Mirror Therapy in Patients with Somatoform Pain Disorders—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Study Procedure
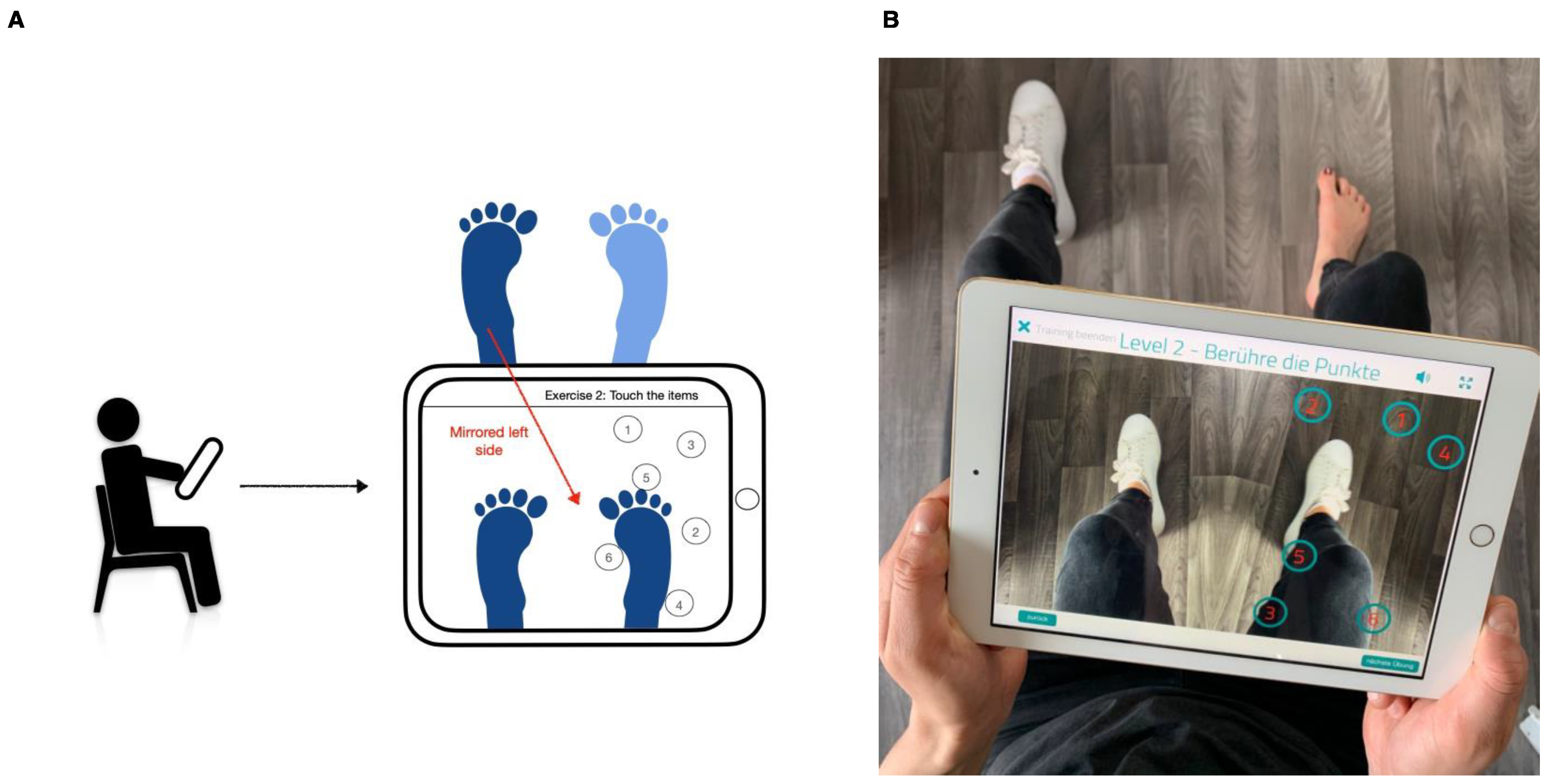
2.3. Mirror Therapy
2.4. Psychometric Parameters
2.5. Physiological Parameters
2.6. Psychophysical Parameters
2.7. Statistical Analysis
3. Results
3.1. Psychometric Parameters
3.1.1. Baseline Measurements at T0
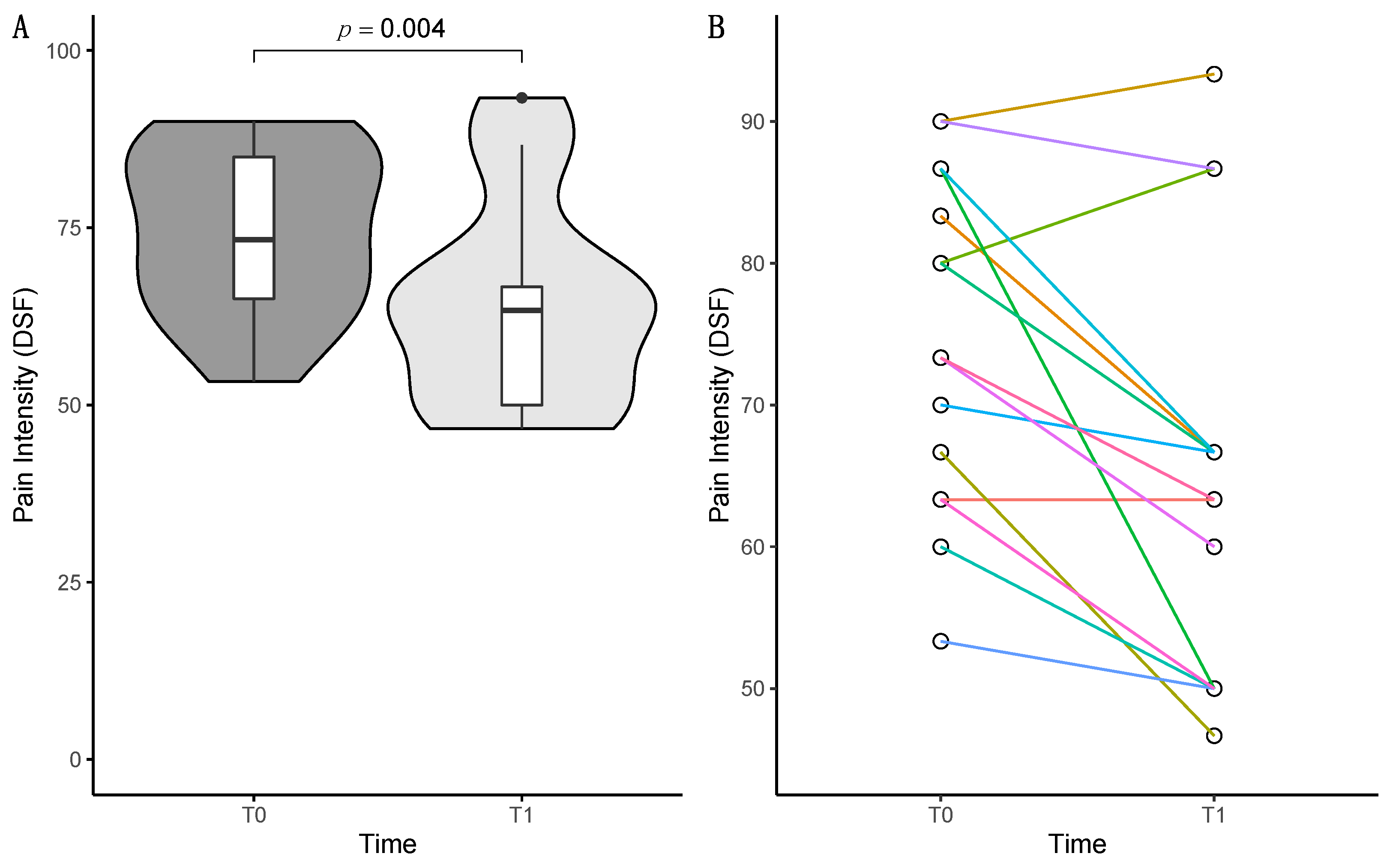
3.1.2. DSF Comparison of T0 and T1
3.1.3. Exploratory Data Analysis of the Reduction in Pain Intensity
3.2. Physiological and Psychophysical Parameters
3.2.1. HRV Comparison of T0 and T1
3.2.2. Thermal Detection and Pain Threshold Comparison of T0 and T1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of Chronic Pain in the UK: A Systematic Review and Meta-Analysis of Population Studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A Classification of Chronic Pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef]
- Egloff, N.; Cámara, R.J.A.; von Känel, R.; Klingler, N.; Marti, E.; Ferrari, M.-L.G. Hypersensitivity and Hyperalgesia in Somatoform Pain Disorders. Gen. Hosp. Psychiatry 2014, 36, 284–290. [Google Scholar] [CrossRef]
- Murray, A.M.; Toussaint, A.; Althaus, A.; Löwe, B. The Challenge of Diagnosing Non-Specific, Functional, and Somatoform Disorders: A Systematic Review of Barriers to Diagnosis in Primary Care. J. Psychosom. Res. 2016, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Landa, A.; Peterson, B.S.; Fallon, B.A. Somatoform Pain. Psychosom. Med. 2012, 74, 717–727. [Google Scholar] [CrossRef]
- Meyer, C.; Rumpf, H.-J.; Hapke, U.; Dilling, H.; John, U. Lebenszeitprävalenz Psychischer Störungen in Der Erwachsenen Allgemeinbevölkerung. Nervenarzt 2000, 71, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hilderink, P.H.; Collard, R.; Rosmalen, J.G.M.; Oude Voshaar, R.C. Prevalence of Somatoform Disorders and Medically Unexplained Symptoms in Old Age Populations in Comparison with Younger Age Groups: A Systematic Review. Ageing Res. Rev. 2013, 12, 151–156. [Google Scholar] [CrossRef]
- Dilling, H.; Mombour, W.; Schmidt, M.H. Internationale Klassifikation Psychischer Störungen: ICD-10 Kapitel V (F). Klinisch-Diagnostische Leitlinien; Huber: Bern, Switzerland, 2011; ISBN 3456850182. [Google Scholar]
- Demyttenaere, K.; Bruffaerts, R.; Lee, S.; Posada-Villa, J.; Kovess, V.; Angermeyer, M.C.; Levinson, D.; de Girolamo, G.; Nakane, H.; Mneimneh, Z.; et al. Mental Disorders among Persons with Chronic Back or Neck Pain: Results from the World Mental Health Surveys. Pain 2007, 129, 332–342. [Google Scholar] [CrossRef]
- Sumathipala, A. What Is the Evidence for the Efficacy of Treatments for Somatoform Disorders? A Critical Review of Previous Intervention Studies. Psychosom. Med. 2007, 69, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Cutler, R.B.; Rosomoff, H.L.; Steele Rosomoff, R. Do Antidepressants Have an Analgesic Effect in Psychogenic Pain and Somatoform Pain Disorder? A Meta-Analysis. Psychosom. Med. 1998, 60, 503–509. [Google Scholar] [CrossRef]
- Kroenke, K.; Swindle, R. Cognitive-Behavioral Therapy for Somatization and Symptom Syndromes: A Critical Review of Controlled Clinical Trials. Psychother. Psychosom. 2000, 69, 205–215. [Google Scholar] [CrossRef]
- Allen, L.A.; Escobar, J.I.; Lehrer, P.M.; Gara, M.A.; Woolfolk, R.L. Psychosocial Treatments for Multiple Unexplained Physical Symptoms: A Review of the Literature. Psychosom. Med. 2002, 64, 939–950. [Google Scholar] [CrossRef] [PubMed]
- van Dessel, N.; den Boeft, M.; van der Wouden, J.C.; Kleinstäuber, M.; Leone, S.S.; Terluin, B.; Numans, M.E.; van der Horst, H.E.; van Marwijk, H. Non-Pharmacological Interventions for Somatoform Disorders and Medically Unexplained Physical Symptoms (MUPS) in Adults. Cochrane Database Syst. Rev. 2014, 11, CD011142. [Google Scholar] [CrossRef]
- Rask, M.T.; Rosendal, M.; Fenger-Grøn, M.; Bro, F.; Ørnbøl, E.; Fink, P. Sick Leave and Work Disability in Primary Care Patients with Recent-Onset Multiple Medically Unexplained Symptoms and Persistent Somatoform Disorders: A 10-Year Follow-up of the FIP Study. Gen. Hosp. Psychiatry 2015, 37, 53–59. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Rogers-Ramachandran, D.; Cobb, S. Touching the Phantom Limb. Nature 1995, 377, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Foell, J.; Bekrater-Bodmann, R.; Diers, M.; Flor, H. Mirror Therapy for Phantom Limb Pain: Brain Changes and the Role of Body Representation. Eur. J. Pain 2014, 18, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; Llewellyn, D.M.E.; Ramachandran, V. Rehabilitation of Hemiparesis after Stroke with a Mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef]
- Pervane Vural, S.; Nakipoglu Yuzer, G.F.; Sezgin Ozcan, D.; Demir Ozbudak, S.; Ozgirgin, N. Effects of Mirror Therapy in Stroke Patients with Complex Regional Pain Syndrome Type 1: A Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2016, 97, 575–581. [Google Scholar] [CrossRef]
- Schwarzer, A.; Glaudo, S.; Zenz, M.; Maier, C. Mirror Feed-Back—A New Method for the Treatment of Neuropathic Pain. Dtsch. Med. Wochenschr. 2007, 132, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Gallace, A.; Spence, C. Is Mirror Therapy All It Is Cracked up to Be? Current Evidence and Future Directions. Pain 2008, 138, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, F.J.A.; Smorenburg, A.R.P.; Benham, A.; Ledebt, A.; Feltham, M.G.; Savelsbergh, G.J.P. Reflections on Mirror Therapy. Neurorehabil. Neural Repair 2015, 29, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, P.G.; Johnson, M.I. Mirror Therapy: A Potential Intervention for Pain Management. Rev. Assoc. Med. Bras. 2017, 63, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S.; Altschuler, E.L. The Use of Visual Feedback, in Particular Mirror Visual Feedback, in Restoring Brain Function. Brain 2009, 132, 1693–1710. [Google Scholar] [CrossRef]
- Moseley, G.L.; Gallace, A.; Spence, C. Bodily Illusions in Health and Disease: Physiological and Clinical Perspectives and the Concept of a Cortical “Body Matrix”. Neurosci. Biobehav. Rev. 2012, 36, 34–46. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The Biopsychosocial Approach to Chronic Pain: Scientific Advances and Future Directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins, Methods, and Interpretative Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-Analytic Evidence for Decreased Heart Rate Variability in Chronic Pain Implicating Parasympathetic Nervous System Dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, P.M.; Reis, F.J.J.; Sequeira, V.C.C.; Chaves, A.C.S.; Fernandes, O.; Arruda-Sanchez, T. Heart Rate Variability in Patients with Low Back Pain: A Systematic Review. Scand. J. Pain 2021, 21, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ying-Chih, C.; Yu-Chen, H.; Wei-Lieh, H. Heart Rate Variability in Patients with Somatic Symptom Disorders and Functional Somatic Syndromes: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 112, 336–344. [Google Scholar] [CrossRef]
- Kalezic, N.; Åsell, M.; Kerschbaumer, H.; Lyskov, E. Physiological Reactivity to Functional Tests in Patients with Chronic Low Back Pain. J. Musculoskelet. Pain 2007, 15, 29–40. [Google Scholar] [CrossRef]
- Zimmermann-Viehoff, F.; Thayer, J.; Bergt, J.; Weber, C.S.; Erdur, L.; Richter, S.; Deter, H.-C. Heart Rate Variability during Inpatient Psychosomatic Treatment—A Naturalistic Observational Study. Z. Psychosom. Med. Psychother. 2016, 62, 20–31. [Google Scholar] [CrossRef]
- Schoenen, J.; Bottin, D.; Hardy, F.; Gerard, P. Cephalic and Extracephalic Pressure Pain Thresholds in Chronic Tension-Type Headache. Pain 1991, 47, 145–149. [Google Scholar] [CrossRef]
- Maixner, W.; Fillingim, R.; Booker, D.; Sigurdsson, A. Sensitivity of Patients with Painful Temporomandibular Disorders to Experimentally Evoked Pain. Pain 1995, 63, 341–351. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Verneuer, B.; Dany, K.; Marziniak, M.; Wolowski, A.; Çolak-Ekici, R.; Schulte, T.L.; Bullmann, V.; Grewe, S.; Gralow, I.; et al. Validation of the Pain Sensitivity Questionnaire in Chronic Pain Patients. Pain 2012, 153, 1210–1218. [Google Scholar] [CrossRef]
- Edwards, R.R.; Haythornthwaite, J.A.; Tella, P.; Max, M.B.; Raja, S. Basal Heat Pain Thresholds Predict Opioid Analgesia in Patients with Postherpetic Neuralgia. Anesthesiology 2006, 104, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.R.; Doleys, D.M.; Lowery, D.; Fillingim, R.B. Pain Tolerance as a Predictor of Outcome Following Multidisciplinary Treatment for Chronic Pain: Differential Effects as a Function of Sex. Pain 2003, 106, 419–426. [Google Scholar] [CrossRef]
- Heathers, J.A.J. Everything Hertz: Methodological Issues in Short-Term Frequency-Domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Selles, R.W.; van der Geest, J.N.; Eckhardt, M.; Yavuzer, G.; Stam, H.J.; Smits, M.; Ribbers, G.M.; Bussmann, J.B.J. Motor Recovery and Cortical Reorganization After Mirror Therapy in Chronic Stroke Patients. Neurorehabil. Neural Repair 2011, 25, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Santos, J.; Soto-Camara, R.; Rodriguez-Fernández, P.; Jimenez-Barrios, M.; Gonzalez-Bernal, J.; Collazo-Riobo, C.; Jahouh, M.; Bravo-Anguiano, Y.; Trejo-Gabriel-Galan, J.M. Effects of Home-Based Mirror Therapy and Cognitive Therapeutic Exercise on the Improvement of the Upper Extremity Functions in Patients with Severe Hemiparesis after a Stroke: A Protocol for a Pilot Randomised Clinical Trial. BMJ Open 2020, 10, e035768. [Google Scholar] [CrossRef] [PubMed]
- Rothgangel, A.; Braun, S.; Winkens, B.; Beurskens, A.; Smeets, R. Traditional and Augmented Reality Mirror Therapy for Patients with Chronic Phantom Limb Pain (PACT Study): Results of a Three-Group, Multicentre Single-Blind Randomized Controlled Trial. Clin. Rehabil. 2018, 32, 1591–1608. [Google Scholar] [CrossRef]
- Petzke, F.; Hüppe, M.; Kohlmann, T.; Kükenshöner, S.; Lindena, G.; Pfingsten, M.; Schneider, K.; Nagel, N. Handbuch Deutscher Schmerz-Fragebogen 2022. 2022. Available online: https://www.schmerzgesellschaft.de/fileadmin/2022/PDFs/DSF_Handbuch_2022.pdf (accessed on 1 May 2023).
- Basler, H.; Herda, C.; Scharfenstein, A. Marburger Fragebogen Zum Habituellen Wohlbefinden. In Diagnostische Verfahren zu Lebensqualität und Wohlbefinden; Schumacher, J., Klaiberg, A., Brähler, E., Eds.; Hogrefe: Göttingen, Germany, 2003; pp. 212–215. [Google Scholar]
- Lovibond, P.F.; Lovibond, S.H. The Structure of Negative Emotional States: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nagel, B.; Gerbershagen, H.U.; Lindena, G.; Pfingsten, M. Entwicklung Und Empirische Überprüfung Des Deutschen Schmerzfragebogens Der DGSS. Schmerz 2002, 16, 263–270. [Google Scholar] [CrossRef]
- Mazurak, N.; Sauer, H.; Weimer, K.; Dammann, D.; Zipfel, S.; Horing, B.; Muth, E.R.; Teufel, M.; Enck, P.; Mack, I. Effect of a Weight Reduction Program on Baseline and Stress-Induced Heart Rate Variability in Children with Obesity. Obesity 2016, 24, 439–445. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M.P. A Robust Algorithm for Heart Rate Variability Time Series Artefact Correction Using Novel Beat Classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Weimer, K.; Hahn, E.; Mönnikes, N.; Herr, A.K.; Stengel, A.; Enck, P. Are Individual Learning Experiences More Important Than Heritable Tendencies? A Pilot Twin Study on Placebo Analgesia. Front. Psychiatry 2019, 10, 679. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, -D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative Sensory Testing in the German Research Network on Neuropathic Pain (DFNS): Standardized Protocol and Reference Values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows 2020; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: New York, NY, USA, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2022. [Google Scholar]
- Méndez-Rebolledo, G.; Gatica-Rojas, V.; Torres-Cueco, R.; Albornoz-Verdugo, M.; Guzmán-Muñoz, E. Update on the Effects of Graded Motor Imagery and Mirror Therapy on Complex Regional Pain Syndrome Type 1: A Systematic Review. J. Back Musculoskelet. Rehabil. 2017, 30, 441–449. [Google Scholar] [CrossRef]
- Achenbach, J.; Tran, A.-T.; Jaeger, B.; Kapitza, K.; Bernateck, M.; Karst, M. Quantitative Sensory Testing in Patients with Multisomatoform Disorder with Chronic Pain as the Leading Bodily Symptom—A Matched Case–Control Study. Pain Med. 2019, 21, e54–e61. [Google Scholar] [CrossRef]
- Gerhardt, A.; Eich, W.; Janke, S.; Leisner, S.; Treede, R.D.; Tesarz, J. Chronic Widespread Back Pain Is Distinct from Chronic Local Back Pain. Clin. J. Pain 2016, 32, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Baron, R. Translation of Symptoms and Signs into Mechanisms in Neuropathic Pain. Pain 2003, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Angelovski, A.; Sattel, H.; Henningsen, P.; Sack, M. Heart Rate Variability Predicts Therapy Outcome in Pain-Predominant Multisomatoform Disorder. J. Psychosom. Res. 2016, 83, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Jarczok, M.N.; Ellis, R.J.; Hillecke, T.K.; Thayer, J.F. Heart Rate Variability and Experimentally Induced Pain in Healthy Adults: A Systematic Review. Eur. J. Pain 2014, 18, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Terkelsen, A.J.; Mølgaard, H.; Hansen, J.; Andersen, O.K.; Jensen, T.S. Acute Pain Increases Heart Rate: Differential Mechanisms during Rest and Mental Stress. Auton. Neurosci. Basic Clin. 2005, 121, 101–109. [Google Scholar] [CrossRef]
- Chouchoul, F.; Pichotl, V.; Perchetl, C.; Legrainl, V.; Garcia-Larreal, L.; Rochel, F.; Bastujil, H. Autonomic Pain Responses during Sleep: A Study of Heart Rate Variability. Eur. J. Pain 2011, 15, 554–560. [Google Scholar] [CrossRef]
- Huang, W.L.; Liao, S.C.; Yang, C.C.H.; Kuo, T.B.J.; Chen, T.T.; Chen, I.M.; Gau, S.S.F. Measures of Heart Rate Variability in Individuals with Somatic Symptom Disorder. Psychosom. Med. 2017, 79, 34–42. [Google Scholar] [CrossRef]
- Heiss, S.; Vaschillo, B.; Vaschillo, E.G.; Timko, C.A.; Hormes, J.M. Heart Rate Variability as a Biobehavioral Marker of Diverse Psychopathologies: A Review and Argument for an “Ideal Range”. Neurosci. Biobehav. Rev. 2021, 121, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A Quantitative Systematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. PACE -Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Galaasen Bakken, A.; Eklund, A.; Hallman, D.M.; Axén, I. The Effect of Spinal Manipulative Therapy and Home Stretching Exercises on Heart Rate Variability in Patients with Persistent or Recurrent Neck Pain: A Randomized Controlled Trial. Chiropr. Man. Ther. 2021, 29, 48. [Google Scholar] [CrossRef]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart Rate Variability Today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mosley, E.; Laborde, S. A scoping review of heart rate variability in sport and exercise psychology. Int. Rev. Sport Exerc. Psychol. 2022, 1–75. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Kuhn, W.; Morsch, K.; Schäfer, A.; Hillecke, T.K.; Thayer, J.F. Impact of Caffeine on Heart Rate Variability: A Systematic Review. J. Caffeine Res. 2013, 3, 22–37. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. In Cochrane Database of Systematic Reviews; Geneen, L.J., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2017; Volume 1, p. CD011279. [Google Scholar]
- Majeed, M.H.; Ali, A.A.; Sudak, D.M. Mindfulness-Based Interventions for Chronic Pain: Evidence and Applications. Asian J. Psychiatr. 2018, 32, 79–83. [Google Scholar] [CrossRef]
| Parameter | Distribution: n (%), Median (Q1–Q3) |
|---|---|
| Female | 9 (60) |
| Age (years) | 39 (28–55) |
| BMI (kg/m2) | 28.5 (26.00–29.25) |
| Children (yes) | 9 (60) |
| Marriage (yes) | 9 (60) |
| Diagnosed Comorbidities | |
| Depressive disorder | 9 (60) |
| Anxiety disorder | 4 (26.7) |
| Medication at Baseline | |
| Antidepressant | 5 (33.3) |
| Antipsychotics | 1 (6.7) |
| Anticonvulsant drugs | 4 (26.7) |
| Questionnaire | T0: M (SD) | T1: M (SD) | Test Value | p Value |
|---|---|---|---|---|
| DSF | ||||
| Pain intensity [0–100] | 74.67 (11.67) | 64.44 (14.67) | z = −2.878 | 0.004 * |
| Current pain [0–10] | 6.33 (1.84) | 5.73 (2.05) | z = −1.897 | 0.058 |
| Average pain [0–10] | 7.00 (1.20) | 5.80 (1.74) | t(14) = 3.850 | 0.002 * |
| Greatest pain [0–10] | 9.07 (1.39) | 7.80 (2.46) | z = −1.812 | 0.070 |
| Disability [0–6] | 4.40 (1.77) | 3.87 (2.03) | z = −1.121 | 0.262 |
| MFHW | ||||
| Wellbeing [0–35] | 12.27 (8.84) | 14.27 (9.53) | t(14) = −1.651 | 121 |
| DASS | ||||
| Depression [0–21] | 15.07 (6.75) | 15.00 (5.92) | t(14) = 0.066 | 948 |
| Anxiety [0–21] | 13.07 (6.03) | 12.67 (5.62) | z = −0.448 | 654 |
| Stress [0–21] | 15.60 (5.55) | 14.80 (5.72) | t(14) = 0.939 | 364 |
| Measure | T0: M (SD) | T1: M (SD) | Test Value | p Value |
|---|---|---|---|---|
| HRV | ||||
| Mean RR (ms) | 804.14 (149.62) | 743.59 (138.23) | t(13) = 1.83 | 0.091 |
| RMSSD (ms) | 23.94 (10.71) | 20.57 (11.64) | t(13) = 0.859 | 0.406 |
| LF absolute (ms2) | 659.64 (473.93) | 372.46 (239.18) | t(13) = 2.536 | 0.025 * |
| HF absolute (ms2) | 230.07 (155.95) | 213.93 (202.75) | z = −0.722 | 0.470 |
| LF normalized (nu) | 72.18 (12.05) | 71.35 (17.31) | t(13) = 0.176 | 0.863 |
| HF normalized (nu) | 27.78 (12.04) | 28.57 (17.20) | t(13) = −0.169 | 0.868 |
| LF/HF ratio | 3.19 (1.63) | 4.35 (5.19) | z = −1.036 | 0.300 |
| Thermal detection thresholds (in °C) | ||||
| CDT | 29.65 (2.56) | 30.44 (1.63) | z = −1.070 | 0.285 |
| WDT | 34.71 (1.84) | 34.81 (2.15) | t(14) = −0.164 | 0.872 |
| Pain thresholds (in °C) | ||||
| CPT | 12.33 (11.30) | 15.37 (11.94) | z = −2.040 | 0.041 * |
| HPT | 43.25 (4.56) | 43.46 (4.32) | z = −0.995 | 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruf, S.P.; Hetterich, L.; Mazurak, N.; Rometsch, C.; Jurjut, A.-M.; Ott, S.; Herrmann-Werner, A.; Zipfel, S.; Stengel, A. Mirror Therapy in Patients with Somatoform Pain Disorders—A Pilot Study. Behav. Sci. 2023, 13, 432. https://doi.org/10.3390/bs13050432
Ruf SP, Hetterich L, Mazurak N, Rometsch C, Jurjut A-M, Ott S, Herrmann-Werner A, Zipfel S, Stengel A. Mirror Therapy in Patients with Somatoform Pain Disorders—A Pilot Study. Behavioral Sciences. 2023; 13(5):432. https://doi.org/10.3390/bs13050432
Chicago/Turabian StyleRuf, Steffen Philipp, Larissa Hetterich, Nazar Mazurak, Caroline Rometsch, Anna-Maria Jurjut, Stephan Ott, Anne Herrmann-Werner, Stephan Zipfel, and Andreas Stengel. 2023. "Mirror Therapy in Patients with Somatoform Pain Disorders—A Pilot Study" Behavioral Sciences 13, no. 5: 432. https://doi.org/10.3390/bs13050432
APA StyleRuf, S. P., Hetterich, L., Mazurak, N., Rometsch, C., Jurjut, A.-M., Ott, S., Herrmann-Werner, A., Zipfel, S., & Stengel, A. (2023). Mirror Therapy in Patients with Somatoform Pain Disorders—A Pilot Study. Behavioral Sciences, 13(5), 432. https://doi.org/10.3390/bs13050432







