The Impact of Regular Physical Exercise on Psychopathology, Cognition, and Quality of Life in Patients Diagnosed with Schizophrenia: A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Critical Appraisal
2.4.1. Risk of Bias
2.4.2. Quality Reporting of Exercise Intervention in the Training Programs
| Study | Participants Characteristics | Design | Exercise Protocol | Outcomes of Interest |
|---|---|---|---|---|
| Andrade e Silva et al. [45] | Sex: 100% male BMI (kg/m2): 27.98 ± 1.67 (RESEX), 29.42 ± 1.93 (CONCEX), 25.38 ± 1.60 (CTRL), Race/Ethnicity: N/A Other diseases: N/A Pharmacological treatment: Yes, antipsychotics, antidepressants (n = 15), benzodiazepine (n = 6), mood stabilizer (n = 6) and anticholinergic (n = 8) Previous physical exercise experience: No (sedentary lifestyle > 1 year) Males with a DSM-IV diagnosis of schizophrenia, 18–50 years (33.36 ± 7.6 years) with stable doses of medication and clinically stable disease. | Design: RCT RESEX CONCEX CTRL Sample size: (n = 47) RESEX: n = 12/CONCEX: n = 9/CTRL: n = 13 Intervention period: 20 weeks | Supervised: Yes Familiarization: 3 sessions RESEX: F: 2 sessions/week IL: 40% to 85% 1RM Ti: 55 min/session Ty: progressive RT. V: 7 exercises from 2 × 15 reps to 3 × 6–8 reps (r: 1–2 min between sets). From 210 reps at week 1 to 147 reps at week 20 P: 2.5% per week CONCEX: F: 2 sessions/week IL: RT: 40–85% 1RM/ET: 40–75% VO2max Ti: 55 min/session Ty: progressive RT + progressive ET. V: RT: 7 exercises from 1 × 15 reps to 2 × 6–8 reps (r: 1–2 min), 98–105 reps per week/ET: 50 min per week P: RT: +2.5% per week (RT)/ET: +1.75% per week CTRL: F: 2 sessions/week IL: minimum load IE: 7 exercise 2 × 15 reps (r: 1 min between sets) Ti: 55 min/session Ty: RT with minimum load V: 210 reps/week P: N/A Attrition of the study: 27.65% Adherence to the study: >75% | Analysis within group RESEX: ↓ PANSS total score after 10 weeks (p = 0.002) ↓ PANSS total score after 20 weeks (p ≤ 0.001) ↓ positive symptoms (PANSS) after 10 weeks (p = 0.039) ↓ positive symptoms (PANSS) after 20 weeks (p ≤ 0.001) ↓ negative symptoms (PANSS) after 10 weeks (p = 0.001) ↓ negative symptoms (PANSS) after 20 weeks (p = 0.002) ↑ SF- 36 (physical role dimension) after 20 weeks (p = 0.011) CONCEX: ↓ PANSS total score after 10 weeks (p = 0.026) ↓ PANSS total score after 20 weeks (p = 0.003) ↓ positive symptoms (PANSS) after 20 weeks (p = 0.016) = negative symptoms (PANSS) (p not reported) ↑ SF- 36 (physical role dimension) after 20 weeks (p = 0.014) CTRL: = PANSS total score (p not reported) = positive symptoms (PANSS) (p not reported) = negative symptoms (PANSS) (p not reported) Analysis between groups Not reported for these outcomes |
| Lo et al. [46] | Sex: 17.64% male (HIIT)/50% male (ENEX)/50% male (CTRL) BMI (kg/m2): 26.72 ± 5.31 (HIIT)/25.56 ± 4.09 (ENEX)/24.82 ± 2.83 (CTRL) Race/Ethnicity: N/A Other diseases: N/A Pharmacological treatment: 16 were prescribed atypical antipsychotics and 1 typical antipsychotic in HIIT group/13 were prescribed atypical antipsychotics, 2 typical antipsychotics and 1 was not prescribed with any antipsychotic in AE group/16 were prescribed atypical antipsychotics, 1 typical antipsychotic and 1 was not prescribed with any antipsychotic in control group Previous physical exercise experience: N/A Outpatients aged 18–55 years with a diagnosis of schizophrenia spectrum disorder (DSM V). | Design: RCT ENEX (HIIT) ENEX CTRL (Psychoeducation) Sample size: (n = 51) HIIT: n = 17/ENEX: n = 16/CTRL: n = 18 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX (HIIT): F: 3 sessions/week IL: number of bouts as long as possible). X > 105% FTP + 1–3 min < 91% FTP Ti: number of bouts up to participants expended 150 kJ Ty: cycling performance V: until reaching 150 kJ (not time, sets or reps reported) P: N/A ENEX: F: 3 sessions/week IL: <91% FTP Ti: X min until reaching 150 kJ Ty: cycling performance V: until reaching 150 kJ (not time, sets or reps reported) P: N/A CTRL (Psychoeducation): F: 3 sessions/week Ti: 15–30 min/session Ty: mental and physical health content was delivered to the participants Attrition of the study: 15.69% (2 drop out the study before participating in the interventions and 6 during the intervention) Adherence of the study: N/A | Analysis within group ENEX (HIIT):↑ procedural memory consolidation (sleep-dependent memory consolidation) (p < 0.001) ↑ logical memory (24 h delayed recall) (p < 0.001) = PANSS total score (p = 0.548) = positive symptoms (PANSS) (p = 0.824) = negative symptoms (PANSS) (p = 0.134) ENEX: ↑ procedural memory consolidation (sleep-dependent memory consolidation) (p < 0.05) = logical memory (24 h delayed recall) (p = 0.077) = PANSS total score (p = 0.460) = positive symptoms (PANSS) (p = 0.594) = negative symptoms (PANSS) (p = 0.700) CTRL: = procedural memory consolidation (sleep-dependent memory consolidation) (p = 0.023) = logical memory (24 h delayed recall) (p = 0.946) = PANSS total score (p = 0.806) = positive symptoms (PANSS) (p = 0.829) = negative symptoms (PANSS) (p = 0.713) Analysis between groups HIIT vs. CTRL HIIT > CTRL at procedural memory consolidation (sleep-dependent memory consolidation) after 12 weeks (p < 0.01) HIIT > CTRL at logical memory (24 h delayed recall) after 12 weeks (p < 0.05) |
| Kern et al. [47] | Sex: 94% male (ENEX)/100% male (CTRL) BMI (kg/m2): Intervention group 30.1/control group 30.0 Race/Ethnicity: Intervention group (66% Black, 11% White, 11% Asian, 11% Hispanic) and control group (67% Black, 17% White, 0% Asian, 11% Hispanic) Other diseases: yes, chronic diseases Pharmacological treatment: Yes, antipsychotics 92% in ENEX and 88% in CTRL. Previous physical exercise experience: No (no participation in an aerobic exercise program in the past 6 months) Veterans aged 40–65 with a psychiatric diagnosis of schizophrenia orschizoaffective disorder (DSM V). | Design: RCT ENEX CTRL Sample size: (n = 53) ENEX: n = 35/CTRL: n = 18 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 3 sessions/week IL: 60–70% HRmax Ti: 20–40 min/session Ty: progressive ET V: 20 min week 1–2, 30 min week 3–4, 40 min week 5–12 P: +10 min every 2 weeks CTRL: F: 3 sessions/week Ti: 40 min/session Ty: stretching exercise. Attrition of ENEX: 22.8% Attrition of CTRL: 27.8% Adherence of ENEX: 81.4% (29.3 of 36 sessions completed) Adherence of CTRL: 77.2% (27.8 of 36 sessions completed) | Analysis within group ENEX: = social functioning (p = 0.09) = social cognition (p = ns) = non-social cognition (p = ns) = BPRS scores for positive and negative symptoms (p = ns) CTRL: Not reported significant effects on the outcomes of interest Analysis between groups ENEX vs. CTRL ↑ ENEX vs. ↓ CTRL of social functioning after 12 weeks (d = 0.35, p = 0.06) |
| Huang et al. [48] | Sex: 45.45% male in ENEX/38.23% male in CTRL BMI (kg/m2): 27.6 ± 4.8 in ENEX/26.1 ± 6.1 in CTRL Race/Ethnicity: N/A Other diseases: N/A Pharmacological treatment: use of antipsychotics in 100% of participants, stable doses for at least 1 month before. Previous physical exercise experience: N/A Patients 20–60 years of age having a diagnosis of schizophrenia (DSM V) with stable psychotic symptoms. | Design: RCT ENEX (aerobic walking + treatment as usual) CTRL (treatment as usual, original lifestyle and psychotropic treatment) Sample size: (n = 67) ENEX: n = 33/CTRL: n = 34 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 3.2 ± 0.8 days /week IL: target 40–60% HRR Ti: 30–50 min/session Ty: walking program V: 128.7 ± 29.1 min P: N/A CTRL (treatment as usual): Ty: original lifestyle and psychotropic treatment. Attrition of ENEX: 15.4% Attrition of CTRL: 10.5% Adherence of groups or study: N/A | Analysis within group ENEX: ↑ performance verbal memory (time effect, p = 0.03; ∆ Z score = 0.52 ± 0.89) CTRL: ↑ performance verbal memory (time effect, p = 0.03; ∆ Z score = 0.47 ± 0.78) Analysis between groups ENEX vs. CTRL No significant time × group interaction effect on BACS scores or any dimension. ENEX: high-intensity vs. low-intensity (cutoff > 40% HRR for high-intensity) No significant time × group interaction effect on BACS score. ↑ ENEX high-intensity vs. ↓ ENEX low-intensity, significant time × group interaction effect on verbal fluency (p = 0.05) after adjusting for duration of illness (MANCOVA). No significant interaction for the rest of dimensions. |
| Kimhy et al. [49] | Sex: 63% male (ENEX)/65% male (CTRL) BMI (kg/m2): 31.60 (ENEX)/30.75 (CTRL) Race/ Ethnicity: 43% Hispanic (ENEX)/29% Hispanic (CTRL) Other diseases: N/A Pharmacological treatment: 100% were prescribed antipsychotics and 6% were prescribed beta-blockers. Previous physical exercise experience: N/A Patients 18–55 years, diagnosis of schizophrenia or related disorders (DSM IV), no changes in the treatment in the last 3 months. | Design: RCT ENEX CTRL (treatment as usual) Sample size: (n = 33) ENEX: n = 16/CTRL: n = 17 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 3 sessions/week IL: 60–70% HRmax Ti: 45 min/session Ty: progressive ET + standard psychiatric care. V: 135 min per week P: +5% HRmax first 4 weeks CTRL: Standard psychiatric, regular meetings with a psychiatrist, psychologists, social workers, and/or psychiatric nurses. Attrition of ENEX: 19% Attrition of CTRL: 23.5% Attrition of the study: 21% (3 dropped out in ENEX and 4 dropped out in CTRL) Adherence of ENEX: 79% (28.5 of 36 sessions) Adherence of CTRL: N/A Adherence of study: N/A | Analysis within group ENEX: Not reported significant effects on the outcomes of interest CTRL: Not reported significant effects on the outcomes of interest Analysis between groups ENEX vs. CTRL ↑ENEX (+23%) vs. ↓CTRL (−4.2%) in social functioning index PSRS (p = 0.012) No significant differences in social functioning index by SANS (p = 0.58) No significant differences in social functioning index by SLOF (p = 0.22) |
| Marzolini et al. [50] | Sex: 51.14% male (CONCEX)/66.66% male (CTRL) BMI (kg/m2): 27.2 ± 1.2 (CONCEX)/29.3 ± 2.2 (CTRL) Race/Ethnicity: N/A Other diseases: At least cardiovascular risk. Pharmacological treatment: 6 used atypical antipsychotics, 5 used typical antipsychotics and 3 used antianxiety in CONCEX and 5 used atypical antipsychotics, 3 used typical antipsychotics, 2 used antidepressants and 4 used antianxiety in CTRL. Previous physical exercise experience: N/A Individuals with a diagnosis of schizophrenia/schizoaffective (DSM IV) and at least 1 cardiovascular risk. | Design: RCT CONCEX CTRL (usual care) Sample size: (n = 13) CONCEX: n = 7/CTRL: n = 6 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A CONCEX: F: 2 sessions/week IL: RT: starting 60% 1RM. 10–15 reps (last set repetition at RPE 15)/ET: 60–80% HRR (RPE 11–14) Ti: 20 min (RT) + 60 min (ET) each session Ty: CT V: RT: 4 exercises upper- and lower-limbs: 1–2 × 10–15 reps, r: >30 s/ET: 1.6km to 6.4 km P: RT: +1–2 kg based on RPE/ET: +3.33% HRR each 2 weeks CTRL: Usual care Attrition of study: 0% (all participants attended, at least, 50% of sessions; no participant dropped-out) Adherence of CONCEX: 72% (±4.4%) | Analysis within group CONCEX: ↑ MHI score after 12 weeks (p = 0.03) ↑ 6MWD = ↑ MHI total score (p = 0.09) ↓ depressive symptoms (MHI subscale) = ↑ 6MWD (p < 0.001) ↓ depressive symptoms (MHI subscale) = ↑ adherence to exercise (p = 0.02) CTRL: = MHI score after 12 weeks (p = 0.57) ↑ 6MWD = ↑ MHI total score (p = 0.09) ↓ depressive symptoms (MHI subscale) = ↑ 6MWD (p < 0.001) Analysis between groups CONCEX vs. CTRL No significant differences in MHI score (p = 0.33) |
| Ryu et al. [51] | Sex: 50% male in ENEX/56.66% male in CTRL BMI (kg/m2): N/A Race/Ethnicity: N/A Other diseases: N/A Pharmacological treatment: 100% were prescribed antipsychotics with a stable dose for at least 4 weeks before intervention. Previous physical exercise experience: No (exclusion of patients who participated in any exercise program 3 months before the study). Outpatients 18–65 years old, with a diagnosis of schizophrenia or schizoaffective disorder (DSM IV). | Design: Single blind RCT ENEX (outdoor cycling) CTRL (occupational therapy) Sample size: (n = 60) ENEX: n = 30/CTRL: n = 30 Intervention period: 16 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 1 session/week IL: 16km/h Ti: 40 min of bike training each session Ty: outdoor cycling. V: 40 min per week P: N/A CTRL: F: 1 session/week Ty: daily living skills, social skills, or creative activities. Ti: 90 min/session Attrition of ENEX: 13.3% Attrition of CTRL: 20% Attrition of the study: 16.7% Adherence of groups or study: N/A | Analysis within group ENEX: ↓ psychotic symptoms (BPRS) (p = 0.042) ↓ thought disturbance (subscales of BPRS) (p = 0.002) ↓ BDI score (p < 0.001) ↓ STAI-state score (p = 0.001) ↓ STAI-trait score (p < 0.001) ↑ GAF score (p = 0.001) ↑ WCST CR (p < 0.001) ↑ WCST CC (p = 0.005) CTRL: = psychotic symptoms (BPRS) (p = 0.136) = BDI score (p = 0.945) = STAI-state score (p = 0.696) = STAI-trait score (p = 0.788) = GAF score (p = 0.556) = WCST CR (p = 0.406) = WCST CC (p = 0.838) Analysis between groups ENEX vs. CTRL No significant group × time interaction in RSES (p = 0.052) No significant group × time interaction in QoL score (p = 0.098) |
| Battaglia et al. [52] | Sex: 100% male BMI (kg/m2): 28.55 ± 4.06 in ENEX/28.65 ± 2.62 in CTRL Race/ Ethnicity: N/A Other diseases: N/A Pharmacological treatment: 100% were prescribed antipsychotics (clozapine, olanzapine or risperidone) with a stable dose. Previous physical exercise experience: at least 1 year of soccer experience. Male patients with a diagnosis of schizophrenia or schizoaffective disorders (DSM IV) > 18 years old. | Design: Double- blind RCT ENEX CTRL Sample size: (n = 18) ENEX: n = 10/CTRL: n = 8 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 2 sessions/week IL: 50–85% HRmax Ti: 40–60 min of training period each session Ty: progressive soccer technical-tactical exercises and soccer games V: 80–120 min per week P: +5 min each game after week 5 and +5 min each game after week 8. CTRL: not performed any organized physical activity. Attrition/Adherence: N/A | Analysis within group ENEX: ↑ MCS-12 score (+10.8%, p < 0.0001) CTRL: Not reported significant effects on the outcomes of interest Analysis between groups ENEX vs. CTRL ENEX > CTRL in MCS-12 score after 12 weeks (p < 0.0001) |
| Nygård et al. [53] | Sex: 58.33% male BMI (kg/m2): 29.7 (CONCEX)/30 (CTRL) Race/Ethnicity: N/A Other diseases: 11 smokers in TG and 8 in CG (without any other chronic disease). Pharmacological treatment: 24 were prescribed antipsychotics, 3 antiepileptics, 8 benzodiazepine, 2 biperiden, 1 levaxine, 1 lithium and 1 SSRI in ENEX/21 were prescribed antipsychotics, 3 antiepileptics, 6 benzodiazepine, 1 levaxine, 2 lithium and 3 SSRI in CTRL. Previous physical exercise experience: N/A Outpatients, 22–59 years old with a diagnosis of schizophrenia spectrum disorders (ICD-10). | Design: RCT CONCEX CTRL Sample size: (n = 36) CONCEX: n = 17/CTRL: n = 19 Intervention period: 12 weeks | Supervised: Yes Familiarization: 1 day with a familiarization session on treadmill and leg press. CONCEX: F: 2 sessions/week IL: RT: 90% of 1RM/ET: 85–95% of HRpeak Ti: 35 min (ET) Ty: endurance interval training on treadmill + leg press MST V: RT: 4 × 4 reps, r: 3–4 min. 8 reps per session/ET: 4 × 4 min, r: 3 min (at 70% of HRpeak). 70 min per week P: +5 kg each session the patient managed to complete 5 reps (RT) CTRL: 2 training sessions; participants were encouraged to train on their own. Attrition of ENEX: 32% Attrition of CTRL: 17% Adherence of groups or study: N/A | Analysis within group CONCEX: = mental health index by SF-36 (p = 0.158) CTRL: = mental health index by SF-36 (p = 0.934) Analysis between groups CONCEX vs. CTRL No group × time significant differences in mental health index by SF-36 (p = 0.277) |
| Massa et al. [54] | Sex: 76.19% male (ENEX) /88.23% male (CTRL) BMI (kg/m2): 31.11 ± 6.87 (ENEX)/29.31 ± 4 (CTRL) Race/Ethnicity: 19 African American (ENEX)/17 African American (CTRL). Other diseases: N/A Pharmacological treatment: 14 were prescribed atypical antipsychotics, 1 typical antipsychotics, 2 both, 6 antidepressants and 4 were not prescribed with any antipsychotic in ENEX/ 13 were prescribed atypical antipsychotics, 2 typical antipsychotics, 1 both, 4 antidepressants and 1 were not prescribed with any antipsychotic in CTRL. Previous physical exercise experience: No, sedentary lifestyle for the last month. Outpatients with a diagnosis of schizophrenia, 18–70 years old. | Design: RCT (assessments were completed by an assessor-blinded to the treatment group): ENEX CTRL Sample size: (n = 38) (only completed 15) ENEX: n = 21 (completed the study 9) CTRL: n = 17 (completed the study 6) Intervention period: 12 weeks (follow-up to week 20) | Supervised: Yes Familiarization: N/A ENEX: F: 3 sessions/week IL: 50–80% of HRmax Ti: 20–45 min Ty: progressive ET on a stationary bicycle ergometer. V: from 60 min to 135 min per week P: +5 min and +5% HRmax per week CTRL: 3 sessions/week Ty: stretching and toning exercise performed for the same amount of time as the AE program. Attrition of ENEX: 68.08% Attrition of CTRL: N/A Adherence of groups or study: N/A | Analysis within group ENEX: No significant difference after week 12 for MCCB composite scores CTRL: No significant difference after week 12 for MCCB composite scores Analysis between groups ENEX vs. CTRL ↑ENEX vs. ↓CTRL in MCCB composite score from week 12 to week 20 (p = 0.03) ↑ENEX vs. ↓CTRL in visual learning domain of MCCB composite score from week 12 to week 20 (p = 0.006) |
| Su et al. [55] | Sex: 45.5% male (ENEX)/45.5% male (CTRL) BMI (kg/m2): 30.72 (ENEX)/34.56 (CTRL) Race/ Ethnicity: N/A Other diseases: N/A Pharmacological treatment: were on stable antipsychotic medication with no major dose changes for at least 3 months before the study. Previous physical exercise experience: N/A Patients with a diagnosis of criteria for schizophrenia or schizoaffective disorder (DSM IV) with a stable medication. | Design: Single-blinded RCT ENEX CTRL (stretching and toning control group) Sample size: (n = 44) ENEX: n = 22/CTRL: n = 22 Intervention period: 12 weeks (follow-up to 6 months) | Supervised: Yes Familiarization: N/A ENEX: F: 4–5 sessions/week IL: 55–69% HRmax/13–16 RPE Ti: 30 min Ty: progressive ET on treadmill V: 120–150 min per week P: Not specified. CTRL: F: 4–5 sessions/week IE: 14 exercises, held for 10 s and repeated 10 times. Ti: 30 min Ty: stretching and toning control program. Attrition of groups or study: N/A Adherence of ENEX: 76.6% (45.95 of the 60 maximum scheduled sessions) Adherence of CTRL: 78.2% (46.91 of the 60 maximum scheduled sessions) | Analysis within group ENEX ↑ processing speed scores at posttest (p = 0.005) ↑ processing speed scores at follow-up (p = 0.009) ↑ attention scores at follow-up > posttest (p = 0.006) ↑ verbal learning scores at follow-up > posttest (p = 0.009) ↑ verbal learning scores at follow-up > pretest (p = 0.3001; ↑25.6%) CTRL: Processing speed scores at follow-up > posttest (p = 0.02) ↑ verbal learning scores at posttest (p = 0.02) Reasoning and problem solving at follow-up > posttest (p = 0.003) Analysis between groups ENEX vs. CTRL ENEX > CTRL In processing speed scores at posttest (p = 0.001) ENEX > CTRL in attention scores at posttest (p = 0.03) CTRL > ENEX in PANSS negative symptoms score at follow-up (p = 0.03) |
| Kimhy et al. [56] | Sex: 63% male (ENEX)/65% male (CTRL) BMI (kg/m2): 31.60 (ENEX)/30.75 (CTRL) Race/Ethnicity: 43% Hispanic (ENEX)/29% Hispanic (CTRL) Other diseases: 25% smokers (ENEX)/23% smokers (CTRL) Pharmacological treatment: 100% used antipsychotics, 44% used antidepressants and 31% used SSRIs in ENEX/100% used antipsychotics, 35% used antidepressants and 23% used SSRIs in CTRL. Previous physical exercise experience: N/A Outpatients with a diagnosis of schizophrenia or related disorders (DSM IV); age 18–55 years. | Design: Single-blind RCT ENEX CTRL (treatment as usual) Sample size: (n = 33) ENEX: n = 16/CTRL: n = 17 Intervention period: 12 weeks | Supervised: Yes Familiarization: N/A ENEX: F: 3 sessions/week IL: 60–75% of HRmax Ti: 45 min Ty: progressive ET with 2 active-play video game systems, 2 treadmill machines, a stationary bike and an elliptical machine. V: 135 min per week P: +5% of HRmax after week 1, +5% after week 2 and +5% after week 3. CTRL: Standard psychiatric care, regular meetings with a psychiatrist, psychologists, social workers or psychiatric nurses. Attrition of ENEX: 18.7% (dropped-out 3) Attrition of CTRL: 23.5% (dropped-out 4) Attrition of the study: 21% Adherence of ENEX: 79% (28.5 of 36 sessions) Adherence of study: N/A | Analysis within group ENEX: Not reported CTRL: Not reported Analysis between groups ENEX vs. CTRL ↑ENEX (+15%) > ↓CTRL (−2%) in MCCB composite scores after 12 weeks (p = 0.031) |
| Nygård et al., 2023 [57] | Sex: 58.33% male BMI (kg/m2): 27.98 ± 1.67 (CONCEX), 25.5 ± 5.0 (CTRL) Race/Ethnicity: N/A Other diseases: N/A Pharmacological treatment: First generation antipsychotics (n = 7), second generation antipsychotics (n = 42), clozapine (n = 18), 2 antipsychotics (n = 17) and without antipsychotics (n = 3) Previous physical exercise experience: N/A Outpatients with a schizophrenia spectrum disorders diagnosis (International Statistical Classification of Diseases (ICD)-10) between 18 and 65 years with clinically stable disease. | Design: RCT CONCEX CTRL Sample size: (n = 48) CONCEX: n = 25/CTRL: n = 23 Intervention period: 1 year | Supervised: Yes Familiarization: 1 session CONCEX: 2 sessions/week IL: RT: 90% 1RM/ET: 85–95% HRpeak Ti: RT: N/A/ET: 35 min Ty: leg press MST + interval ET. V: RT: 4 × 4 reps of leg press (r: 3–4 min), 32 reps per week/ET: 4 × 4 min treadmill walking/running (r: 3 min) P: RT: +5 kg if 5 reps in last set/ET: +1.75% per week CTRL: 2 introductory training sessions to inform them of the benefits of regular exercise and encourage them to train on their own. Attrition of CONCEX: 40% Attrition of CTRL: 21.74% Adherence of study: 64.58% (62 ± 16 attended sessions of 96) | Analysis within group CONCEX: = SF-36 score after 3 months (p = 0.720) = SF-36 score after 1 year (p = 0.336) CTRL: = SF-36 score after 3 months (p = 0.720) = SF-36 score after 1 year (p = 0.336) Analysis between groups CONCEX vs. CTRL No group × time significant differences in SF-36 scores after 3 months (p = 0.436) or 1 year (p = 0.304) |
3. Results
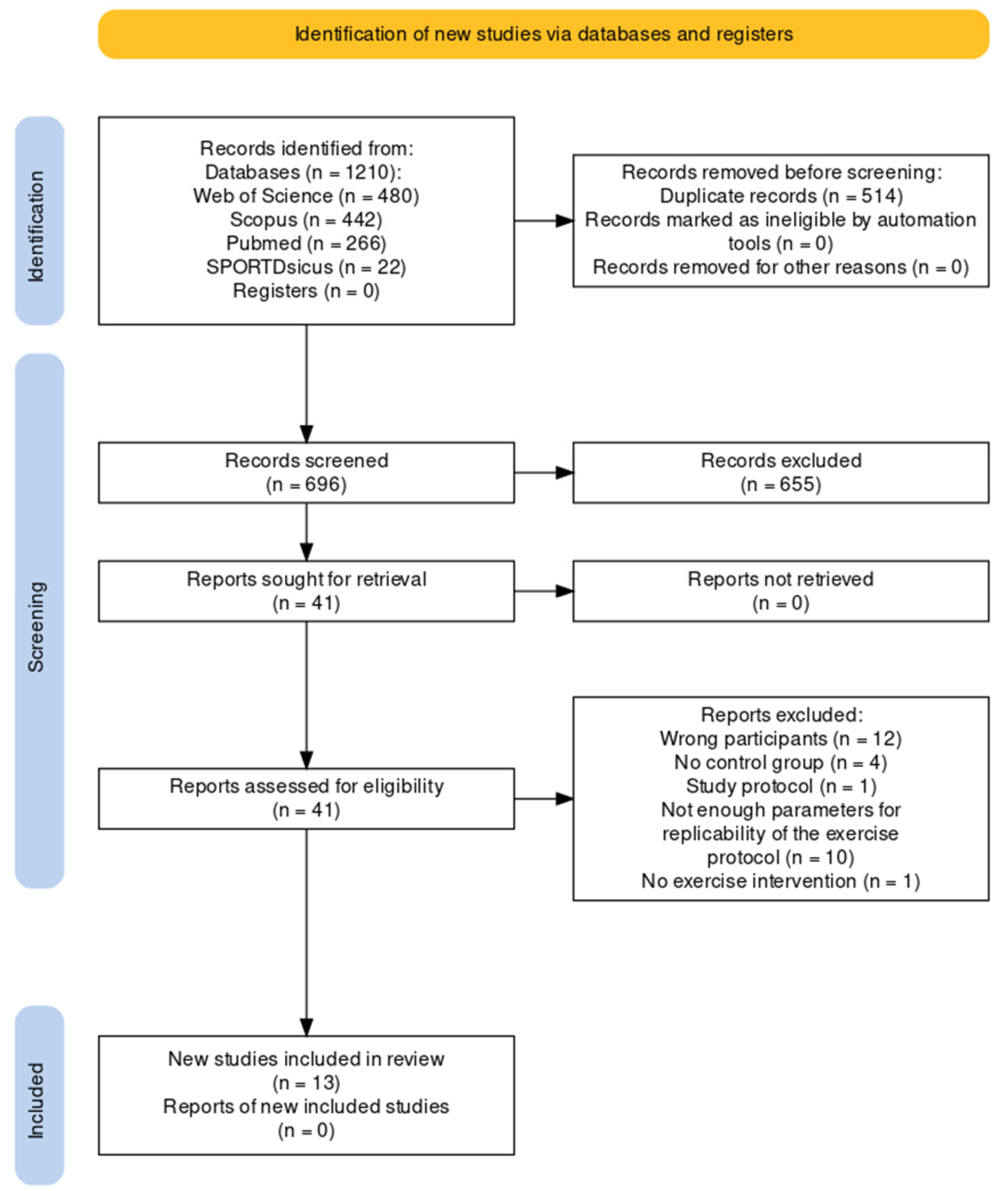
3.1. Study Selection
3.2. Study Characteristics
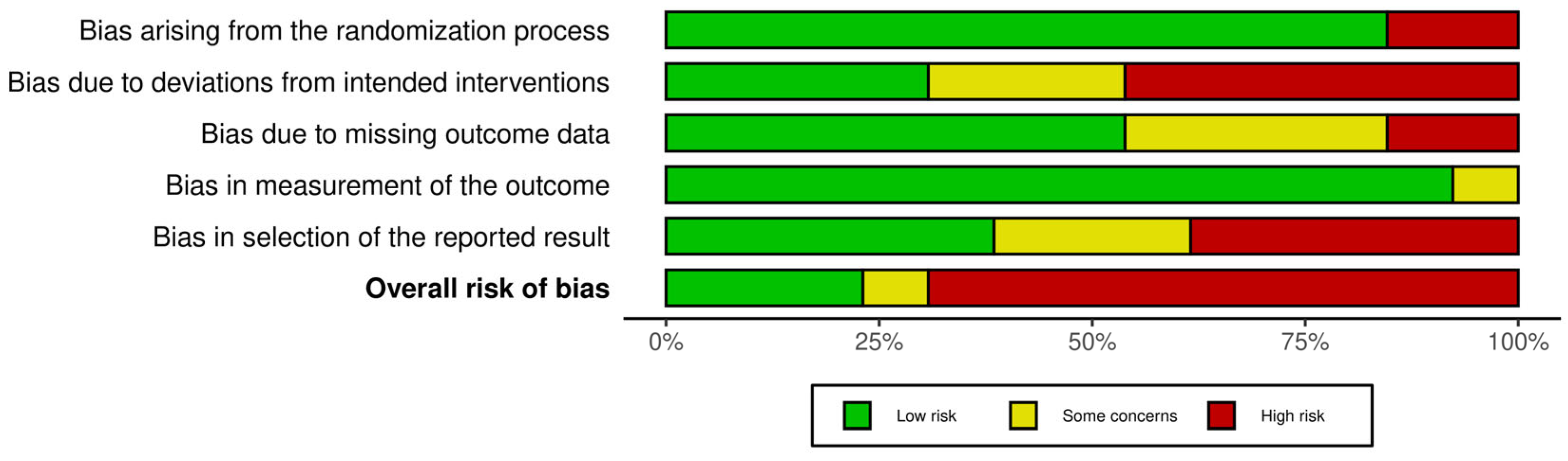
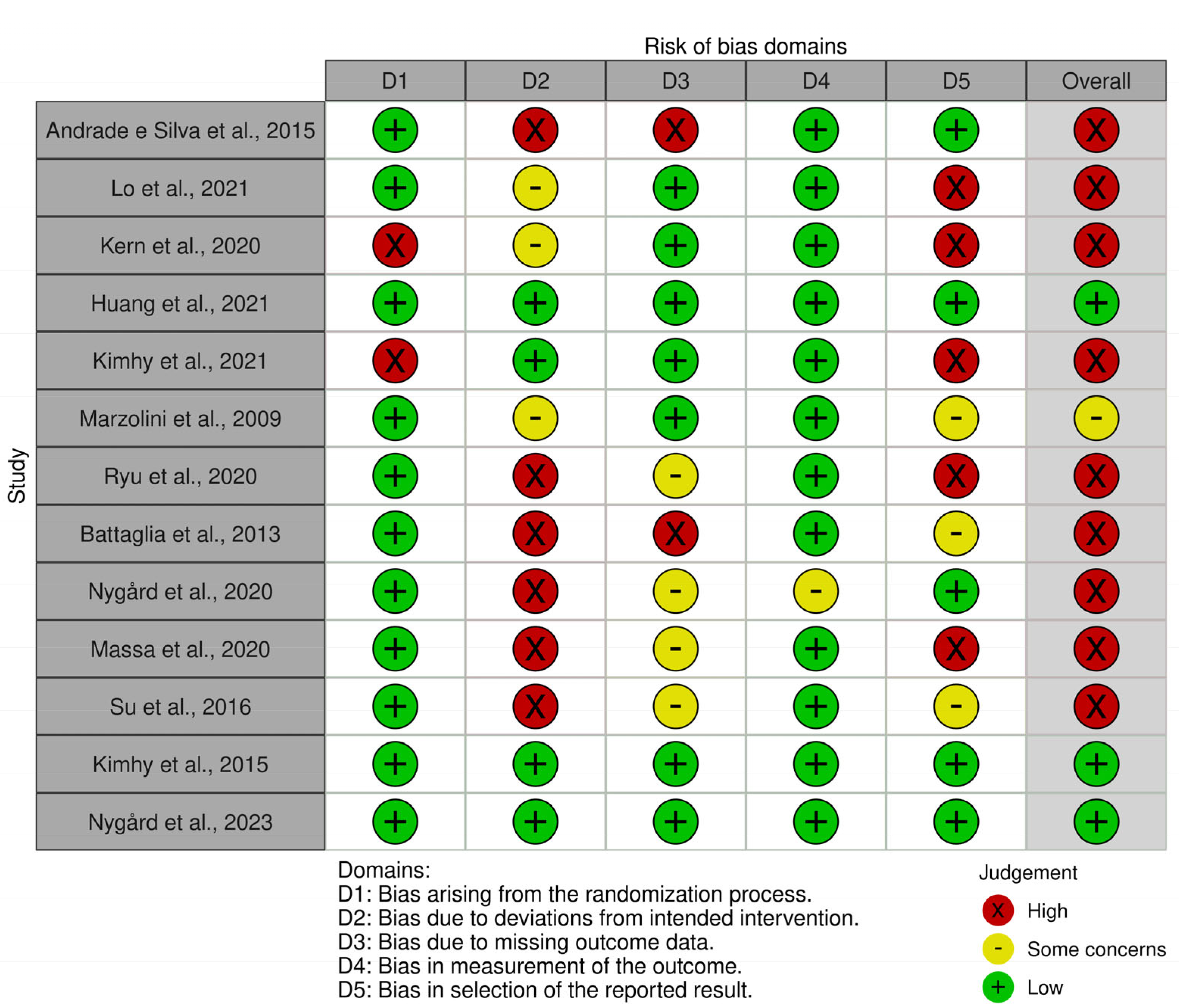
3.3. Critical Appraisal
3.3.1. Risk of Bias
3.3.2. Quality Reporting of Exercise Intervention in the Training Programs
3.4. Summary of Evidence
3.4.1. Psychopathology
3.4.2. Cognition
3.4.3. Quality of Life
4. Discussion
4.1. Training Characteristics of the Included Studies
4.2. Effects of Regular Exercise on Psychopathology, Cognition, and Quality of Life of Patients with Schizophrenia
4.2.1. Psychopathology
4.2.2. Cognition
4.2.3. Quality of Life and Functioning
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- ICD-11 for Mortality and Morbidity Statistics [Internet]. Available online: https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1683919430 (accessed on 26 November 2022).
- American Psychiatric Association. Guía de Consulta de los Criterios Diagnósticos del DSM-5; American Psychiatric Publishing: Arlington, VA, USA, 2014. [Google Scholar]
- GBD Results [Internet]. Institute for Health Metrics and Evaluation. Available online: https://vizhub.healthdata.org/gbd-results (accessed on 28 November 2022).
- Jin, H.; Mosweu, I. The Societal Cost of Schizophrenia: A Systematic Review. PharmacoEconomics 2017, 35, 25–42. [Google Scholar] [CrossRef]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojtabai, R.; Servis, M.; Walaszek, A.; Buckley, P.; Lenzenweger, M.F.; et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. Am. J. Psychiatry 2020, 177, 868–872. [Google Scholar] [CrossRef]
- Pennington, M.; McCrone, P. The Cost of Relapse in Schizophrenia. PharmacoEconomics 2017, 35, 921–936. [Google Scholar] [CrossRef]
- Correll, C.U.; Ng-Mak, D.S.; Stafkey-Mailey, D.; Farrelly, E.; Rajagopalan, K.; Loebel, A. Cardiometabolic comorbidities, readmission, and costs in schizophrenia and bipolar disorder: A real-world analysis. Ann. Gen. Psychiatry 2017, 16, 9. [Google Scholar] [CrossRef]
- Ride, J.; Kasteridis, P.; Gutacker, N.; Aragon, M.J.A.; Jacobs, R. Healthcare Costs for People with Serious Mental Illness in England: An Analysis of Costs Across Primary Care, Hospital Care, and Specialist Mental Healthcare. Appl. Health Econ. Health Policy 2020, 18, 177–188. [Google Scholar] [CrossRef]
- Auquier, P.; Lançon, C.; Rouillon, F.; Lader, M. Mortality in schizophrenia. Pharmacoepidemiol. Drug Safe 2007, 16, 1308–1312. [Google Scholar] [CrossRef]
- Connolly, M.; Kelly, C. Lifestyle and physical health in schizophrenia. Adv. Psychiatr. Treat. 2005, 11, 125–132. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D.; Casey, D.E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, G.; Kim, C.-E.; Ryu, S. Physical Activity of Patients with Chronic Schizophrenia and Related Clinical Factors. Psychiatry Investig. 2018, 15, 811–817. [Google Scholar] [CrossRef]
- Vancampfort, D.; Firth, J.; Schuch, F.B.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.B.; Gaughran, F.; De Hert, M.; et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry 2017, 16, 308–315. [Google Scholar] [CrossRef]
- Vancampfort, D.; Probst, M.; Knapen, J.; Carraro, A.; De Hert, M. Associations between sedentary behaviour and metabolic parameters in patients with schizophrenia. Psychiatry Res. 2012, 200, 73–78. [Google Scholar] [CrossRef]
- Soundy, A.; Wampers, M.; Probst, M.; De Hert, M.; Stubbs, B.; Vancampfort, D.; Attux, C.; Leutwyler, H.; Ströhle, A. Physical activity and sedentary behaviour in outpatients with schizophrenia: A systematic review and meta-analysis. Int. J. Ther. Rehabil. 2013, 20, 588–595. [Google Scholar] [CrossRef]
- Lebiecka, Z.; Łopuszko, A.; Rudkowski, K.; Dańczura, E. Effects of physical activity on treatmentof schizophrenia. Arch. Psychiatry Psychother. 2019, 21, 28–35. [Google Scholar] [CrossRef]
- Shimada, T.; Ito, S.; Makabe, A.; Yamanushi, A.; Takenaka, A.; Kawano, K.; Kobayashi, M. Aerobic exercise and cognitive functioning in schizophrenia: An updated systematic review and meta-analysis. Psychiatry Res. 2022, 314, 114656. [Google Scholar] [CrossRef]
- Girdler, S.J.; Confino, J.E.; Woesner, M.E. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol. Bull. 2019, 49, 56–69. [Google Scholar]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess Early Mortality in Schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Laursen, T.M. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr. Res. 2011, 131, 101–104. [Google Scholar] [CrossRef]
- World Health Organization. Programme on Mental Health: WHOQOL User Manual; World Health Organization: Geneva, Switzerland, 2023; Available online: https://apps.who.int/iris/handle/10665/77932 (accessed on 16 May 2023).
- Galuppi, A.; Turola, M.C.; Nanni, M.G.; Mazzoni, P.; Grassi, L. Schizophrenia and quality of life: How important are symptoms and functioning? Int. J. Ment. Health Syst. 2010, 4, 31. [Google Scholar] [CrossRef]
- Acil, A.A.; Dogan, S.; Dogan, O. The effects of physical exercises to mental state and quality of life in patients with schizophrenia. J. Psychiatr. Ment. Health Nurs. 2008, 15, 808–815. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Tandon, R.; Lenderking, W.R.; Weiss, C.; Shalhoub, H.; Barbosa, C.D.; Chen, J.; Greene, M.; Meehan, S.R.; Duvold, L.B.; Arango, C.; et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: A worldwide, cross-sectional, web-based survey. Ann. Gen. Psychiatry 2020, 19, 42. [Google Scholar] [CrossRef]
- Hansford, H.J.; A Wewege, M.; Cashin, A.G.; Hagstrom, A.D.; Clifford, B.K.; McAuley, J.H.; Jones, M.D. If exercise is medicine, why don’t we know the dose? An overview of systematic reviews assessing reporting quality of exercise interventions in health and disease. Br. J. Sports Med. 2022, 56, 692–700. [Google Scholar] [CrossRef]
- Bayles, M.P.; Swank, A.M. General Principles of Exercise Prescription. In ACSM’s Exercise Testing and Prescription; American College of Sports Medicine; Wolters Kluwer: Philadelphia, PA, USA, 2018; pp. 126–141. [Google Scholar]
- Sabe, M.; Kaiser, S.; Sentissi, O. Physical exercise for negative symptoms of schizophrenia: Systematic review of randomized controlled trials and meta-analysis. Gen. Hosp. Psychiatry 2020, 62, 13–20. [Google Scholar] [CrossRef]
- Martin, H.; Beard, S.; Clissold, N.; Andraos, K.; Currey, L. Combined aerobic and resistance exercise interventions for individuals with schizophrenia: A systematic review. Ment. Health Phys. Act. 2017, 12, 147–155. [Google Scholar] [CrossRef]
- Firth, J.; Cotter, J.; Elliott, R.; French, P.; Yung, A.R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med. 2015, 45, 1343–1361. [Google Scholar] [CrossRef]
- Bredin, S.S.D.; Kaufman, K.L.; Chow, M.I.; Lang, D.J.; Wu, N.; Kim, D.D.; Warburton, D.E.R. Effects of Aerobic, Resistance, and Combined Exercise Training on Psychiatric Symptom Severity and Related Health Measures in Adults Living with Schizophrenia: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 8, 753117. [Google Scholar] [CrossRef]
- Yu, Q.; Wong, K.-K.; Lei, O.-K.; Nie, J.; Shi, Q.; Zou, L.; Kong, Z. Comparative Effectiveness of Multiple Exercise Interventions in the Treatment of Mental Health Disorders: A Systematic Review and Network Meta-Analysis. Sports Med. Open 2022, 8, 135. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Heringa, S.M.; Sommer, I.E. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2016, 42, 588–599. [Google Scholar] [CrossRef]
- Cella, M.; Roberts, S.; Pillny, M.; Riehle, M.; O’Donoghue, B.; Lyne, J.; Tomlin, P.; Valmaggia, L.; Preti, A. Psychosocial and behavioural interventions for the negative symptoms of schizophrenia: A systematic review of efficacy meta-analyses. Br. J. Psychiatry 2023, 223, 321–331. [Google Scholar] [CrossRef]
- Gallardo-Gómez, D.; Noetel, M.; Álvarez-Barbosa, F.; Alfonso-Rosa, R.M.; Ramos-Munell, J.; Cruz, B.d.P.; del Pozo-Cruz, J. Exercise to treat psychopathology and other clinical outcomes in schizophrenia: A systematic review and meta-analysis. Eur. Psychiatry 2023, 66, e40. [Google Scholar] [CrossRef]
- Korman, N.; Stanton, R.; Vecchio, A.; Chapman, J.; Parker, S.; Martland, R.; Siskind, D.; Firth, J. The effect of exercise on global, social, daily living and occupational functioning in people living with schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2023, 256, 98–111. [Google Scholar] [CrossRef]
- Oliva, H.N.; Monteiro-Junior, R.S.; Oliva, I.O.; Powers, A.R. Effects of exercise intervention on psychotic symptoms: A meta-analysis and hypothetical model of neurobiological mechanisms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 125, 110771. [Google Scholar] [CrossRef]
- Tavares, V.D.d.O.; Rossell, S.L.; Schuch, F.B.; Herring, M.; de Sousa, G.M.; Galvão-Coelho, N.L.; Hallgren, M. Effects of exercise on cognitive functioning in adults with serious mental illness: A meta analytic review. Psychiatry Res. 2023, 321, 115081. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res Methodol. 2016, 16, 15. [Google Scholar] [CrossRef]
- Zotero [Software], Version 6.0.26; Corporation for Digital Scholarship: Vienna, VA, USA, 2023. Available online: https://www.zotero.org/(accessed on 5 April 2022).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Silva, B.A.; Cassilhas, R.C.; Attux, C.; Cordeiro, Q.; Gadelha, A.L.; Telles, B.A.; Bressan, R.A.; Ferreira, F.N.; Rodstein, P.H.; Daltio, C.S.; et al. A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: Results of a blind, randomized controlled trial. Rev. Bras. Psiquiatr. 2015, 37, 271–279. [Google Scholar] [CrossRef]
- Lo, L.L.H.; Lee, E.H.M.; Hui, C.L.M.; Chong, C.S.Y.; Chang, W.C.; Chan, S.K.W.; Lin, J.J.; Lo, W.T.L.; Chen, E.Y.H. Effect of high-endurance exercise intervention on sleep-dependent procedural memory consolidation in individuals with schizophrenia: A randomized controlled trial. Psychol. Med. 2021, 53, 1708–1720. [Google Scholar] [CrossRef]
- Kern, R.S.; Reddy, L.F.; Cohen, A.N.; Young, A.S.; Green, M.F. Effects of aerobic exercise on cardiorespiratory fitness and social functioning in veterans 40 to 65 years old with schizophrenia. Psychiatry Res. 2020, 291, 113258. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hung, C.-F.; Hsu, S.-T.; Lin, P.-Y.; Lee, Y.; Chong, M.-Y.; Chen, C.-C.; Kuo, Y.-H.; Wang, L.-J. Effects of aerobic walking on cognitive function in patients with schizophrenia: A randomized controlled trial. J. Psychiatr. Res. 2021, 134, 173–180. [Google Scholar] [CrossRef]
- Kimhy, D.; Tay, C.; Vakhrusheva, J.; Beck-Felts, K.; Ospina, L.H.; Ifrah, C.; Parvaz, M.; Gross, J.J.; Bartels, M.N. Enhancement of aerobic fitness improves social functioning in individuals with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 367–376. [Google Scholar] [CrossRef]
- Marzolini, S.; Jensen, B.; Melville, P. Feasibility and effects of a group-based resistance and aerobic exercise program for individuals with severe schizophrenia: A multidisciplinary approach. Ment. Health Phys. Act. 2009, 2, 29–36. [Google Scholar] [CrossRef]
- Ryu, J.; Jung, J.H.; Kim, J.; Kim, C.-H.; Lee, H.-B.; Kim, D.-H.; Lee, S.-K.; Shin, J.-H.; Roh, D. Outdoor cycling improves clinical symptoms, cognition and objectively measured physical activity in patients with schizophrenia: A randomized controlled trial. J. Psychiatr. Res. 2020, 120, 144–153. [Google Scholar] [CrossRef]
- Battaglia, G.; Alesi, M.; Inguglia, M.; Roccella, M.; Caramazza, G.; Bellafiore, M.; Palma, A. Soccer practice as an add-on treatment in the management of individuals with a diagnosis of schizophrenia. Neuropsychiatr. Dis. Treat. 2013, 9, 595–603. [Google Scholar] [CrossRef]
- Nygård, M.; Brobakken, M.F.; Taylor, J.L.; Reitan, S.K.; Güzey, I.C.; Morken, G.; Lydersen, S.; Vedul-Kjelsås, E.; Wang, E.; Heggelund, J. Strength training restores force-generating capacity in patients with schizophrenia. Scand. J. Med. Sci. Sports 2021, 31, 665–678. [Google Scholar] [CrossRef]
- Massa, N.; Alrohaibani, A.; Mammino, K.; Bello, M.; Taylor, N.; Cuthbert, B.; Fargotstein, M.; Coulter, M.M.; Boatright, J.H.; Nocera, J.; et al. The Effect of Aerobic Exercise on Physical and Cognitive Outcomes in a Small Cohort of Outpatients with Schizophrenia. Brain Plast. 2020, 5, 161–174. [Google Scholar] [CrossRef]
- Su, C.-Y.; Wang, P.-W.; Lin, Y.-J.; Tang, T.-C.; Liu, M.-F.; Chen, M.-D. The effects of aerobic exercise on cognition in schizophrenia: A 3-month follow-up study. Psychiatry Res. 2016, 244, 394–402. [Google Scholar] [CrossRef]
- Kimhy, D.; Vakhrusheva, J.; Bartels, M.N.; Armstrong, H.F.; Ballon, J.S.; Khan, S.; Chang, R.W.; Hansen, M.C.; Ayanruoh, L.; Lister, A.; et al. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr. Bull. 2015, 41, 859–868. [Google Scholar] [CrossRef]
- Nygård, M.; Brobakken, M.F.; Lydersen, S.; Güzey, I.C.; Morken, G.; Heggelund, J.; Wang, E. Strength training integrated in long term collaborative care of patients with schizophrenia. Schizophr. Res. 2023, 260, 67–75. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, J. The effect of exercise on physical and mental health for adults with schizophrenia: A review of clinical aerobic exercise. Ethiop. J. Health Dev. 2020, 34, 35–43. [Google Scholar]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People with Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2016, 43, 546–556. [Google Scholar] [CrossRef]
- Stanton, R.; Happell, B. A systematic review of the aerobic exercise program variables for people with schizophrenia. Curr. Sports Med. Rep. 2014, 13, 260–266. [Google Scholar] [CrossRef]
- Heggelund, J.; Morken, G.; Helgerud, J.; E Nilsberg, G.; Hoff, J. Therapeutic effects of maximal strength training on walking efficiency in patients with schizophrenia—A pilot study. BMC Res. Notes 2012, 5, 344. [Google Scholar] [CrossRef]
- Maurus, I.; Mantel, C.; Keller-Varady, K.; Schmitt, A.; Lembeck, M.; Röh, A.; Papazova, I.; Falkai, P.; Schneider-Axmann, T.; Hasan, A.; et al. Resistance training in patients with schizophrenia: Concept and proof of principle trial. J. Psychiatr. Res. 2020, 120, 72–82. [Google Scholar] [CrossRef]
- Westcott, W.L. Resistance Training is Medicine: Effects of Strength Training on Health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef]
- Maestroni, L.; Read, P.; Bishop, C.; Papadopoulos, K.; Suchomel, T.J.; Comfort, P.; Turner, A. The Benefits of Strength Training on Musculoskeletal System Health: Practical Applications for Interdisciplinary Care. Sports Med. 2020, 50, 1431–1450. [Google Scholar] [CrossRef]
- Rastad, C.; Martin, C.; Åsenlöf, P. Barriers, Benefits, and Strategies for Physical Activity in Patients with Schizophrenia. Phys. Ther. 2014, 94, 1467–1479. [Google Scholar] [CrossRef]
- Gross, J.; Vancampfort, D.; Stubbs, B.; Gorczynski, P.; Soundy, A. A narrative synthesis investigating the use and value of social support to promote physical activity among individuals with schizophrenia. Disabil. Rehabil. 2016, 38, 123–150. [Google Scholar] [CrossRef]
- Arnautovska, U.; Kesby, J.P.; Korman, N.; Rebar, A.L.; Chapman, J.; Warren, N.; Rossell, S.L.; Dark, F.L.; Siskind, D. Biopsychology of Physical Activity in People with Schizophrenia: An Integrative Perspective on Barriers and Intervention Strategies. Neuropsychiatr. Dis. Treat. 2022, 18, 2917–2926. [Google Scholar] [CrossRef]
- Wang, P.-W.; Lin, H.-C.; Su, C.-Y.; Chen, M.-D.; Lin, K.C.; Ko, C.-H.; Yen, C.-F. Effect of Aerobic Exercise on Improving Symptoms of Individuals with Schizophrenia: A Single Blinded Randomized Control Study. Front. Psychiatry 2018, 9, 167. [Google Scholar] [CrossRef]
- Curcic, D.; Lazarevic, B.C.; Stojmenovic, T.; Djukic-Dejanovic, S.; Dikic, N.; Vesic-Vukasinovic, M.; Radivojevic, N.; Andjelkovic, M.; Borovcanin, M.; Djokic, G. Positive impact of prescribed physical activity on symptoms of schizophrenia: Randomized clinical trial. Psychiatr. Danub. 2017, 29, 459–465. [Google Scholar] [CrossRef]
- Loh, S.Y.; Abdullah, A.; Abu Bakar, A.K.; Thambu, M.; Jaafar, N.R.N. Structured Walking and Chronic Institutionalized Schizophrenia Inmates: A pilot RCT Study on Quality of Life. Glob. J. Health Sci. 2015, 8, p238–p248. [Google Scholar] [CrossRef]
- Pelham, T.W.; Campagna, P.D.; Ritvo, P.G.; Birnie, W.A. The effects of exercise therapy on clients in a psychiatric rehabilitation program. Psychosoc. Rehabil. J. 1993, 16, 75–84. [Google Scholar] [CrossRef]
- Ho, R.T.; Fong, T.C.; Wan, A.H.; Au-Yeung, F.S.; Wong, C.P.; Ng, W.Y.; Cheung, I.K.; Lo, P.H.; Ng, S.; Chan, C.L.; et al. A randomized controlled trial on the psychophysiological effects of physical exercise and Tai-chi in patients with chronic schizophrenia. Schizophr. Res. 2016, 171, 42–49. [Google Scholar] [CrossRef]
- Schmitt, A.; Maurus, I.; Rossner, M.J.; Röh, A.; Lembeck, M.; von Wilmsdorff, M.; Takahashi, S.; Rauchmann, B.; Keeser, D.; Hasan, A.; et al. Effects of Aerobic Exercise on Metabolic Syndrome, Cardiorespiratory Fitness, and Symptoms in Schizophrenia Include Decreased Mortality. Front. Psychiatry 2018, 9, 690. [Google Scholar] [CrossRef]
- Ashdown-Franks, G.; Firth, J.; Carney, R.; Carvalho, A.F.; Hallgren, M.; Koyanagi, A.; Rosenbaum, S.; Schuch, F.B.; Smith, L.; Solmi, M.; et al. Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sports Med. 2020, 50, 151–170. [Google Scholar] [CrossRef]
- Gallardo-Gómez, D.; del Pozo-Cruz, J.; Noetel, M.; Álvarez-Barbosa, F.; Alfonso-Rosa, R.M.; Cruz, B.d.P. Optimal dose and type of exercise to improve cognitive function in older adults: A systematic review and bayesian model-based network meta-analysis of RCTs. Ageing Res. Rev. 2022, 76, 101591. [Google Scholar] [CrossRef]
- Papadea, D.; Dalla, C.; Tata, D.A. Exploring a Possible Interplay between Schizophrenia, Oxytocin, and Estrogens: A Narrative Review. Brain Sci. 2023, 13, 461. [Google Scholar] [CrossRef]
- Bhatia, T.; Mazumdar, S.; Wood, J.; He, F.; Gur, R.E.; Gur, R.C.; Nimgaonkar, V.L.; Deshpande, S.N. A randomised controlled trial of adjunctive yoga and adjunctive physical exercise training for cognitive dysfunction in schizophrenia. Acta Neuropsychiatr. 2016, 29, 102–114. [Google Scholar] [CrossRef]
- Nasution, N.M.; Effendy, E.; Amin, M.M.; Siregar, I.R. Effect of Aerobic Exercise in Positive and Negative Symptoms in Schizophrenia. Open Access Maced. J. Med. Sci. 2021, 9, 178–181. [Google Scholar] [CrossRef]
- Malchow, B.; Keeser, D.; Keller, K.; Hasan, A.; Rauchmann, B.-S.; Kimura, H.; Schneider-Axmann, T.; Dechent, P.; Gruber, O.; Ertl-Wagner, B.; et al. Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr. Res. 2016, 173, 182–191. [Google Scholar] [CrossRef]
- Chen, M.-D.; Kuo, Y.-H.; Chang, Y.-C.; Hsu, S.-T.; Kuo, C.-C.; Chang, J.-J. Influences of Aerobic Dance on Cognitive Performance in Adults with Schizophrenia. Occup. Ther. Int. 2016, 23, 346–356. [Google Scholar] [CrossRef]
- Sisman, F.N.; Büber, B.; Taş, F.; Turan, H. Randomized Controlled Trial for the Effects of an Exercise Program for Functional Remission and Weight Control in Schizophrenia: A Community Mental Health Study. Issues Ment. Health Nurs. 2022, 43, 603–612. [Google Scholar] [CrossRef]
- Le, T.P.; Ventura, J.; Ruiz-Yu, B.; McEwen, S.C.; Subotnik, K.L.; Nuechterlein, K.H. Treatment engagement in first-episode schizophrenia: Associations between intrinsic motivation and attendance during cognitive training and an aerobic exercise program. Schizophr. Res. 2023, 251, 59–65. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; McEwen, S.C.; Ventura, J.; Subotnik, K.L.; Turner, L.R.; Boucher, M.; Casaus, L.R.; Distler, M.G.; Hayata, J.N. Aerobic exercise enhances cognitive training effects in first-episode schizophrenia: Randomized clinical trial demonstrates cognitive and functional gains. Psychol. Med. 2022, 53, 4751–4761. [Google Scholar] [CrossRef]
- Ventura, J.; McEwen, S.; Subotnik, K.L.; Hellemann, G.S.; Ghadiali, M.; Rahimdel, A.; Seo, M.J.; Irwin, M.R.; Nuechterlein, K.H. Changes in inflammation are related to depression and amount of aerobic exercise in first episode schizophrenia. Early Interv. Psychiatry 2021, 15, 213–216. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Ventura, J.; McEwen, S.C.; Gretchen-Doorly, D.; Vinogradov, S.; Subotnik, K.L. Enhancing Cognitive Training Through Aerobic Exercise After a First Schizophrenia Episode: Theoretical Conception and Pilot Study. Schizophr. Bull. 2016, 42 (Suppl. S1), S44–S52. [Google Scholar] [CrossRef]
- Bang-Kittilsen, G.; Egeland, J.; Holmen, T.L.; Bigseth, T.T.; Andersen, E.; Mordal, J.; Ulleberg, P.; Engh, J.A. High-intensity interval training and active video gaming improve neurocognition in schizophrenia: A randomized controlled trial. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 339–353. [Google Scholar] [CrossRef]
- Cristiano, V.B.; Szortyka, M.F.; Belmonte-De-Abreu, P. A controlled open clinical trial of the positive effect of a physical intervention on quality of life in schizophrenia. Front. Psychiatry 2023, 14, 1066541. [Google Scholar] [CrossRef]
- Arietaleanizbeaskoa, M.S.; on behalf of the EfiKroniK group; Sancho, A.; Olazabal, I.; Moreno, C.; Gil, E.; Garcia-Alvarez, A.; Mendizabal, N.; de la Fuente, I.; Dominguez, S.; et al. Effectiveness of physical exercise for people with chronic diseases: The EFIKRONIK study protocol for a hybrid, clinical and implementation randomized trial. BMC Fam. Pract. 2020, 21, 227. [Google Scholar] [CrossRef]
- Nyboe, L.; Moeller, M.K.; Vestergaard, C.H.; Lund, H.; Videbech, P. Physical activity and anomalous bodily experiences in patients with first-episode schizophrenia. Nord. J. Psychiatry 2016, 70, 514–520. [Google Scholar] [CrossRef]
- Shimada, T.; Ito, S.; Makabe, A.; Yamanushi, A.; Takenaka, A.; Kobayashi, M. Aerobic exercise and cognitive functioning in schizophrenia: A pilot randomized controlled trial. Psychiatry Res. 2019, 282, 112638. [Google Scholar] [CrossRef]
- Shimada, T.; Ito, S.; Makabe, A.; Yamanushi, A.; Takenaka, A.; Kawano, K.; Kobayashi, M. Aerobic exercise and cognitive functioning in schizophrenia: Results of a 1-year follow-up from a randomized controlled trial. Psychiatry Res. 2020, 286, 112854. [Google Scholar] [CrossRef]
- Khonsari, N.M.; Badrfam, R.; Mohammdi, M.R.; Rastad, H.; Etemadi, F.; Vafaei, Z.; Zandifar, A. Effect of Aerobic Exercise as Adjunct Therapy on the Improvement of Negative Symptoms and Cognitive Impairment in Patients with Schizophrenia: A Randomized, Case-Control Clinical Trial. J. Psychosoc. Nurs. Ment. Health Serv. 2022, 60, 38–43. [Google Scholar] [CrossRef]
- Şenormancı, G.; Korkmaz, N.; Şenormancı, Ö.; Uğur, S.; Topsaç, M.; Gültekin, O. Effects of Exercise on Resilience, Insight and Functionality in Patients with Chronic Schizophrenia in a Psychiatric Nursing Home Setting: A Randomized Controlled Trial. Issues Ment. Health Nurs. 2021, 42, 690–698. [Google Scholar] [CrossRef]
- Heggelund, J.; Nilsberg, G.E.; Hoff, J.; Morken, G.; Helgerud, J. Effects of high aerobic intensity training in patients with schizophrenia—A controlled trial. Nord. J. Psychiatry 2011, 65, 269–275. [Google Scholar] [CrossRef]
- Maggouritsa, G.; Kokaridas, D.; Theodorakis, I.; Patsiaouras, A.; Mouzas, O.; Dimitrakopoulos, S.; Diggelidis, N. The effect of a physical activity programme on improving mood profile of patients with schizophrenia. Int. J. Sport Exerc. Psychol. 2014, 12, 273–284. [Google Scholar] [CrossRef]
- Areshtanab, H.N.; Ebrahimi, H.; Abdi, M.; Mohammadian, R.; Asl, A.M.; Piri, S. The Effect of Aerobic Exercise on the Quality of Life of Male Patients Who Suffer from Chronic Schizophrenia: Double-Blind, Randomized Control Trial. Iran. J. Psychiatry Behav. Sci. 2020, 14, 529–535. [Google Scholar] [CrossRef]
- Areshtanab, H.; Ebrahimi, H.; Farnam, A.; Mohammadpoorasl, A.; Jamali, B.; Piri, S. The effect of regular aerobic exercise on both positive and negative symptoms of male patients with chronic Schizophrenia: A double blinded study. Int. J. Med. Res. Health Sci. 2016, 5, 529–535. [Google Scholar]
- Ho, R.T.H.; Wan, A.H.Y.; Au-Yeung, F.S.W.; Lo, P.H.Y.; Siu, P.J.C.Y.; Wong, C.P.K.; Ng, W.Y.H.; Cheung, I.K.M.; Ng, S.M.; Chan, C.L.W.; et al. The psychophysiological effects of Tai-chi and exercise in residential schizophrenic patients: A 3-arm randomized controlled trial. BMC Complement. Altern. Med. 2014, 14, 364. [Google Scholar] [CrossRef]
- Mullor, D.; Gallego, J.; Cangas, A.; Aguilar-Parra, J.; Trigueros, R.; Lopez-Pardo, A. Psychological and social impact of an inclusive sports program among students and people with severe mental disorder. Rev. Psicol. Deporte 2020, 29, 8–15. [Google Scholar]
- Hasson-Ohayon, I.; Kravetz, S.; Roe, D.; Rozencwaig, S.; Weiser, M. Qualitative assessment of verbal and non-verbal psychosocial interventions for people with severe mental illness. J. Ment. Health 2006, 15, 343–353. [Google Scholar] [CrossRef]
| PICOS Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Participants >18 years and diagnosed with schizophrenia in a non-residential environment. | While including participants with schizophrenia, did not perform a differentiated sub-group analysis. |
| Intervention | Studies whose exercise protocol had specified, at least, the first five parameters to configure the exercise dose (i.e., F.I.T.T.−V.P.): frequency, intensity, type, time (duration) and volume. Furthermore, the training period must last, at least, three weeks. | Exercise interventions whose protocols were unstructured or did not report, at least, the first five parameters of F.I.T.T.−V.P. previously mentioned. |
| Comparator | One control group (i.e., not exposed to a regular physical exercise program). | No control group or a control group with an active intervention. |
| Outcomes | Data evaluating adaptations of regular exercise interventions on psychopathology, QoL or cognition in patients diagnosed with schizophrenia. | No reported measures of psychopathology, QoL or cognition in patients diagnosed with schizophrenia. |
| Study design | Experimental studies with randomized participants. | Non-experimental and/or non-randomized studies. |
| CERT Item | TS (n = 13) n (%) | ET (n = 9) n (%) | CT (n = 4) n (%) | RT † (n = 1) n (%) |
|---|---|---|---|---|
| Item 1. What (materials) | 9 (69.2%) | 6 (66.66%) | 3 (75%) | 1 (100%) |
| Item 2. Who (provider) | 3 (23%) | 3 (33.33%) | 0 (0%) | 0 (0%) |
| Item 3. Individually or in a group | 6 (46.2%) | 5 (55.55%) | 1 (25%) | 0 (0%) |
| Item 4. Supervised or unsupervised | 6 (46.2%) | 5 (55.55%) | 1 (25%) | 0 (0%) |
| Item 5. Adherence report | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Item 6. Motivation strategies | 1 (7.7%) | 1 (11.11%) | 0 (0%) | 0 (0%) |
| Item 7a. Exercise progression | 6 (46.2%) | 3 (33.33%) | 3 (75%) | 0 (0%) |
| Item 7b. Program progression | 7 (53.8%) | 4 (44.44%) | 3 (75%) | 1 (100%) |
| Item 8. Exercise replication | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Item 9. Home components | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Item 10. Non-exercise components | 4 (30.8%) | 4 (44.44%) | 0 (0%) | 0 (0%) |
| Item 11. Adverse events report | 2 (15.4%) | 1 (11.11%) | 1 (25%) | 0 (0%) |
| Item 12. Setting | 4 (30.8%) | 3 (33.33%) | 1 (25%) | 0 (0%) |
| Item 13. Description of the exercise | 13 (100%) | 9 (100%) | 4 (100%) | 1 (100%) |
| Item 14a. Exercises generic or tailored? | 10 (76.9%) | 6 (66.66%) | 4 (100%) | 1 (100%) |
| Item 14b. Description of the adaptation made in the exercises | 9 (69.2%) | 6 (66.66%) | 3 (75%) | 1 (100%) |
| Item 15. Rules for starting level | 2 (15.4%) | 2 (22.22%) | 0 (0%) | 0 (0%) |
| Item 16a. How adherence to exercise was measured | 5 (38.5%) | 5 (55.55%) | 0 (0%) | 0 (0%) |
| Item 16b. Is the Intervention carried out according to how it was planned? | 10 (76.9%) | 7 (77.77%) | 3 (75%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Barrios, L.; Carballeira, E.; Varela-Sanz, A.; Iglesias-Soler, E.; Dopico-Calvo, X. The Impact of Regular Physical Exercise on Psychopathology, Cognition, and Quality of Life in Patients Diagnosed with Schizophrenia: A Scoping Review. Behav. Sci. 2023, 13, 959. https://doi.org/10.3390/bs13120959
Vila-Barrios L, Carballeira E, Varela-Sanz A, Iglesias-Soler E, Dopico-Calvo X. The Impact of Regular Physical Exercise on Psychopathology, Cognition, and Quality of Life in Patients Diagnosed with Schizophrenia: A Scoping Review. Behavioral Sciences. 2023; 13(12):959. https://doi.org/10.3390/bs13120959
Chicago/Turabian StyleVila-Barrios, Lucía, Eduardo Carballeira, Adrián Varela-Sanz, Eliseo Iglesias-Soler, and Xurxo Dopico-Calvo. 2023. "The Impact of Regular Physical Exercise on Psychopathology, Cognition, and Quality of Life in Patients Diagnosed with Schizophrenia: A Scoping Review" Behavioral Sciences 13, no. 12: 959. https://doi.org/10.3390/bs13120959
APA StyleVila-Barrios, L., Carballeira, E., Varela-Sanz, A., Iglesias-Soler, E., & Dopico-Calvo, X. (2023). The Impact of Regular Physical Exercise on Psychopathology, Cognition, and Quality of Life in Patients Diagnosed with Schizophrenia: A Scoping Review. Behavioral Sciences, 13(12), 959. https://doi.org/10.3390/bs13120959









