Impact of General Practitioner Education on Acceptance of an Adjuvanted Seasonal Influenza Vaccine among Older Adults in England
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection Instruments
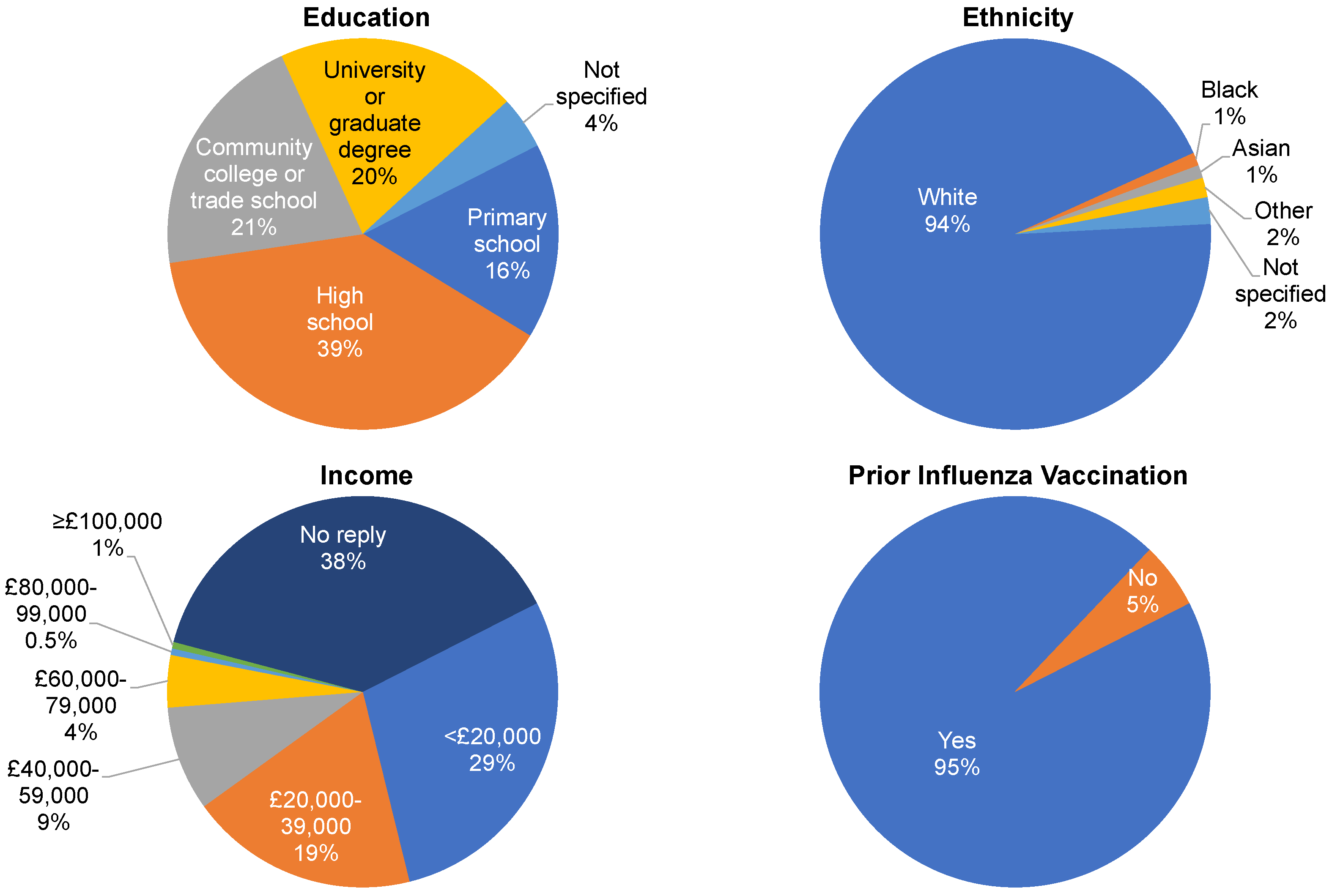
2.3. Study Participants
2.4. Study Objectives
2.5. Statistical Methods
3. Results
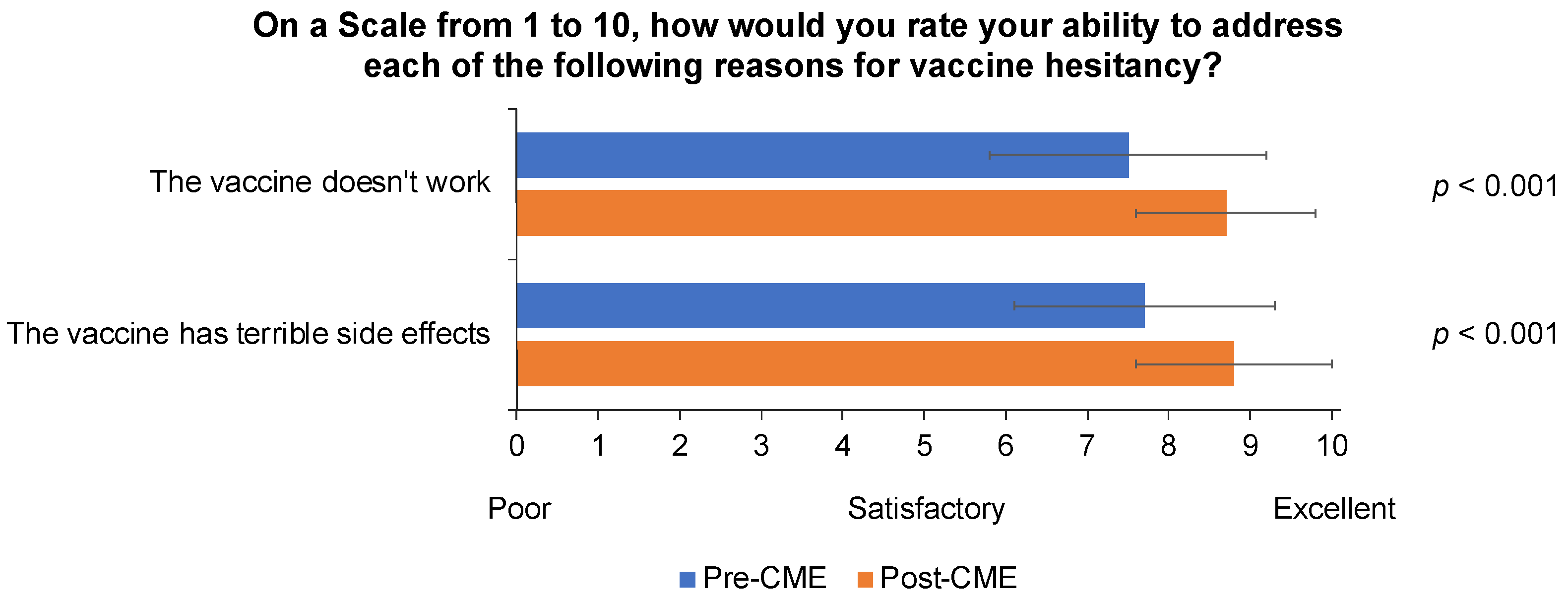
3.1. GP Knowledge, Attitudes, and Beliefs about Influenza Disease and Vaccination before and after CME Module
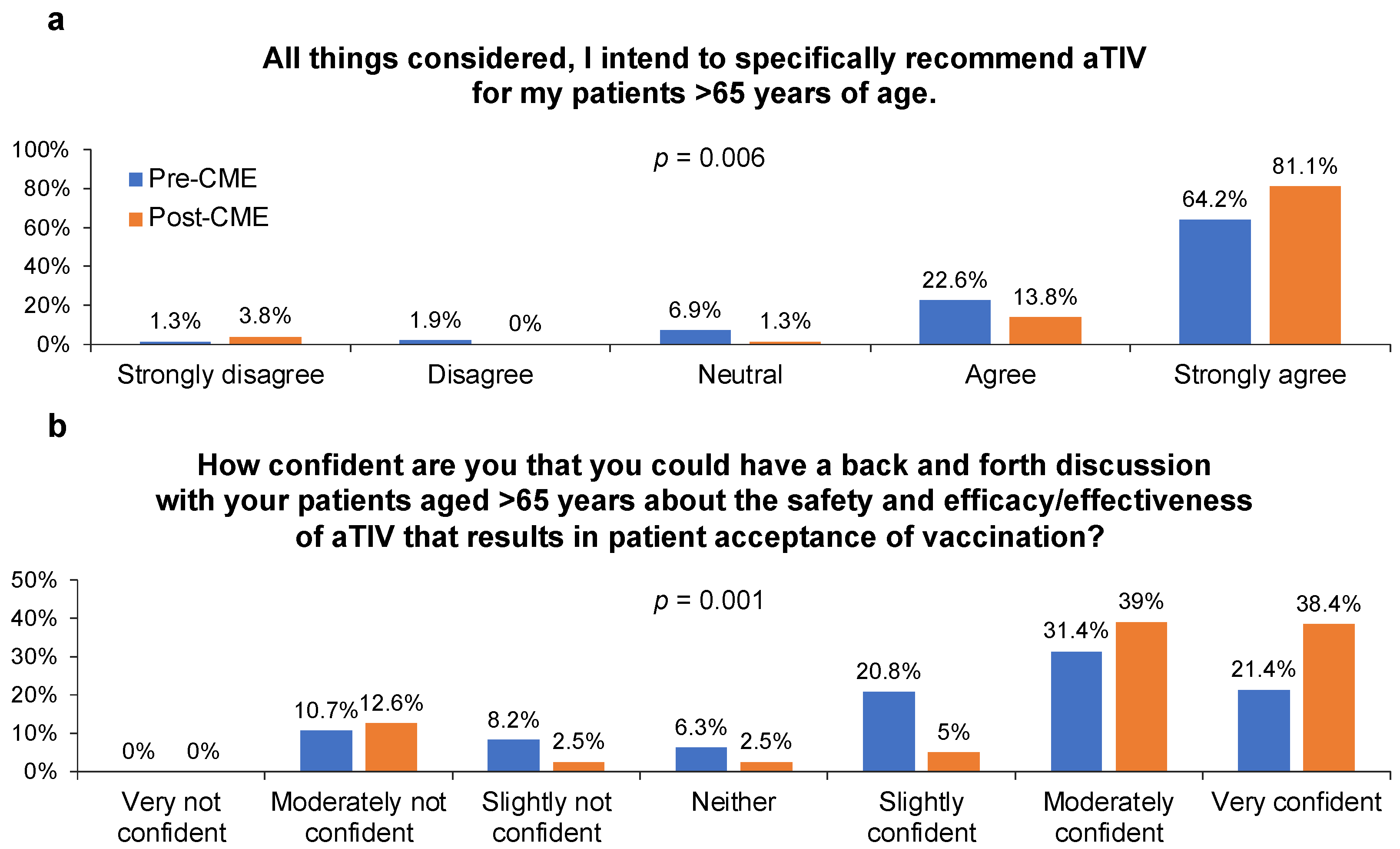
3.2. GP Recommendations and Actions Regarding Influenza Vaccination during Patient Visits
3.3. Patients’ Knowledge, Attitudes, and Beliefs about Influenza Disease and Vaccination before and after Interactions with Their GP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Public Health England. Coronavirus (COVID-19) in the UK. Available online: https://coronavirus.data.gov.uk (accessed on 17 May 2022).
- Whitaker, H.J.; Tsang, R.S.M.; Byford, R.; Andrews, N.J.; Sherlock, J.; Sebastian Pillai, P.; Williams, J.; Button, E.; Campbell, H.; Sinnathamby, M.; et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups. J. Infect. 2022, 84, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Surveillance of Influenza and Other Respiratory Viruses in the UK: Winter 2019 to 2020. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20220401215804/https://www.gov.uk/government/statistics/annual-flu-reports (accessed on 1 December 2022).
- Pebody, R.G.; Green, H.K.; Warburton, F.; Sinnathamby, M.; Ellis, J.; Mølbak, K.; Nielsen, J.; de Lusignan, S.; Andrews, N. Significant spike in excess mortality in England in winter 2014/15-influenza the likely culprit. Epidemiol. Infect. 2018, 146, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Davidson, C.; Mattock, R.; Gibbons, I.; Mealing, S.; Carroll, S. Quantifying the direct secondary health care cost of seasonal influenza in England. BMC Public Health 2020, 20, 1464. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Keri, V.C.; Hooda, A.; Kodan, P.; Brunda, R.L.; Jorwal, P.; Wig, N. Intricate interplay between Covid-19 and cardiovascular diseases. Rev. Med. Virol. 2020, 31, e2188. [Google Scholar] [CrossRef]
- Yedlapati, S.H.; Khan, S.U.; Talluri, S.; Lone, A.N.; Khan, M.Z.; Khan, M.S.; Navar, A.M.; Gulati, M.; Johnson, H.; Baum, S.; et al. Effects of Influenza Vaccine on Mortality and Cardiovascular Outcomes in Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019636. [Google Scholar] [CrossRef]
- Meza-Torres, B.; Delanerolle, G.; Okusi, C.; Mayer, N.; Anand, S.; McCartney, J.; Gatenby, P.; Glampson, B.; Chapman, M.; Curcin, V.; et al. Differences in clinical presentation with long covid following community and hospital infection, and associations with all-cause mortality: English sentinel network database study. JMIR Public Health Surveill. 2022, 8, e37668. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2017–2018 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2017-2018.htm (accessed on 29 August 2022).
- Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Effectiveness, 2017–2018. Available online: https://www.cdc.gov/flu/vaccines-work/2017-2018.html (accessed on 24 March 2022).
- Rolfes, M.A.; Flannery, B.; Chung, J.R.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.K.; Jackson, M.L.; Monto, A.S.; et al. Effects of Influenza Vaccination in the United States During the 2017-2018 Influenza Season. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 1845–1853. [Google Scholar] [CrossRef]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar] [CrossRef]
- Smedley, J.; Poole, J.; Waclawski, E.; Stevens, A.; Harrison, J.; Watson, J.; Hayward, A.; Coggon, D. Influenza immunisation: Attitudes and beliefs of UK healthcare workers. Occup. Environ. Med. 2007, 64, 223–227. [Google Scholar] [CrossRef]
- Shrikrishna, D.; Williams, S.; Restrick, L.; Hopkinson, N.S. Influenza vaccination for NHS staff: Attitudes and uptake. BMJ Open Respir. Res. 2015, 2, e000079. [Google Scholar] [CrossRef]
- Royal Society for Public Health. Moving the Needle: Promoting Vaccination Uptake Across the Life Course. Available online: https://www.rsph.org.uk/static/uploaded/3b82db00-a7ef-494c-85451e78ce18a779.pdf (accessed on 17 May 2022).
- Nagata, J.M.; Hernández-Ramos, I.; Kurup, A.S.; Albrecht, D.; Vivas-Torrealba, C.; Franco-Paredes, C. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: A systematic review of qualitative and quantitative data. BMC Public Health 2013, 13, 388. [Google Scholar] [CrossRef]
- Ruiz, J.B.; Bell, R.A. Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine 2021, 39, 1080–1086. [Google Scholar] [CrossRef]
- Allington, D.; McAndrew, S.; Moxham-Hall, V.; Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol. Med. 2021, 53, 1–12. [Google Scholar] [CrossRef]
- Office for National Statistics. COVID-19 Vaccine Refusal, UK: February to March 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/bulletins/covid19vaccinerefusaluk/februarytomarch2021 (accessed on 26 August 2022).
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Kim, J.; Krishnan, A.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert Rev. Vaccines 2018, 17, 435–443. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Frey, S.E.; Reyes, M.R.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.; Whitaker, H.; Zhao, H.; Andrews, N.; Ellis, J.; Donati, M.; Zambon, M. Protection provided by influenza vaccine against influenza-related hospitalisation in ≥65 year olds: Early experience of introduction of a newly licensed adjuvanted vaccine in England in 2018/19. Vaccine 2019, 38, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of Adjuvanted Trivalent Inactivated Influenza Vaccine Versus Egg-Derived Quadrivalent Inactivated Influenza Vaccines and High-Dose Trivalent Influenza Vaccine in Preventing Influenza-Related Medical Encounters in US Adults >/=65 Years During the 2017–2018 and 2018–2019 Influenza Seasons. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 816–823. [Google Scholar] [CrossRef]
- de Lusignan, S.; Tsang, R.S.M.; Amirthalingam, G.; Akinyemi, O.; Sherlock, J.; Tripathy, M.; Deeks, A.; Ferreira, F.; Howsam, G.; Hobbs, F.D.R.; et al. Adverse events of interest following influenza vaccination, a comparison of cell culture-based with egg-based alternatives: English sentinel network annual report paper 2019/20. Lancet Reg. Health Eur. 2021, 2, 100029. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunisation. Advice on Influenza Vaccines for 2022/23; Joint Committee on Vaccination and Immunisation: London, UK, 2021.
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Public Health England. National Flu Immunisation Programme 2018/19. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20210701160234/https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan (accessed on 26 August 2022).
- de Lusignan, S.; Correa, A.; Smith, G.E.; Yonova, I.; Pebody, R.; Ferreira, F.; Elliot, A.J.; Fleming, D. RCGP Research and Surveillance Centre: 50 years’ surveillance of influenza, infections, and respiratory conditions. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2017, 67, 440–441. [Google Scholar] [CrossRef]
- Pebody, R.; Whitaker, H.; Ellis, J.; Andrews, N.; Marques, D.; Cottrell, S.; Reynolds, A.; Gunson, R.; Thompson, C.; Galiano, M.; et al. End of season influenza vaccine effectiveness in primary care in adults and children in the United Kingdom in 2018/19. Vaccine 2020, 38, 489–497. [Google Scholar] [CrossRef]
- Pebody, R.; Djennad, A.; Ellis, J.; Andrews, N.; Marques, D.F.P.; Cottrell, S.; Reynolds, A.J.; Gunson, R.; Galiano, M.; Hoschler, K.; et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Euro Surveill. 2019, 24, 1800488. [Google Scholar] [CrossRef]
- Fisher, W.A.; Fisher, J.D.; Shuper, P.A. Chapter Three—Social Psychology and the Fight Against AIDS: An Information–Motivation–Behavioral Skills Model for the Prediction and Promotion of Health Behavior Change. In Advances in Experimental Social Psychology; Olson, J.M., Zanna, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 50, pp. 105–193. [Google Scholar]
- Fisher, W.A.; Fisher, J.D. The Information-Motivation-Behavioral Skills Model: A general social psychological approach to understanding and promoting health behaviour. In Social Psychological Foundations of Health and Illness; Suls, J., Wallston, K., Eds.; Blackwell: London, UK, 2003; pp. 82–106. [Google Scholar]
- Zimmerman, R.K.; Santibanez, T.A.; Janosky, J.E.; Fine, M.J.; Raymund, M.; Wilson, S.A.; Bardella, I.J.; Medsger, A.R.; Nowalk, M.P. What affects influenza vaccination rates among older patients? An analysis from inner-city, suburban, rural, and Veterans Affairs practices. Am. J. Med. 2003, 114, 31–38. [Google Scholar] [CrossRef]
- Shemesh, A.A.; Rasooly, I.; Horowitz, P.; Lemberger, J.; Ben-Moshe, Y.; Kachal, J.; Danziger, J.; Clarfield, A.M.; Rosenberg, E. Health behaviors and their determinants in multiethnic, active Israeli seniors. Arch. Gerontol. Geriatr. 2008, 47, 63–77. [Google Scholar] [CrossRef]
- Lasser, K.E.; Kelly, B.; Maier, J.; Murillo, J.; Hoover, S.; Isenberg, K.; Osber, D.; Pilkauskas, N.; Willis, B.C.; Hersey, J. Discussions about preventive services: A qualitative study. BMC Fam. Pract. 2008, 9, 49. [Google Scholar] [CrossRef]
- Payaprom, Y.; Bennett, P.; Burnard, P.; Alabaster, E.; Tantipong, H. Understandings of influenza and influenza vaccination among high-risk urban dwelling Thai adults: A qualitative study. J. Public Health 2010, 32, 26–31. [Google Scholar] [CrossRef]
- Lau, L.; Lau, Y.; Lau, Y.H. Prevalence and correlates of influenza vaccination among non-institutionalized elderly people: An exploratory cross-sectional survey. Int. J. Nurs. Stud. 2009, 46, 768–777. [Google Scholar] [CrossRef]
- National Health Service England. We’re Here to Help You Stay Well This Winter; Public Health England: London, UK, 2020.
- National Health Service England. Investment and Impact Fund 2020/21: Guidance. Available online: https://www.england.nhs.uk/wp-content/uploads/2020/09/IIF-Implementation-Guidance-2020-21-Final.pdf (accessed on 22 September 2022).
- Public Health England. Seasonal Flu Vaccine Uptake in GP Patients: Provisional Monthly Data for 1 September 2018 to 28 February 2019 by Local Team. Available online: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-gp-patients-monthly-data-2018-to-2019 (accessed on 25 March 2022).
- Public Health England. Seasonal Flu Vaccine Uptake in GP Patients: Winter 2020 to 2021. Available online: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-gp-patients-winter-2020-to-2021 (accessed on 25 March 2022).
- Public Health England. Seasonal Flu Vaccine Uptake in GP Patients: Monthly Data, 2021 to 2022. Available online: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-gp-patients-monthly-data-2021-to-2022 (accessed on 25 March 2022).
- Watkinson, R.A.-O.; Williams, R.A.-O.; Gillibrand, S.; Sanders, C.A.-O.X.; Sutton, M.A.-O. Ethnic Inequalities in COVID-19 Vaccine Uptake and Comparison to Seasonal Influenza Vaccine Uptake in Greater Manchester, UK: A cohort study. PLoS Med. 2022, 19, e1003932. [Google Scholar] [CrossRef]


| Before GP Visit | After GP Visit |
|---|---|
| What do you know about seasonal flu infection? | What do you know about seasonal flu infection? |
| What do you know about seasonal flu vaccination? | What do you know about the adjuvanted seasonal flu vaccine? |
| Last winter, did you get a seasonal flu vaccine? Why? | What concerns do you have about getting the adjuvanted seasonal flu vaccine? |
| This current winter, will you get a seasonal flu vaccine? Why? | Some of the good things about getting the adjuvanted seasonal flu vaccine |
| Some of the bad things about getting the adjuvanted seasonal flu vaccine | |
| Who would approve of you getting the adjuvanted seasonal flu vaccine? | |
| Who would disapprove of you getting the adjuvanted seasonal flu vaccine? |
| Reasons for Refusal |
|---|
| Because I didn’t want it and I don’t normally get flu |
| Had once and had a bad reaction |
| Didn’t think it was necessary. Not keen on needles |
| Because I had whooping cough vaccine |
| I consider myself for my age really fit and able to cope. Some elderly peoplemay need it; I could fight it off |
| Don’t think about flu so won’t bother |
| Using homeopathy; some side effects for some people |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lusignan, S.; Ashraf, M.; Ferreira, F.; Tripathy, M.; Yonova, I.; Rafi, I.; Kassianos, G.; Joy, M. Impact of General Practitioner Education on Acceptance of an Adjuvanted Seasonal Influenza Vaccine among Older Adults in England. Behav. Sci. 2023, 13, 130. https://doi.org/10.3390/bs13020130
de Lusignan S, Ashraf M, Ferreira F, Tripathy M, Yonova I, Rafi I, Kassianos G, Joy M. Impact of General Practitioner Education on Acceptance of an Adjuvanted Seasonal Influenza Vaccine among Older Adults in England. Behavioral Sciences. 2023; 13(2):130. https://doi.org/10.3390/bs13020130
Chicago/Turabian Stylede Lusignan, Simon, Mansoor Ashraf, Filipa Ferreira, Manasa Tripathy, Ivelina Yonova, Imran Rafi, George Kassianos, and Mark Joy. 2023. "Impact of General Practitioner Education on Acceptance of an Adjuvanted Seasonal Influenza Vaccine among Older Adults in England" Behavioral Sciences 13, no. 2: 130. https://doi.org/10.3390/bs13020130
APA Stylede Lusignan, S., Ashraf, M., Ferreira, F., Tripathy, M., Yonova, I., Rafi, I., Kassianos, G., & Joy, M. (2023). Impact of General Practitioner Education on Acceptance of an Adjuvanted Seasonal Influenza Vaccine among Older Adults in England. Behavioral Sciences, 13(2), 130. https://doi.org/10.3390/bs13020130







