | X | X | X | X | X | X | X | ||
| Shata et al., 2017 [52] | X | X | X | X | X | X | |||||
| Zhang et al., 2020 [56] | X | X | X | X | X | X | X | X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, I.; Patel, R.; Stoner, C.R.; Melville, M.; Spector, A. A Systematic Review of Educational Interventions for Informal Caregivers of People Living with Dementia in Low and Middle-Income Countries. Behav. Sci. 2024, 14, 177. https://doi.org/10.3390/bs14030177
Evans I, Patel R, Stoner CR, Melville M, Spector A. A Systematic Review of Educational Interventions for Informal Caregivers of People Living with Dementia in Low and Middle-Income Countries. Behavioral Sciences. 2024; 14(3):177. https://doi.org/10.3390/bs14030177
Chicago/Turabian StyleEvans, Isabelle, Ria Patel, Charlotte R. Stoner, Mel Melville, and Aimee Spector. 2024. "A Systematic Review of Educational Interventions for Informal Caregivers of People Living with Dementia in Low and Middle-Income Countries" Behavioral Sciences 14, no. 3: 177. https://doi.org/10.3390/bs14030177
APA StyleEvans, I., Patel, R., Stoner, C. R., Melville, M., & Spector, A. (2024). A Systematic Review of Educational Interventions for Informal Caregivers of People Living with Dementia in Low and Middle-Income Countries. Behavioral Sciences, 14(3), 177. https://doi.org/10.3390/bs14030177





