Working-Memory-Guided Attention Competes with Exogenous Attention but Not with Endogenous Attention
Abstract
1. Introduction
2. Experiment 1
2.1. Method
2.1.1. Participants
2.1.2. Apparatus and Stimuli
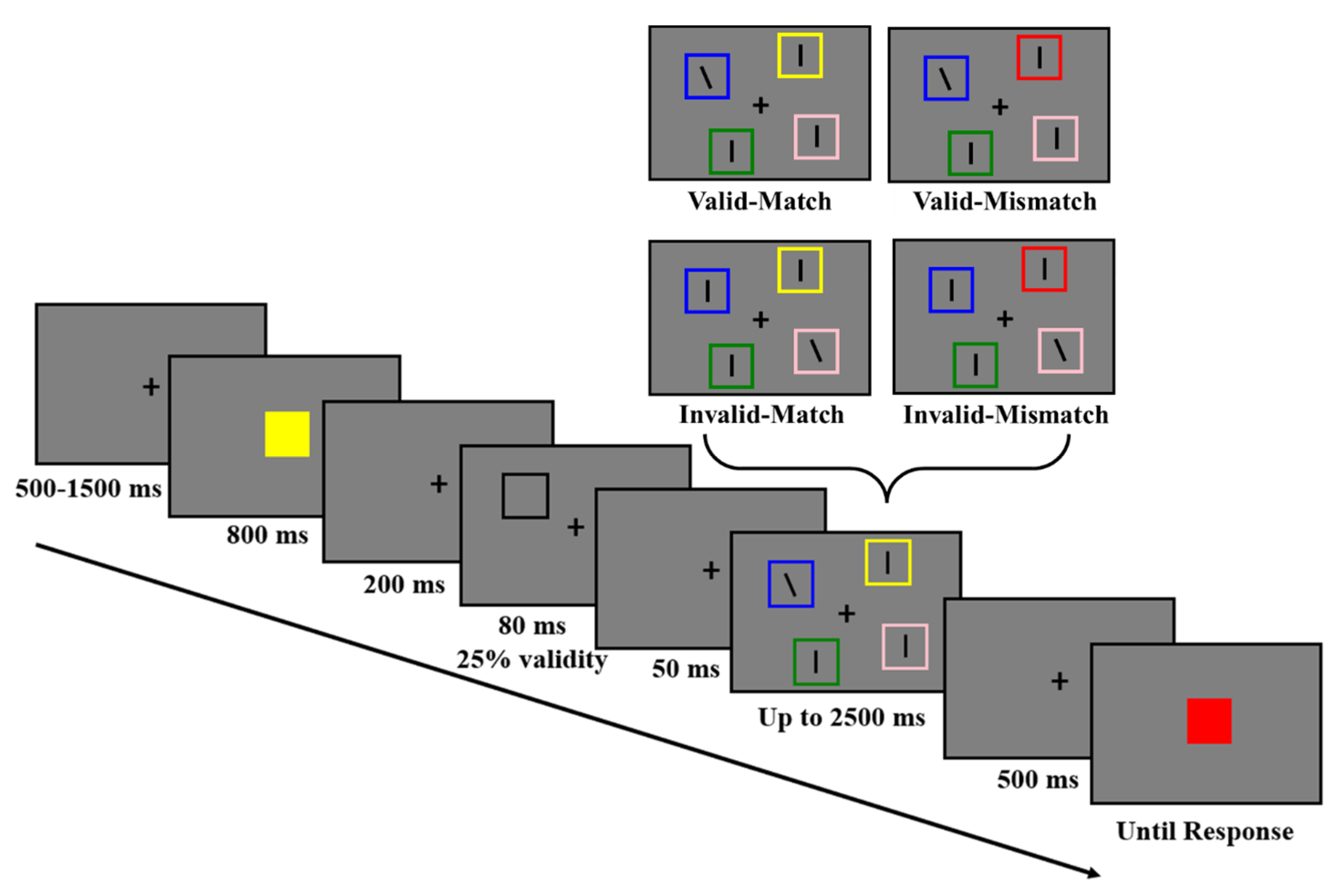
2.1.3. Procedures and Design
2.1.4. Data Analysis
2.2. Results
3. Experiment 2
3.1. Method
3.1.1. Participants
3.1.2. Procedures and Design
3.2. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awh, E.; Vogel, E.K.; Oh, S.H. Interactions between attention and working memory. Neuroscience 2006, 139, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gazzaley, A. Influence of early attentional modulation on working memory. Neuropsychologia 2011, 49, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Gazzaley, A.; Nobre, A.C. Top-down modulation: Bridging selective attention and working memory. Trends Cogn. Sci. 2012, 16, 129–135. [Google Scholar] [CrossRef]
- Olivers, C.N.L. What Drives Memory-Driven Attentional Capture? The Effects of Memory Type, Display Type, and Search Type. J. Exp. Psychol.-Hum. Percept. Perform. 2009, 35, 1275–1291. [Google Scholar] [CrossRef]
- Olivers, C.N.L.; Meijer, F.; Theeuwes, J. Feature-Based Memory-Driven Attentional Capture. J. Exp. Psychol.-Hum. Percept. Perform. 2006, 32, 1243–1265. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Humphreys, G.W. Automatic guidance of visual attention from verbal working memory. J. Exp. Psychol.-Hum. Percept. Perform. 2007, 33, 730–737. [Google Scholar] [CrossRef]
- Soto, D.; Hodsoll, J.; Rotshtein, P.; Humphreys, G.W. Automatic guidance of attention from working memory. Trends Cogn. Sci. 2008, 12, 342–348. [Google Scholar] [CrossRef]
- Downing, P.E. Interactions between visual working memory and selective attention. Psychol. Sci. 2000, 11, 467–473. [Google Scholar] [CrossRef]
- Carlisle, N.B.; Woodman, G.F. When memory is not enough: Electrophysiological evidence for goal-dependent use of working memory representations in guiding visual attention. J. Cogn. Neurosci. 2011, 23, 2650–2664. [Google Scholar] [CrossRef]
- Kumar, S.; Soto, D.; Humphreys, G.W. Electrophysiological evidence for attentional guidance by the contents of working memory. Eur. J. Neurosci. 2009, 30, 307–317. [Google Scholar] [CrossRef]
- Kiss, M.; Van Velzen, J.; Eimer, M. The N2pc component and its links to attention shifts and spatially selective visual processing. Psychophysiology 2008, 45, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Woodman, G.F.; Luck, S.J. Electrophysiological measurement of rapid shifts of attention during visual search. Nature 1999, 400, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Woodman, G.F.; Luck, S.J. Serial deployment of attention during visual search. J. Exp. Psychol.-Hum. Percept. Perform. 2003, 29, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, N.; Doradzińska, Ł.; Bola, M. Attentional Prioritization of Complex, Naturalistic Stimuli Maintained in Working-Memory–A Dot-Probe Event-Related Potentials Study. Front. Hum. Neurosci. 2022, 16, 838338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, B.; Zhou, D.; Fu, Y.; Chen, L.; Liu, H.; Zheng, J.; Guan, C.; Shen, M.; Chen, H. The Dynamic Process of Supranormal Attention in Schizophrenia: Hyperfocusing on Relevant Information and Hyperfiltering on Irrelevant Information Emerge over Time. PsyArXiv 2023. [Google Scholar] [CrossRef]
- Luck, S.J.; McClenon, C.; Beck, V.M.; Hollingworth, A.; Leonard, C.J.; Hahn, B.; Robinson, B.M.; Gold, J.M. Hyperfocusing in schizophrenia: Evidence from interactions between working memory and eye movements. J. Abnorm. Psychol. 2014, 123, 783–795. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J.; Xu, M.; Zhu, P.; Shen, M.; Chen, H. The object as the unit of interaction between visual working memory and visual attention. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Hollingworth, A.; Beck, V.M. Memory-Based Attention Capture When Multiple Items Are Maintained in Visual Working Memory. J. Exp. Psychol.-Hum. Percept. Perform. 2016, 42, 911–917. [Google Scholar] [CrossRef]
- Houtkamp, R.; Roelfsema, P.R. Matching of visual input to only one item at any one time. Psychol. Res. 2009, 73, 317–326. [Google Scholar] [CrossRef]
- Olivers, C.N.; Peters, J.; Houtkamp, R.; Roelfsema, P.R. Different states in visual working memory: When it guides attention and when it does not. Trends Cogn. Sci. 2011, 15, 327–334. [Google Scholar] [CrossRef]
- Downing, P.; Dodds, C. Competition in visual working memory for control of search. Vis. Cogn. 2004, 11, 689–703. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, Y.; Zhou, J.; Shen, M.; Chen, H. More attention with less working memory: The active inhibition of attended but outdated information. Sci. Adv. 2021, 7, eabj4985. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Heinke, D.; Humphreys, G.W.; Blanco, M.J. Early, Involuntary Top-Down Guidance of Attention from Working Memory. J. Exp. Psychol.-Hum. Percept. Perform. 2005, 31, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Bahle, B.; Thayer, D.D.; Mordkoff, J.T.; Hollingworth, A. The architecture of working memory: Features from multiple remembered objects produce parallel, coactive guidance of attention in visual search. J. Exp. Psychol.-Gen. 2020, 149, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Grubert, A.; Eimer, M. All set, indeed! N2pc components reveal simultaneous attentional control settings for multiple target colors. J. Exp. Psychol.-Hum. Percept. Perform. 2016, 42, 1215–1230. [Google Scholar] [CrossRef]
- Zhou, C.; Lorist, M.M.; Mathôt, S. Concurrent guidance of attention by multiple working memory items: Behavioral and computational evidence. Atten. Percept. Psychophys. 2020, 82, 2950–2962. [Google Scholar] [CrossRef]
- Beck, V.M.; Vickery, T.J. Multiple states in visual working memory: Evidence from oculomotor capture by memory-matching distractors. Psychon. Bull. Rev. 2019, 26, 1340–1346. [Google Scholar] [CrossRef]
- van Moorselaar, D.; Theeuwes, J.; Olivers, C.N.L. In Competition for the Attentional Template: Can Multiple Items within Visual Working Memory Guide Attention? J. Exp. Psychol.-Hum. Percept. Perform. 2014, 40, 1450–1464. [Google Scholar] [CrossRef]
- Williams, J.R.; Brady, T.F.; Störmer, V.S. Guidance of attention by working memory is a matter of representational fidelity. J. Exp. Psychol.-Hum. Percept. Perform. 2022, 48, 202–231. [Google Scholar] [CrossRef]
- Carrasco, M. Visual attention: The past 25 years. Vision Res. 2011, 51, 1484–1525. [Google Scholar] [CrossRef]
- Wyble, B.; Callahan-Flintoft, C.; Chen, H.; Marinov, T.; Sarkar, A.; Bowman, H. Understanding visual attention with RAGNAROC: A reflexive attention gradient through neural AttRactOr competition. Psychol. Rev. 2020, 127, 1163–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wyble, B. The neglected contribution of memory encoding in spatial cueing: A new theory of costs and benefits. Psychol. Rev. 2018, 125, 936–968. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, J.; Decaix, C.; Siéroff, E.; Chokron, S.; Milliken, B.; Bartolomeo, P. Independent effects of endogenous and exogenous spatial cueing: Inhibition of return at endogenously attended target locations. Exp. Brain Res. 2004, 159, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- Giordano, A.M.; McElree, B.; Carrasco, M. On the automaticity and flexibility of covert attention: A speed-accuracy trade-off analysis. J. Vision 2009, 9, 30. [Google Scholar] [CrossRef]
- Hein, E.; Rolke, B.; Ulrich, R. Visual attention and temporal discrimination: Differential effects of automatic and voluntary cueing. Vis. Cogn. 2006, 13, 29–50. [Google Scholar] [CrossRef]
- McCormick, P.A. Orienting Attention without Awareness. J. Exp. Psychol.-Hum. Percept. Perform. 1997, 23, 168–180. [Google Scholar] [CrossRef]
- Liu, T.; Stevens, S.T.; Carrasco, M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Res. 2007, 47, 108–113. [Google Scholar] [CrossRef]
- Posner, M.I.; Cohen, Y.; Choate, L.S.; Hockey, R.; Maylor, E. Sustained Concentration: Passive Filtering or Active Orienting? In Preparatory States & Processes; Psychology Press: London, UK, 1984; pp. 49–65. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Lauritzen, T.Z.; D’Esposito, M.; Heeger, D.J.; Silver, M.A. Top–down flow of visual spatial attention signals from parietal to occipital cortex. J. Vision 2009, 9, 18. [Google Scholar] [CrossRef]
- Serences, J.T.; Shomstein, S.; Leber, A.B.; Golay, X.; Egeth, H.E.; Yantis, S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol. Sci. 2005, 16, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kiyonaga, A.; Egner, T. Resource-sharing between internal maintenance and external selection modulates attentional capture by working memory content. Front. Hum. Neurosci. 2014, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.X.; Huang, S.; Kong, L.; Wang, S. Effects of load on the guidance of visual attention from working memory. Vision Res. 2011, 51, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Henik, A.; Rafal, R. Competition between Endogenous and Exogenous Orienting of Visual Attention. J. Exp. Psychol.-Gen. 2005, 134, 207–221. [Google Scholar] [CrossRef]
- Slessor, G.; Finnerty, A.; Papp, J.; Smith, D.T.; Martin, D. Gaze-cueing and endogenous attention operate in parallel. Acta Psychol. 2019, 192, 172–180. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spatial Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef]
- JASP Team. JASP (Version 0.16.3), [Computer software]. 2022.
- Lee, M.D.; Wagenmakers, E. Bayesian Cognitive Modeling; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Wagenmakers, E.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Love, J.; Selker, R.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon. Bull. Rev. 2018, 25, 35–57. [Google Scholar] [CrossRef]
- Olivers, C.N.; Eimer, M. On the difference between working memory and attentional set. Neuropsychologia 2011, 49, 1553–1558. [Google Scholar] [CrossRef]
- Woodman, G.F.; Luck, S.J. Do the contents of visual working memory automatically influence attentional selection during visual search? J. Exp. Psychol.-Hum. Percept. Perform. 2007, 33, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Dowd, E.W.; Kiyonaga, A.; Egner, T.; Mitroff, S.R. Attentional guidance by working memory differs by paradigm: An individual-differences approach. Atten. Percept. Psychophys. 2015, 77, 704–712. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Yang, Q.; Chen, L.; Guan, C.; Zhou, J.; Shen, M.; Chen, H. Working-Memory-Guided Attention Competes with Exogenous Attention but Not with Endogenous Attention. Behav. Sci. 2023, 13, 426. https://doi.org/10.3390/bs13050426
Zhu P, Yang Q, Chen L, Guan C, Zhou J, Shen M, Chen H. Working-Memory-Guided Attention Competes with Exogenous Attention but Not with Endogenous Attention. Behavioral Sciences. 2023; 13(5):426. https://doi.org/10.3390/bs13050426
Chicago/Turabian StyleZhu, Ping, Qingqing Yang, Luo Chen, Chenxiao Guan, Jifan Zhou, Mowei Shen, and Hui Chen. 2023. "Working-Memory-Guided Attention Competes with Exogenous Attention but Not with Endogenous Attention" Behavioral Sciences 13, no. 5: 426. https://doi.org/10.3390/bs13050426
APA StyleZhu, P., Yang, Q., Chen, L., Guan, C., Zhou, J., Shen, M., & Chen, H. (2023). Working-Memory-Guided Attention Competes with Exogenous Attention but Not with Endogenous Attention. Behavioral Sciences, 13(5), 426. https://doi.org/10.3390/bs13050426




