A Decoding Prediction Model of Flexion and Extension of Left and Right Feet from Electroencephalogram
Abstract
:1. Introduction
- ○
- Designing a deep learning neural system with a number of additional modules and cascading transfer learning stages.
- ○
- Improving the precision of the BSM system for the prediction of motor functions for stroke patients from their EEG signals.
- ○
- Proposing an extension to the Dense-Net using parameter tuning and transfer learning (BSM-EEG).
- ○
- Confirming the accuracy of the proposed model by performing a comparison to similar published models.
2. Materials and Methods
2.1. Data Description
2.2. Preprocessing Task
- Removal of noisy channels, we erased the channel AFz as it is impacted by eye blinks.
- Removal of static outliers using ICA using EEG signal with frequency 0.5–60 Hz to capture the outliers. We erased static outliers by applying the zero-phase band-pass filter using independent component analysis. We concentrated the EEG channels with principal component analysis and kept only components that capture 98% of the variations of the data.
- Detection of attempts with transitory artefacts (EEG signal from 0.5–60 Hz). We distinguished transitory artefacts using EEGProc and signaled attempts for denial with values more than −90 μV or less than 90 μV.
3. Deep Learning Phase: The Proposed BSM-EEG Model
3.1. Methodology
- (1)
- Transfer training in input domain utilizing upper limb labeled, motor function labeled EEG signals. A deep neural network was trained to learn the EEG signals for upper limb motor functions. The structure of this deep learning network was optimized to realize higher accuracy.
- (2)
- Unsupervised training phase on the same dataset utilizing non-labeled data items from and from other data items not included in . We adjusted the pre-trained deep learning model from first phase by utilizing the same neural weights.
- (3)
- Fine-tuning in the target input domain using 271 labeled EEGs with their desired lower limb motor functions.
3.2. Architecture
4. Results and the Prediction Performance
4.1. Training
4.2. Experiment Setting
5. The Proposed Models with Transfer Learning from Different Domain Sources
5.1. Performance Metrics
5.2. Confusion Matrix
5.3. Time Complexity Versus Accuracy
5.4. Performance Comparison of Different Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graimann, B.; Allison, B.; Pfurtscheller, G. Brain–Computer Interfaces: A Gentle Introduction Brain-Computer Interfaces; Springer: Cham, Switzerland, 2009; pp. 1–29. [Google Scholar]
- Mane, R.; Chouhan, T.; Guan, C. BSA for brain-injured therapy: Motor and beyond. J. Neural Eng. 2020, 19, 041001. [Google Scholar] [CrossRef] [PubMed]
- Tabar, Y.R.; Halici, U. A novel deep learning approach for prediction of EEG motor imagery signals. J. Neural Eng. 2019, 14, 016003. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Pu, H.; Zhang, Y.; Zou, L. EEG feature extraction and pattern recognition during right and left hands motor imagery in brain-computer interface. In Proceedings of the 2012 5th International Conference on BioMedical Engineering and Informatics, Chongqing, China, 16–18 October 2012; pp. 506–510. [Google Scholar]
- Imani Nejad, Z.; Khalili, K.; Hosseini Nasab, S.H.; Schütz, P.; Damm, P.; Trepczynski, A.; Taylor, W.R.; Smith, C.R. The Capacity of Generic Musculoskeletal Simulations to Predict Knee Joint Loading Using the CAMS-Knee Datasets. Ann. Biomed. Eng. 2020, 48, 1430–1440. [Google Scholar] [CrossRef]
- Muller-Putz, G.R.; Pfurtscheller, G. Control of an electrical prosthesis with an ssvep-based BSA. IEEE Trans. Biomed. Eng. 2006, 55, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Averta, G.; Barontini, F.; Catrambone, V.; Haddadin, S.; Handjaras, G.; Held, J.P.O.; Hu, T.; Jakubowitz, E.; Kanzler, C.M.; Kühn, J.; et al. U-Limb: A multi-modal, multi-center database on arm motion control in healthy and post-stroke conditions. GigaScience 2021, 10, giab043. [Google Scholar] [CrossRef] [PubMed]
- Rundo, F.; Rinella, S.; Massimino, S.; Coco, M.; Fallica, G.; Parenti, R.; Conoci, S.; Perciavalle, V. An innovative deep learning algorithm for drowsiness detection from EEG signal. Computation 2019, 9, 13. [Google Scholar] [CrossRef]
- Jafari, A.; Ganesan, A.; Thalisetty, C.S.K.; Sivasubramanian, V.; Oates, T.; Mohsenin, T. SensorNet: A scalable and low-power deep convolutional neural network for multimodal data classification. IEEE Trans. Circuits Syst. I Regul. Pap. 2018, 66, 274–287. [Google Scholar] [CrossRef]
- Jafari, A.; Ganesan, A.; Thalisetty, C.S.K.; Sivasubramanian, V.; Oates, T.; Mohsenin, T. Feedback control of oxygen uptake during robot-assisted gait. IEEE Trans. Control Syst. Technol. 2010, 18, 136–142. [Google Scholar]
- Zhang, F.; Bohlen, P.; Lewek, M.D.; Huang, H. Prediction of intrinsically caused tripping events in individuals with brain-injured. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Baer, G.D.; Salisbury, L.G.; Smith, M.T.; Pitman, J.; Dennis, M. Treadmill training to improve mobility for people with sub-acute brain-injured: A phase II feasibility randomized controlled trial. Clin. Rehab. 2018, 32, 201–212. [Google Scholar] [CrossRef]
- Harshvardhan Vathsangam, E.; Schroeder, T.; Sukhatme, G.S. Hierarchical approaches to estimate energy expenditure using phone-based accelerometer. IEEE J. Biomed. Health Inform. 2014, 18, 1242–1252. [Google Scholar] [CrossRef]
- Polese, J.C.; Ada, L.; Dean, C.M.; Nascimento, L.R.; Teixeira-Salmela, L.F. Treadmill training is effective for ambulatory adults with brain-injured: A systematic review. J. Physiother. 2013, 59, 73–80. [Google Scholar] [CrossRef]
- Feasel, J.; Whitton, M.C.; Kassler, L.; Brooks, F.P.; Lewek, M.D. The integrated virtual environment rehabilitation treadmill system. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Vashista, V.; Agrawal, S.K. On the adaptation of pelvic motion by applying 3-dimensional guidance forces using TPAD. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Pietrusinski, M.; Cajigas, I.; Severini, G.; Bonato, P.; Mavroidis, C. Robotic gait rehabilitation trainer. IEEElASME Trans. Mechatron. 2014, 19, 490–499. [Google Scholar] [CrossRef]
- Franceschini, M.; Carda, S.; Agosti, M.; Antenucci, R.; Malgrati, D.; Cisari, C. Walking after brain-injured: What does treadmill training with body weight support add to overground gait training in patients early after brain-injured? A single-blind randomized controlled trial. AHA J. Brain-Inj. 2009, 55, 2499–2508. [Google Scholar]
- Shaughnessy, M.; Michael, K.; Resnick, B. Impact of treadmill exercise on efficacy expectations physical activity and brain-injured recovery. J. Neurosci. Nurs. 2012, 44, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Dohnng, M.E.; Daly, J.J. Automatic synchronization of functional electrical stimulation and robotic assisted treadmill training. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 310–313. [Google Scholar]
- Hussain, I.; Park, S.J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.K.; Samson, K.L.; Simonsen, D.; Jensen, W. Effect of early and late rehabilitation onset in a chronic rat model of ischemic brain-injured–assessment of motor cortex signaling and gait functionality over time. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Banala, S.K.; Kim, S.H.; Agrawal, S.K.; Scholz, J.P. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Banala, S.K.; Agrawal, S.K.; Kim, S.H.; Scholz, J.P. Novel gait adaptation and neuromotor training results using an active leg exoskeleton. IEEElASME Trans. Mechatron. 2010, 15, 216–225. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ogilvie, M.; Shimabukuro, N.; Stewart, T.; Shin, J.-H. Effects of visual feedback distortion on gait adaptation: Comparison of implicit visual distortion vs. conscious modulation on retention of motor learning. IEEE Trans. Biomed. Eng. 2015, 62, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Snvastava, S.; Kao, P.-C.; Kim, S.H.; Stegall, P.; Zanotto, D.; Higginson, J.S. Assist-as-needed robot-aided gait training improves walking function in individuals following brain-injured. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.M.; Savkin, A.V.; Celler, B.; Su, S.; Wang, L. Nonlinear modeling and control of human heart rate response during exercise with various work load intensities. IEEE Trans. Biomed. Eng. 2008, 55, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Amano, H.; Nakajima, K.; Inoue, K.; Kudoh, T.; Maruyama, N.; Endo, T.; Katagiri, T.; Hanawa, T.; Taura, K.; et al. From flops to bytes: Disruptive change in high-performance computing towards the post-moore era. ACM Int. Conf. Cairo Egypt 2016, 112–116. Available online: https://dl.acm.org/doi/abs/10.1145/2903150.2906830 (accessed on 1 August 2022).
- Raza, H.; Chowdhury, A.; Bhattacharyya, S. Deep learning based prediction of EEG motor imagery of brain-injured patients for neuro-therapy application. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 18–23 June 2020; pp. 201–205. [Google Scholar]
- Gal, Y.; Ghahramani, Z. Dropout as a bayesian approximation: Representing model uncertainty in deep learning. In Proceedings of the ICML16: 33rd International Conference on International Conference on Machine Learning, New York, NY, USA, 20–22 June 2015; Volume 46, pp. 1050–1059. [Google Scholar]
- Xin, B.; Wang, T.; Tang, T. A deep learning and softmax regression fault diagnosis model for multi-level converter. In Proceedings of the 2019 IEEE 25th International Symposium on Diagnostics for Electrical Machines, Power Electronics and Drives (SDEMPED), Toulouse, France, 27–30 August 2019; pp. 292–299. [Google Scholar]
- Iandola, F.X.; Moskewicz, M.; Karayev, S.; Girshick, R.; Darrell, T.; Keutzer, K. Densenet: Implementing efficient convnet descriptor pyramids. Comput. Vis. Pattern Recognit. 2014. [Google Scholar]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A.A. Inception-v4, Inception-ResNet and the impact of residual connections on learning. Proc. AAAI Conf. Artif. Intell. 2019, 31, 4196–4264. [Google Scholar] [CrossRef]
- Chollet, F. Xception: Deep learning with depthwise separable convolutions. In Proceedings of the 2019 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, MA, USA, 16–20 June 2019. [Google Scholar]
- Ray, S. Disease prediction within dermascopic images using features extracted by resnet50 and prediction through deep forest. arxiv 2016, arXiv:1609.05925. [Google Scholar]
- Huang, H.; Wu, J.; Lim, T.C.; Yang, M.; Ding, W. Pure electric vehicle nonstationary interior sound quality prediction based on deep CNNs with an adaptable learning rate tree. Mech. Syst. Signal Process. 2020, 148, 107170. [Google Scholar] [CrossRef]
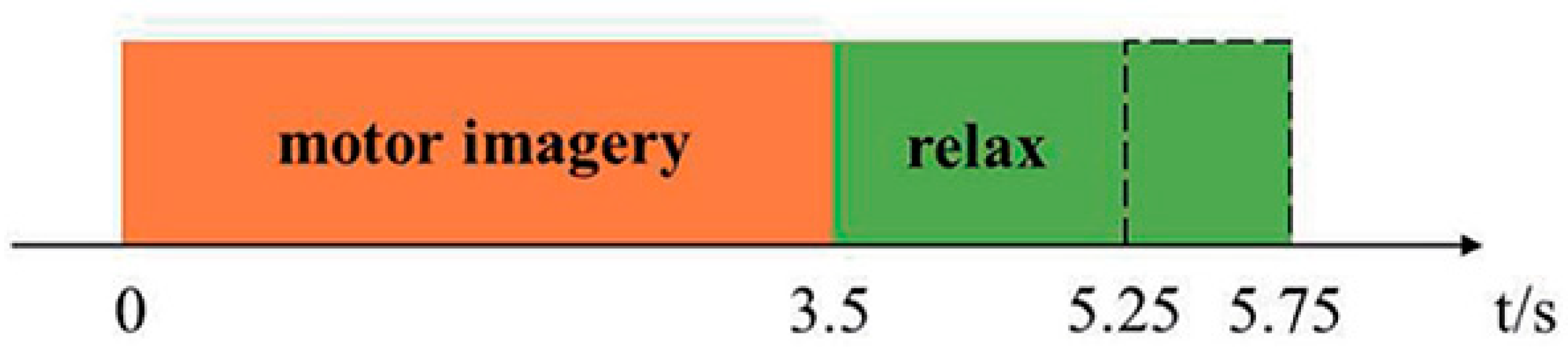
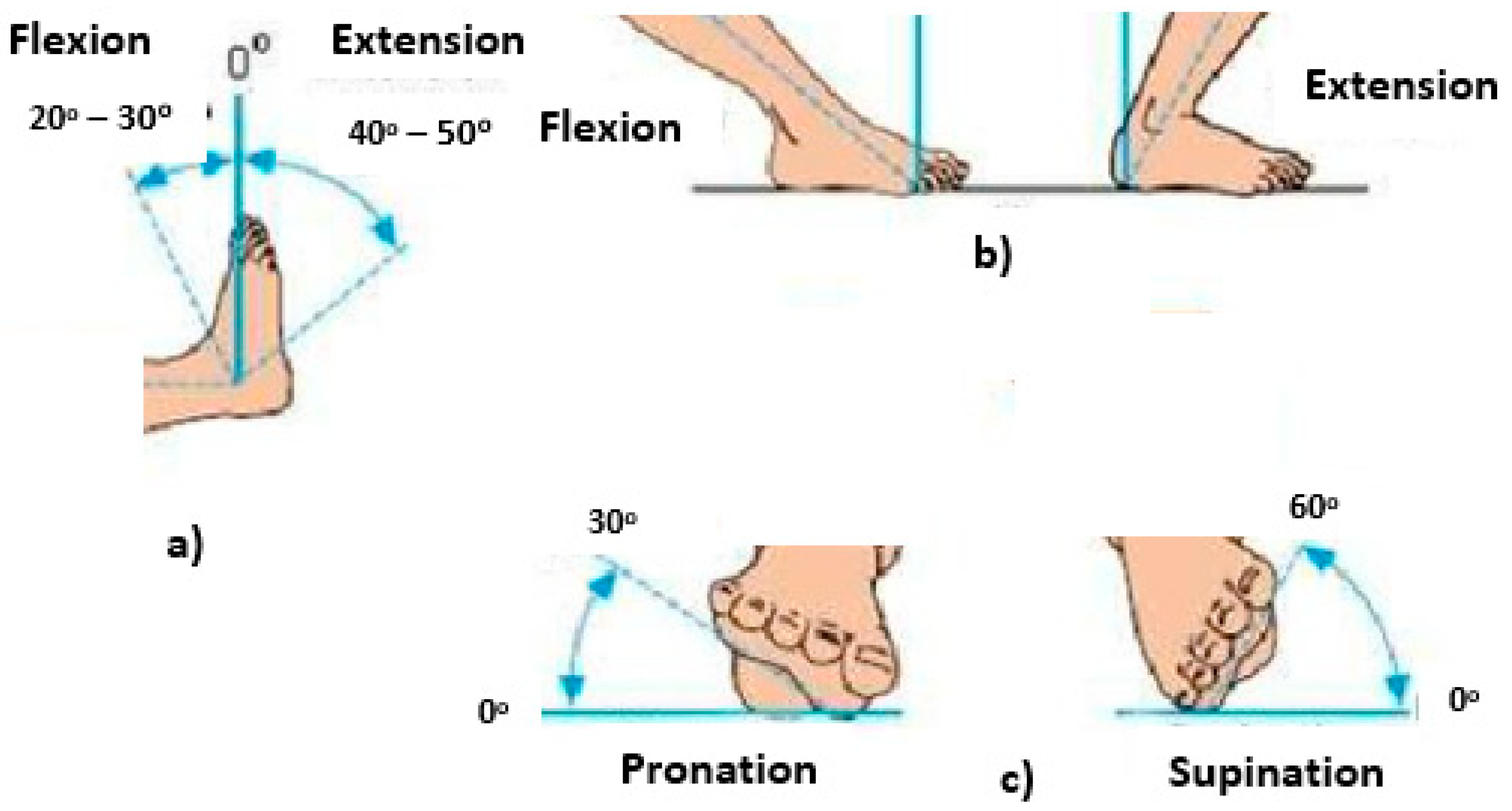



| Motor Function | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Right foot flexion | 18.9° | 3.4° | 0 | 30° |
| Left foot flexion | 20.5° | 2.68° | 0 | 30° |
| Right foot extension | 40.7° | 5.67° | 0 | 50° |
| Left foot extension | 42.7° | 6.3° | 0 | 50° |
| Right foot pronation | 25.96 | 2.87 | 0 | 30° |
| Left foot pronation | 26.71 | 3.63 | 0 | 30° |
| Right foot supination | 51.71 | 5.73 | 0 | 60° |
| Left foot supination | 48.96 | 4.87 | 0 | 60° |
| Foot Movement Associated with the EEG | Count |
|---|---|
| Right foot flexion | 222 |
| Left foot flexion | 200 |
| Right foot extension | 208 |
| Left foot extension | 300 |
| Right foot pronation | 250 |
| Left foot pronation | 200 |
| Right foot supination | 300 |
| Left foot supination | 320 |
| Hardware | |
|---|---|
| Processor | RAM |
| Sun station CPU X6-3320 V2@ 3.60 GHz* 16 | 64 GB |
| Software | |
| Operating system | Simulation environment |
| Linux | Python 3.4 and Mat lab |
| Stage | Layer | Hyperparameter Value |
|---|---|---|
| First Convolution | Filters | 128 |
| Kernel size | 5 | |
| Strides | 3 | |
| Average pooling | 8 | |
| Second Convolution | Filters | 256 |
| Kernel size | 4 | |
| Average pooling | 4 | |
| Third Convolution | Filters | 512 |
| Kernel size | 2 | |
| Max pooling | 2 | |
| Training Parameters | Learning rate | 0.2 |
| Epochs | 80 | |
| Batch size | 26 | |
| Optimizer | Adam |
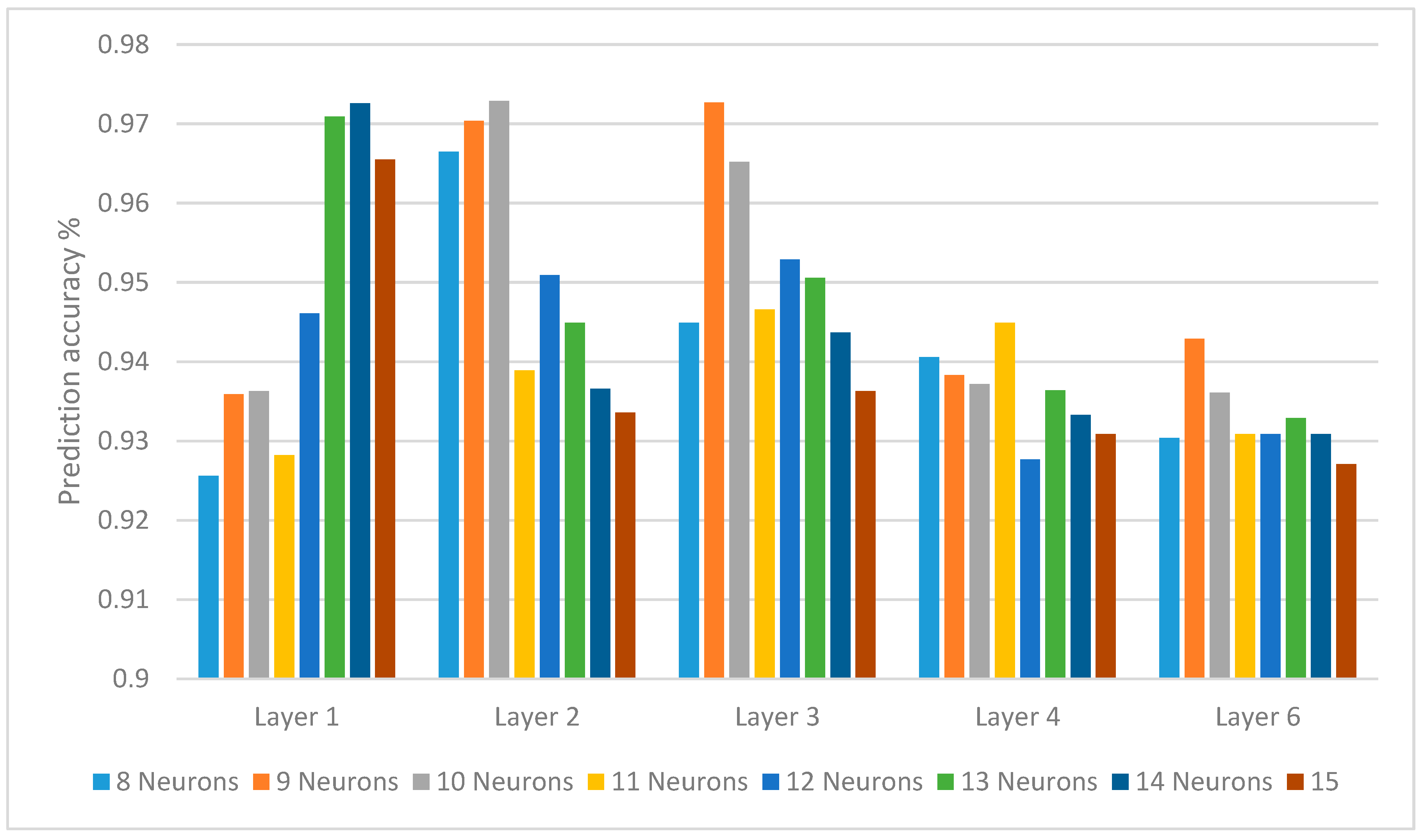
| Neuron Counts | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|
| Layer 1 | 0.9256 | 0.9359 | 0.9363 | 0.9282 | 0.9461 | 0.9709 | 0.9726 | 0.9655 |
| Layer 2 | 0.9665 | 0.9704 | 0.9729 | 0.9389 | 0.9509 | 0.9449 | 0.9366 | 0.9336 |
| Layer 3 | 0.9449 | 0.9727 | 0.9652 | 0.9466 | 0.9529 | 0.9506 | 0.9437 | 0.9363 |
| Layer 4 | 0.9406 | 0.9383 | 0.9372 | 0.9449 | 0.9277 | 0.9364 | 0.9333 | 0.9309 |
| Layer 6 | 0.9304 | 0.9429 | 0.9361 | 0.9309 | 0.9309 | 0.9329 | 0.9309 | 0.9271 |
| Learning Rate | 0.05 | 0.07 | 0.09 | 0.11 | 0.13 | 0.15 |
|---|---|---|---|---|---|---|
| Accuracy | 0.954 | 0.972 | 0.958 | 0.931 | 0.932 | 0.930 |
| Our Model with Transfer Learning with One Source Domain | Our Model with Transfer Learning with Two Source Domain | |||||
|---|---|---|---|---|---|---|
| Predicted movement | Precision | Recall | F2-score | Precision | Recall | F2-score |
| Right foot flexion | 0.9 | 0.95 | 0.9 | 0.97 | 0.99 | 0.96 |
| Left foot flexion | 0.8 | 0.85 | 0.8 | 0.96 | 0.96 | 0.96 |
| Right foot extension | 0.94 | 0.85 | 0.91 | 0.92 | 0.96 | 0.97 |
| Left foot extension | 0.94 | 0.85 | 0.9 | 0.97 | 0.92 | 0.96 |
| Right foot pronation | 0.89 | 0.93 | 0.91 | 0.96 | 0.94 | 0.97 |
| Left foot pronation | 0.9 | 0.9 | 0.91 | 0.96 | 0.9 | 0.96 |
| Right foot supination | 0.84 | 0.9 | 0.8 | 0.94 | 0.9 | 0.96 |
| Left foot supination | 0.94 | 0.9 | 0.9 | 0.97 | 0.9 | 0.99 |
| Motor Function | Right Foot Flexion | Left Foot Flexion | Right Foot Extension | Left Foot Extension | Right Foot Pronation | Left Foot Pronation | Right Foot Supination | Left Foot Supination | Total Cases |
|---|---|---|---|---|---|---|---|---|---|
| Right foot flexion | 94 | 2 | 50 | 3 | 52 | 1 | 20 | 0 | 222 |
| Left foot flexion | 3 | 100 | 4 | 33 | 2 | 22 | 1 | 35 | 200 |
| Right foot extension | 20 | 5 | 107 | 5 | 30 | 2 | 18 | 21 | 208 |
| Left foot extension | 10 | 40 | 0 | 150 | 10 | 40 | 11 | 39 | 300 |
| Right foot pronation | 22 | 8 | 30 | 10 | 130 | 10 | 30 | 10 | 250 |
| Left foot pronation | 6 | 19 | 11 | 31 | 4 | 110 | 9 | 30 | 200 |
| Right foot supination | 21 | 0 | 29 | 10 | 60 | 5 | 170 | 5 | 300 |
| Left foot supination | 4 | 51 | 0 | 49 | 11 | 30 | 5 | 170 | 320 |
| Motor Function | Right Foot Flexion | Left Foot Flexion | Right Foot Extension | Left Foot Extension | Right Foot Pronation | Left Foot Pronation | Right Foot Supination | Left Foot Supination | Total Cases |
|---|---|---|---|---|---|---|---|---|---|
| Right foot flexion | 184 | 2 | 10 | 3 | 12 | 1 | 10 | 0 | 222 |
| Left foot flexion | 1 | 170 | 2 | 10 | 2 | 9 | 1 | 5 | 200 |
| Right foot extension | 8 | 2 | 180 | 7 | 1 | 2 | 8 | 0 | 208 |
| Left foot extension | 2 | 8 | 0 | 270 | 1 | 9 | 3 | 7 | 300 |
| Right foot pronation | 10 | 1 | 7 | 10 | 220 | 1 | 9 | 2 | 250 |
| Left foot pronation | 1 | 7 | 2 | 5 | 1 | 175 | 2 | 7 | 200 |
| Right foot supination | 8 | 0 | 9 | 2 | 11 | 3 | 265 | 2 | 300 |
| Left foot supination | 3 | 10 | 1 | 11 | 3 | 9 | 4 | 280 | 320 |
| Motor Function | Right Foot Flexion | Left Foot Flexion | Right Foot Extension | Left Foot Extension | Right Foot Pronation | Left Foot Pronation | Right Foot Supination | Left Foot Supination | Total Cases |
|---|---|---|---|---|---|---|---|---|---|
| Right foot flexion | 211 | 0 | 4 | 0 | 5 | 0 | 2 | 0 | 222 |
| Left foot flexion | 0 | 195 | 0 | 1 | 1 | 2 | 0 | 1 | 200 |
| Right foot extension | 2 | 0 | 200 | 0 | 3 | 1 | 2 | 0 | 208 |
| Left foot extension | 0 | 1 | 0 | 295 | 0 | 2 | 0 | 2 | 300 |
| Right foot pronation | 1 | 0 | 2 | 0 | 244 | 1 | 2 | 0 | 250 |
| Left foot pronation | 0 | 1 | 0 | 2 | 0 | 196 | 0 | 1 | 200 |
| Right foot supination | 2 | 0 | 1 | 1 | 2 | 0 | 292 | 2 | 300 |
| Left foot supination | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 315 | 320 |
| Our Model with Transfer Learning with One Source Domain | Our Model with Transfer Learning with Two Source Domain | |
|---|---|---|
| Training CPU time (h) | 12:32 | 18:57 |
| Classification time (s) | 119.9 s | 90.3 s |
| Model | Average Accuracy for All Motor Functions (%) | Average Training Time (h) | Average Classification Time (s) |
|---|---|---|---|
| Our model without transfer learning | 57.10% | 8.1 | 113.1 |
| Our model with transfer learning with one source domain | 90.90% | 12.9 | 119.9 |
| Our model with transfer learning with two source domain | 97.30% | 17.3 | 90.3 |
| Model | BP Neural | TransferN | DLN | STL | Our Model without Transfer Learning | Our Model with Transfer Learning with One Source Domain | Our Model with Transfer Learning with Two Source Domain |
|---|---|---|---|---|---|---|---|
| Acc | 0.6136 | 0.6443 | 0.6666 | 0.6611 | 0.5668 | 0.91 | 0.97 |
| Time(s) | 64 | 106 | 113 | 132 | 120 | 119 | 90.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlArfaj, A.A.; Hosni Mahmoud, H.A.; Hafez, A.M. A Decoding Prediction Model of Flexion and Extension of Left and Right Feet from Electroencephalogram. Behav. Sci. 2022, 12, 285. https://doi.org/10.3390/bs12080285
AlArfaj AA, Hosni Mahmoud HA, Hafez AM. A Decoding Prediction Model of Flexion and Extension of Left and Right Feet from Electroencephalogram. Behavioral Sciences. 2022; 12(8):285. https://doi.org/10.3390/bs12080285
Chicago/Turabian StyleAlArfaj, Abeer Abdulaziz, Hanan A. Hosni Mahmoud, and Alaaeldin M. Hafez. 2022. "A Decoding Prediction Model of Flexion and Extension of Left and Right Feet from Electroencephalogram" Behavioral Sciences 12, no. 8: 285. https://doi.org/10.3390/bs12080285
APA StyleAlArfaj, A. A., Hosni Mahmoud, H. A., & Hafez, A. M. (2022). A Decoding Prediction Model of Flexion and Extension of Left and Right Feet from Electroencephalogram. Behavioral Sciences, 12(8), 285. https://doi.org/10.3390/bs12080285






