Heart Rate as a Correlate for the Emotional Processing of Body Stimuli in Anorexia Nervosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
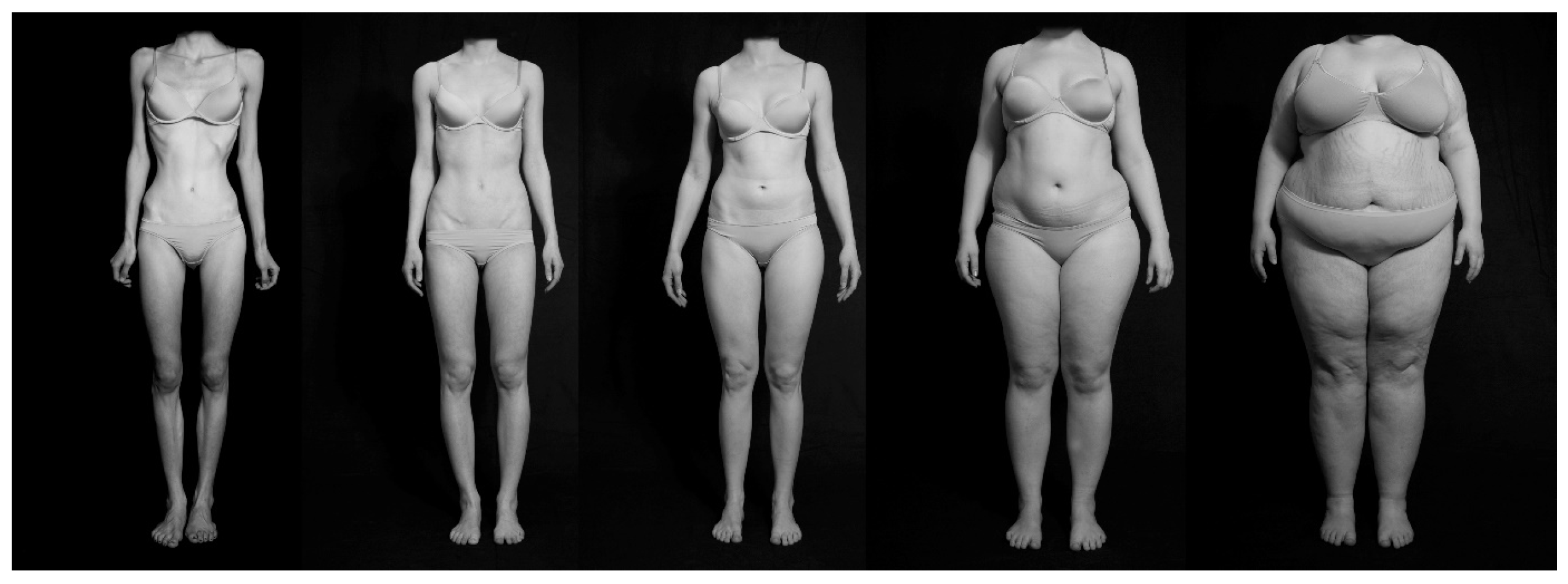
2.3. Stimulus Material
2.4. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Heart Rate
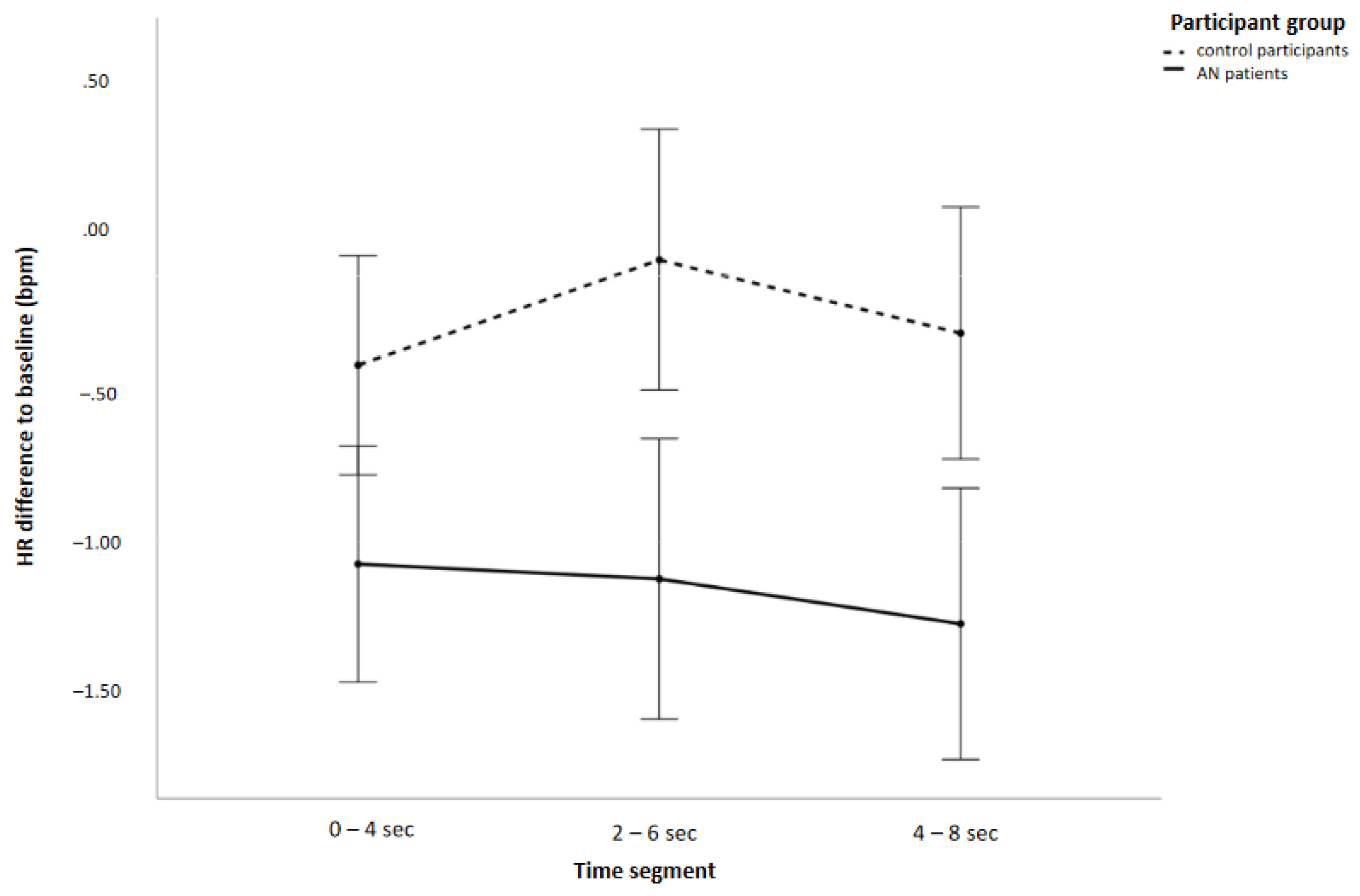
3.2.1. General HR Effects
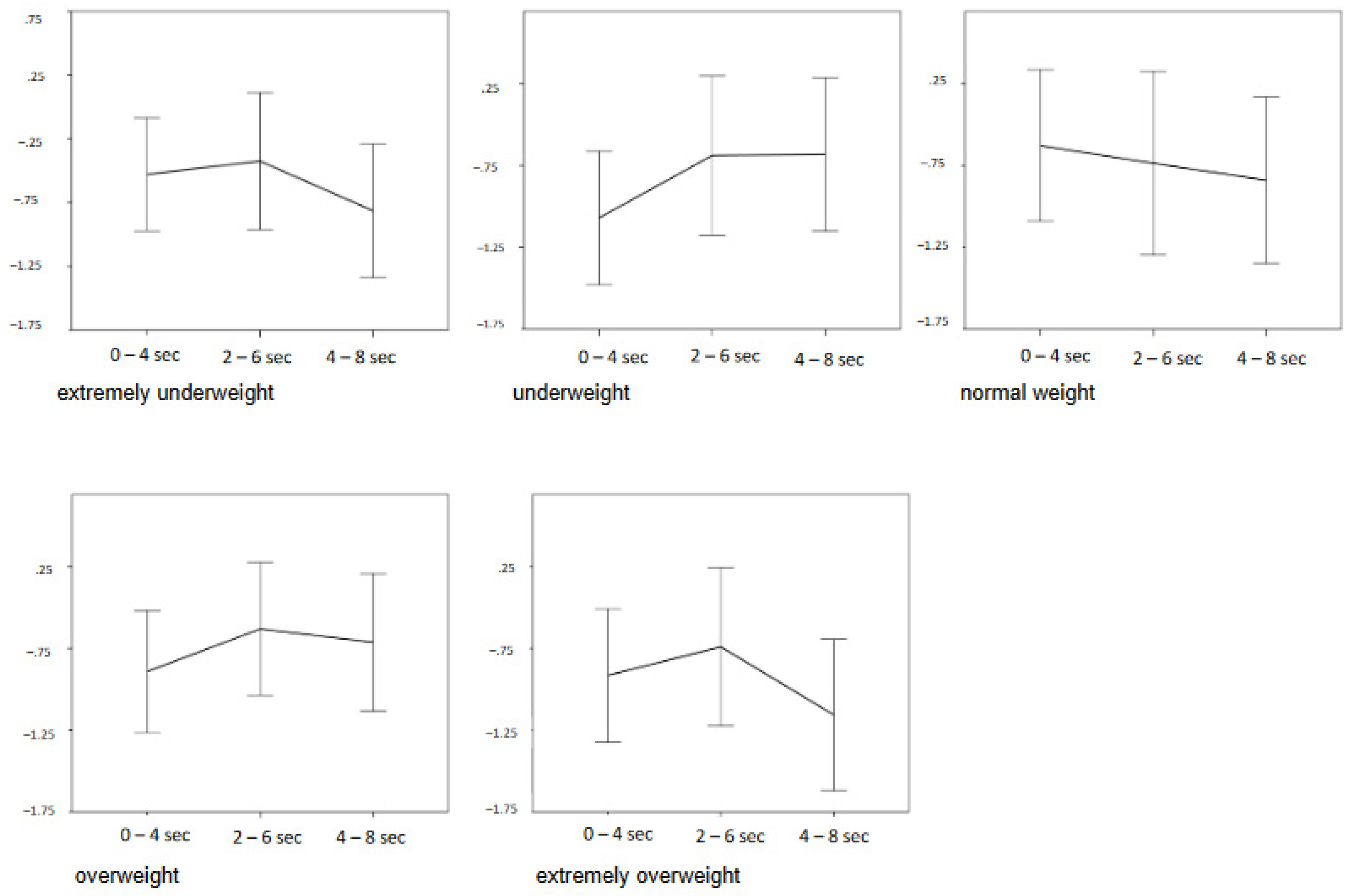
3.2.2. Stimulus-Specific Effects
4. Discussion
4.1. General HR Effects
4.2. Participant Group Effects
4.3. Effects of Extreme Weight Categories
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horndasch, S.; Heinrich, H.; Kratz, O.; Mai, S.; Graap, H.; Moll, G.H. Perception and evaluation of women’s bodies in adolescents and adults with anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Vocks, S.; Stahn, C.; Loenser, K.; Legenbauer, T. Eating and body image disturbances in male-to-female and female-to-male transsexuals. Arch. Sex Behav. 2009, 38, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Codispoti, M.; Cuthbert, B.N.; Lang, P.J. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion 2001, 1, 276–298. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Greenwald, M.K.; Hamm, A.O. Affective picture processing. In The Organization of Emotions; Birbaumer, N.Ö.A., Ed.; Hogrefe-Huber: Toronto, ON, Canada, 1993; pp. 48–65. [Google Scholar]
- Lacey, J.I. Somatic response patterns and stress: Some revisions of activation theory. In Psychological Stress: Issues in Research; Appley, M.H., Trumbull, R., Eds.; Appleton-Century-Crofts: New York, NY, USA, 1967; pp. 4–44. [Google Scholar]
- Pollatos, O.; Herbert, B.M.; Matthias, E.; Schandry, R. Heart rate response after emotional picture presentation is modulated by interoceptive awareness. Int. J. Psychophysiol. 2007, 63, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Burmester, V.; Graham, E.; Nicholls, D. Physiological, emotional and neural responses to visual stimuli in eating disorders: A review. J. Eat. Disord. 2021, 9, 23. [Google Scholar] [CrossRef]
- Svaldi, J.; Tuschen-Caffier, B.; Peyk, P.; Blechert, J. Information processing of food pictures in binge eating disorder. Appetite 2010, 55, 685–694. [Google Scholar] [CrossRef]
- Drobes, D.J.; Miller, E.J.; Hillman, C.H.; Bradley, M.M.; Cuthbert, B.N.; Lang, P.J. Food deprivation and emotional reactions to food cues: Implications for eating disorders. Biol. Psychol. 2001, 57, 153–177. [Google Scholar] [CrossRef]
- Laberg, J.C.; Wilson, G.T.; Eldredge, K.; Nordby, H. Effects of mood on heart rate reactivity in bulimia nervosa. Int. J. Eat. Disord. 1991, 10, 169–178. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Craske, M.G.; Li, W.; Vangala, S.; Strober, M.; Feusner, J.D. Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. Int. J. Eat. Disord. 2015, 48, 889–897. [Google Scholar] [CrossRef]
- Mauler, B.I.; Hamm, A.O.; Weike, A.I.; Tuschen-Caffier, B. Affect regulation and food intake in bulimia nervosa: Emotional responding to food cues after deprivation and subsequent eating. J. Abnorm. Psychol. 2006, 115, 567–579. [Google Scholar] [CrossRef]
- Wollast, R.; Fossion, P.; Kotsou, I.; Rebrasse, A.; Leys, C. Interoceptive Awareness and Anorexia Nervosa: When Emotions Influence the Perception of Physiological Sensations. J. Nerv. Ment. Dis. 2022, 210, 390–393. [Google Scholar] [CrossRef]
- Green, M.A.; Ohrt, T.K. Psychophysiological reactions to objectified weight-classified stimuli. Psychol. Pop. Media Cult. 2013, 2, 61. [Google Scholar] [CrossRef]
- Overduin, J.; Jansen, A.; Eilkes, H. Cue reactivity to food- and body-related stimuli in restrained and unrestrained eaters. Addict. Behav. 1997, 22, 395–404. [Google Scholar] [CrossRef]
- Reichel, V.A.; Schneider, N.; Grunewald, B.; Kienast, T.; Pfeiffer, E.; Lehmkuhl, U.; Korte, A. “Glass fairies” and “bone children”: Adolescents and young adults with anorexia nervosa show positive reactions towards extremely emaciated body pictures measured by the startle reflex paradigm. Psychophysiology 2014, 51, 168–177. [Google Scholar] [CrossRef]
- Brockmeyer, T.; Pellegrino, J.; Maier, C.; Munch, H.M.; Harmer, C.J.; Walther, S.; Herzog, W.; Friederich, H.C. Blunted emotion-modulated startle reflex in anorexia nervosa. Int. J. Eat. Disord. 2019, 52, 270–277. [Google Scholar] [CrossRef] [PubMed]
- French, M.N.; Chen, E.Y. Emotion and Psychophysiological Responses During Emotion-Eliciting Film Clips in an Eating Disorders Sample. Front. Psychol. 2021, 12, 630426. [Google Scholar] [CrossRef] [PubMed]
- Knejzlikova, T.; Svetlak, M.; Malatincova, T.; Roman, R.; Chladek, J.; Najmanova, J.; Theiner, P.; Linhartova, P.; Kasparek, T. Electrodermal Response to Mirror Exposure in Relation to Subjective Emotional Responses, Emotional Competences and Affectivity in Adolescent Girls With Restrictive Anorexia and Healthy Controls. Front. Psychol. 2021, 12, 673597. [Google Scholar] [CrossRef] [PubMed]
- Couton, C.; Gorwood, P.; Pham-Scottez, A.; Poupon, D.; Duriez, P. Pupil psychosensory reflex in response to own and standardised silhouettes in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2022, 30, 135–145. [Google Scholar] [CrossRef]
- Prefit, A.B.; Candea, D.M.; Szentagotai-Tatar, A. Emotion regulation across eating pathology: A meta-analysis. Appetite 2019, 143, 104438. [Google Scholar] [CrossRef]
- Nalbant, K.; Kalayci, B.M.; Akdemir, D.; Akgul, S.; Kanbur, N. Emotion regulation, emotion recognition, and empathy in adolescents with anorexia nervosa. Eat. Weight Disord. 2019, 24, 825–834. [Google Scholar] [CrossRef]
- Schneider, S.; Pflug, V.; In-Albon, T.; Margraf, J. Kinder-DIPS Open Access: Diagnostisches Interview bei psychischen Störungen im Kindes-und Jugendalter; Forschungs-und Behandlungszentrum für psychische Gesundheit, Ruhr-Universität Bochum: Bochum, Germany, 2017. [Google Scholar]
- Schneider, S.; Margraf, J. Diagnostisches Interview bei Psychischen Störungen, 3rd ed.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Paul, T.; Thiel, A. Eating Disorder Inventory-2; Hogrefe: Göttingen, Germany, 2005. [Google Scholar]
- Thiel, A.; Jacobi, C.; Horstmann, S.; Paul, T.; Nutzinger, D.O.; Schussler, G. A German version of the Eating Disorder Inventory EDI-2. Psychother. Psychosom. Med. Psychol. 1997, 47, 365–376. [Google Scholar] [PubMed]
- Knauss, C.; Paxton, S.J.; Alsaker, F.D. Validation of the German version of the Sociocultural Attitudes Towards Appearance Questionnaire (SATAQ-G). Body Image 2009, 6, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes-und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Pluddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef]
- Peyser, D.; Scolnick, B.; Hildebrandt, T.; Taylor, J.A. Heart rate variability as a biomarker for anorexia nervosa: A review. Eur. Eat. Disord. Rev. 2021, 29, 20–31. [Google Scholar] [CrossRef]
- Friederich, H.C.; Kumari, V.; Uher, R.; Riga, M.; Schmidt, U.; Campbell, I.C.; Herzog, W.; Treasure, J. Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: A startle reflex paradigm. Psychol. Med. 2006, 36, 1327–1335. [Google Scholar] [CrossRef]
- Jönsson, P.; Sonnby-Borgstrom, M. The effects of pictures of emotional faces on tonic and phasic autonomic cardiac control in women and men. Biol. Psychol. 2003, 62, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Kollei, I.; Leins, J.; Rinck, M.; Waldorf, M.; Kuhn, M.; Rauh, E.; Steins-Loeber, S. Implicit approach-avoidance tendencies toward food and body stimuli absent in individuals with anorexia nervosa, bulimia nervosa, and healthy controls. Int. J. Eat. Disord. 2022, 55, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, T.; Burdenski, K.; Anderle, A.; Voges, M.M.; Vocks, S.; Schmidt, H.; Wunsch-Leiteritz, W.; Leiteritz, A.; Friederich, H.C. Approach and avoidance bias for thin-ideal and normal-weight body shapes in anorexia nervosa. Eur. Eat. Disord. Rev. 2020, 28, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Renner, B. The implicit nature of the anti-fat bias. Front. Hum. Neurosci. 2011, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.C.; Meneguzzo, P.; Favaro, A.; Teufel, M.; Skoda, E.M.; Lindner, M.; Walder, L.; Quiros Ramirez, A.; Zipfel, S.; Mohler, B.; et al. Weight bias and linguistic body representation in anorexia nervosa: Findings from the BodyTalk project. Eur. Eat. Disord. Rev. 2021, 29, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.B.; Steding, L.H.; Webb, A.K. Reduced emotional and cardiovascular reactivity to emotionally evocative stimuli in major depressive disorder. Int. J. Psychophysiol. 2015, 97, 66–74. [Google Scholar] [CrossRef]
- Preis, M.A.; Schlegel, K.; Stoll, L.; Blomberg, M.; Schmidt, H.; Wunsch-Leiteritz, W.; Leiteritz, A.; Brockmeyer, T. Improving emotion recognition in anorexia nervosa: An experimental proof-of-concept study. Int. J. Eat. Disord. 2020, 53, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, T.; Friederich, H.C.; Schmidt, U. Advances in the treatment of anorexia nervosa: A review of established and emerging interventions. Psychol. Med. 2018, 48, 1228–1256. [Google Scholar] [CrossRef] [PubMed]
| Age Group | Adolescents (n = 37) | Adults (n = 43) | ||
|---|---|---|---|---|
| Participant Group | AN (n = 19) | CO (n = 18) | AN (n = 18) | CO (n = 25) |
| Age (years) | 15.5 (1.9) | 15.9 (1.5) | 27.3 (7.9) | 25.8 (7.1) |
| BMI (kg/m2) | 15.9 (1.5) | 20.8 (2.5) | 16.5 (1.8) | 21.8 (2.2) |
| BMI age percentile | 5.5 (9.8) | 52.4 (23.1) | ||
| EDI-2 total score | 277.8 (71.6) | 205.6 (50.0) | 347.7 (34.5) | 213.1 (45.1) |
| EDI-2 body dissatisfaction | 36.6 (11.6) | 26.2 (9.4) | 44.0 (8.0) | 29.3 (8.7) |
| SATAQ-G total score | 53.3 (21.4) | 41.9 (11.5) | 59.1 (12.1) | 40.4 (10.2) |
| Dependent Variable | Age Group Effect | Participant Group Effect | Age Group * Participant Group Interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Df | F | Partial η2 | p | Df | F | Partial η2 | p | Df | F | Partial η2 | p | |
| Age | 1 | 79.31 | 0.511 | <0.001 * | 1 | 0.61 | 0.008 | 0.43 | 1 | 0.26 | 0.003 | 0.61 |
| BMI (kg/m2) | 1 | 58.56 | 0.613 | <0.001 * | ||||||||
| BMI age percentile | 1 | 63.07 | 0.612 | <0.001 * | ||||||||
| SATAQ-G total score | 1 | 0.43 | 0.006 | 0.52 | 1 | 20.85 | 0.227 | <0.001 * | 1 | 1.24 | 0.017 | 0.27 |
| EDI-2 total score | 1 | 9.37 | 0.131 | 0.003 * | 1 | 66.74 | 0.518 | <0.001 * | 1 | 6.08 | 0.089 | 0.016 |
| EDI-2 body dissatisfaction | 1 | 5.51 | 0.073 | 0.022 | 1 | 32.04 | 0.314 | <0.001 * | 0.94 | 0.013 | 0.34 | |
| Factor/Interaction | Df | F | Partial η2 | p |
|---|---|---|---|---|
| Group | 1 | 10.62 | 0.120 | 0.002 * |
| Category | 4 | 0.42 | 0.005 | 0.78 |
| Time segment | 2 | 2.07 | 0.026 | 0.15 |
| Group * time segment | 2 | 2.14 | 0.027 | 0.14 |
| Group * category | 4 | 1.24 | 0.016 | 0.29 |
| Category * time segment | 8 | 2.96 | 0.037 | 0.010 * |
| Group * category * time segment | 8 | 1.83 | 0.023 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horndasch, S.; Sharon, E.; Eichler, A.; Graap, H.; Moll, G.H.; Kratz, O. Heart Rate as a Correlate for the Emotional Processing of Body Stimuli in Anorexia Nervosa. Behav. Sci. 2023, 13, 215. https://doi.org/10.3390/bs13030215
Horndasch S, Sharon E, Eichler A, Graap H, Moll GH, Kratz O. Heart Rate as a Correlate for the Emotional Processing of Body Stimuli in Anorexia Nervosa. Behavioral Sciences. 2023; 13(3):215. https://doi.org/10.3390/bs13030215
Chicago/Turabian StyleHorndasch, Stefanie, Elisabeth Sharon, Anna Eichler, Holmer Graap, Gunther H. Moll, and Oliver Kratz. 2023. "Heart Rate as a Correlate for the Emotional Processing of Body Stimuli in Anorexia Nervosa" Behavioral Sciences 13, no. 3: 215. https://doi.org/10.3390/bs13030215
APA StyleHorndasch, S., Sharon, E., Eichler, A., Graap, H., Moll, G. H., & Kratz, O. (2023). Heart Rate as a Correlate for the Emotional Processing of Body Stimuli in Anorexia Nervosa. Behavioral Sciences, 13(3), 215. https://doi.org/10.3390/bs13030215





