Abstract
Agastache rugosa (Korean mint) is an important medicinal and aromatic plant and its aerial parts have a pleasant fragrance. A. rugosa leaves are used as an ingredient in salads and soups for enhancing the aroma and taste of foods in Korea. However, there is no report on the influence of the aroma of A. rugosa on human psychophysiological activity. Therefore, the present study aimed to investigate the effect of exposure to the essential oil of Korean A. rugosa on human electroencephalographic (EEG) activity. The essential oil of A. rugosa was isolated using steam distillation extraction and its composition was determined by gas chromatography and mass spectrometry (GC–MS) analysis. In the EEG study, 38 healthy volunteers (19 men and 19 women) participated. The EEG readings were analyzed for 25 EEG indices from 29 electrodes placed on the scalp according to the international 10–20 system. The major component in the essential oil of A. rugosa was estragole (89.49%) followed by D-limonene (3.40%), menthone (1.80%), and pulegone (1.86%). In the EEG study, significant decreases in absolute theta (AT) and relative theta (RT) power spectra were observed during the exposure to A. rugosa essential oil when compared to that of no odor exposure. Whereas relative alpha (RA), relative slow alpha (RSA), spectral edge frequency 50% (SEF50), and spectral edge frequency 50% of alpha (ASEF) power spectra values significantly increased. These results reveal that the EEG power spectra changes incurred during the exposure to the essential oil of A. rugosa may be associated with the enhancement of freshness and concentration states of the human brain.
1. Introduction
In aromatherapy, essential oils from aromatic plants have been utilized to heal various psychophysiological disorders, including depression, anxiety, insomnia, and tension, and restoring physical, as well as emotional, conditions since the ancient era [1]. It is well known that the inhalation of essential oils can produce positive psychological and physiological functions in humans by reducing mental stress, and increasing mind relaxation and cognitive functions via stimulation of the central nervous system [2,3,4]. Previous studies have also proved that fragrances from essential oils can influence the mental condition of human beings [3,5,6,7,8,9,10]. In particular, the aromatic volatile components in essential oils, such as mono- and sesquiterpene hydrocarbons and their oxygenated derivatives, are mainly responsible for their characteristic fragrances. In day-to-day life, these fragrant molecules effectively influence the mood, stress, and working capacity of individuals [11].
The physiological changes stimulated through the fragrance inhalation of essential oils are highly associated with the regulation of the olfactory nervous system [12]. In the olfactory system, the olfactory mucosa finds fragrance molecules via inhalation of essential oils in the posterior and superior parts of the nasal cavity. The olfactory bulb receives the fragrance signal via the olfactory sensory neurons and supplies input to other brain regions that modify neuronal activity [13]. A number of electrophysiological techniques have been developed to examine brain function. Among them, the changes in neuronal activity due to the stimulation of fragrance inhalation can be easily measured by electroencephalography (EEG) [14,15]. EEG is an extensively used technique to determine the impact of fragrance on human brain functions. EEG is used to measure neuronal electrical activity in terms of brain waves. Brain waves are natural processes in the brain that appear during active, as well as resting, states. Our thoughts, emotions, and behavior mirror neuronal activity within the brain. EEG calculates these neuronal electrical activities and represents them as waves. The EEG spectra bands were categorized into five major waves: delta (0–4 Hz), theta (4–8 Hz), alpha (8–13 Hz) beta (13–30 Hz), and gamma (>30 Hz). Generally, the alterations in brain wave activities are highly interlinked with specific functions of the brain [13,16]. Previously, several studies have found that essential oils from a variety of plants, such as peppermint, lavender, rosemary, sandalwood, neroli, jasmine, bergamot, and Abies species, significantly altered EEG activity, resulting in the positive psychological and physiological functions of humans [4,11].
Agastache rugosa (Fisch. & C.A.Mey.) Kuntze (Lamiaceae) is a perennial herb and is widely distributed in East Asian Countries, including Korea, Japan, and China [17,18]. In Korea, A. rugosa is commonly called Korean mint (Baechohyang). The aerial parts of the plant have a unique scent, and the leaves are used as an ingredient for enhancing the aroma and taste of Korean dishes, especially in salads and soups [19,20,21]. A. rugosa has been traditionally used to cure various ailments, including anorexia, anxiety, bacterial infections, colds, cholera, diarrhea, dispel damp, fever, gas, halitosis, headaches, halitosis, nausea, miasma, and vomiting, etc. [18,19,21,22]. Previous studies indicated that the crude extracts and compounds of A. rugosa possess numerous therapeutic properties, such as antimicrobial [23], anti-inflammatory [24], cardiovascular [25,26], antioxidant [27], anti-atherogenic [28], anti-HIV [29], melanogenesis [30], anticancer [31], anti-photoaging [32], anti-adipogenic, and anti-lipogenic effects [33]. Furthermore, various phytochemical studies revealed the presence of methyl hexadecanoate, β-sitosterol, phenolic acid, ursolic acid, apigenin, protocatechuic acid [22], sterols, phenylpropanoids, flavonoids, lignans, and terpenoids [32] in A. rugosa. The essential oil obtained from the stem of A. rugosa contains various groups of aromatic components, such as alcohols, aldehydes, ketones, esters, and terpenoids [34]. In addition, rosmarinic acid, tilianin [35], and acacetin [36] are the main compounds in the extracts of A. rugosa. In particular, acacetin 7-O-β-d-glucoside (tilianin) attenuates house dust mite-induced allergic asthma in mice [37].
There are some reports on the essential oil composition of A. rugosa (Baechohyang) in South Korea [27,38]. The essential oil composition of plant species may vary depending on various ecological factors [39]. Although the essential oil of A. rugosa exhibits various biological activities, including antioxidant, antimicrobial, and antitumor potentials, etc. [26], the effect of exposure to A. rugosa essential oil on human EEG activity is still unknown. Therefore, the present study was initiated to determine the essential oil composition of Korean A. rugosa and to evaluate the effects of exposure to its essential oil on human EEG activity in order to utilize this essential oil in aromatherapy.
2. Materials and Methods
2.1. Plant Material and Cultivation
One hundred seeds of Korean domestic wild Baechohyang (A. rugosa) were collected in 2019–2020 and they were stored in a refrigerator before sowing. The seeds were sown in seedling trays (17 cm3, Seoul Bio, Seoul, Korea) filled with horticultural topsoil (rich farms) during April 2020. All seedlings in trays were grown until the plants reached the 3.5 leaf stage in the glass greenhouse of the Agricultural Research Institute, Gangwon-do Agricultural Research and Development Institute, where the proper temperature (23–25 °C day) and humidity were maintained. The seedlings were planted in a phosphorus field. After that, aerial parts of A. rugosa were harvested when it reached the flowering period and used for essential oil extraction.
2.2. Steam Distillation Extraction
The essential oil was extracted from the aerial parts of A. rugosa using the steam distillation extraction method. The steam distillation extraction was carried out with a 1 kg sample of A. rugosa for 90 min using a Clevenger-type apparatus. After extraction, water and impurities in the extracted essential oil were removed using anhydrous sodium sulfate. The yield (%) of the essential oil was calculated in triplicate as the volume (mL) of the extracted essential oil relative to the amount of the fresh plant sample (1 kg).
2.3. GC–MS Analysis of A. rugosa Essential Oil
The volatile aromatic components in the essential oil of A. rugosa were identified by gas chromatography and mass spectrometry (GC–MS) analysis. GC–MS analysis was carried out with a Varian CP-3800 (GC)/Varian 1200 L (MS) equipped with a VF-5MS polydimethylsiloxane capillary column (30 m × 0.25 mm × 0.25 μm). The GC oven temperature was kept at 50 °C for 5 min, then heated to 250 °C at a rate of 3 °C/min, and maintained for 15 min. One μL of the sample was injected with a split ratio of 10:1, and helium was used as a carrier gas at the rate of 1 mL/min. The injector temperature was set at 250 °C and the ion source temperature was set at 200 °C. For MS analysis, the ionization voltage was set to 70 eV, and the mass range was set to 50–500 m/z. The components in the essential oil of A. rugosa were identified by comparing the mass spectrum data of the NIST library and the retention indices (RI) relative to a homologous series of n-alkanes (C8–C20) with those reported in the literature [40].
2.4. Odor Evaluation
A sensory evaluation was performed to determine the fragrance of A. rugosa essential oil. For sensory evaluation, 1% of dilute A. rugosa essential oil was prepared using a colorless and odorless dipropylene glycol (DPG) solvent, and it was evaluated by three expert panels with olfactory training. The diluted essential oil was placed on the lower part of the commercial odor paper strip. Then the odor characteristics of the essential oil were recorded according to the odor type felt by the professional panel.
2.5. EEG Study
The study followed the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the ethics committee from the Kangwon National University (KWNUIRB-2021-11-007-002), Chuncheon, Korea.
2.5.1. Subjects
Thirty-eight right-handed healthy volunteers (19 men and 19 women) aged between 20 and 30 years participated in this study. The mean ages of men and women were 23.5 ± 3.2 and 22.8 ± 2.7, respectively. The inclusion criteria for the subjects were non-smokers and right-handed without any abnormalities in olfaction. None of the subjects had olfactory diseases or abused drugs. Alcohol consumption or medications were prohibited from 2 days before the experiment. There were no statistically significant differences between men and women. All the subjects were students and no one refused to participate in this study. All subjects gave their informed consent before participation in the EEG study.
2.5.2. Experimental Design
In this study, a single group pre-test and post-test experimental design was used for 38 subjects. A careful measurement was carried out before and during the exposure to the essential oil. The subjects were informed that the aim of this study was to evaluate the changes of EEG activity during no odor and essential oil exposure. The subjects were instructed to sit quietly, close their eyes, and breathe normally during the EEG measurement. After the measurement, the subjects were requested to provide their preference and impression of the tested essential oil.
2.5.3. EEG Recordings
For EEG measurement, a Quick-30 Dry EEG Headset (Cognionics Inc., San Diego, CA, USA) was used, and EEG data were recorded with Cognionics Data Acquisition Software (Cognionics Inc., USA). The EEG recordings were made using an electrode cap from 29 channels positioned on the scalp at Fp1, Fp2, Af3, Af4, F3, F4, Fz, F7, F8, Fc5, Fc6, C3, C4, T7, T8, Cp5, Cp6, P3, P4, P7, P8, Cz, Pz, O1, O2, Po3, Po4, Po5, and Po6 regions according to the International 10–20 System (Figure 1A). The electrodes were referenced to the ipsilateral earlobe electrodes. The EEG sampling rate of the measured subjects was 500 Hz, filtered in the range of 4–50 Hz, and the readings were stored in a computer by 24-bit analog-to-digital conversion. The electrodes (silver/silver chloride) were applied over an elastic cap with plastic electrode holders. The ECI electrode gel (Electro-gel™, Electro-Cap International Inc., Eaton, OH, USA) was applied to each electrode to connect with the surface of the scalp to drop the electric resistance of the scalp below 5 kΩ [41].
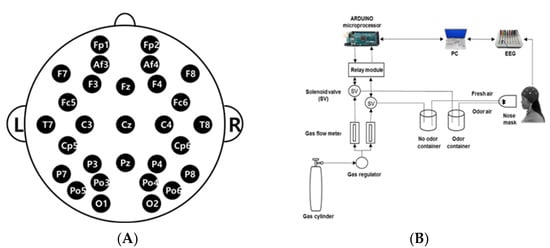
Figure 1.
EEG experiment: (A) EEG electrode placement locations using the International 10–20 system; (B) Schematic diagram of the experimental procedure.
2.5.4. Fragrance Administration
The essential oil of A. rugosa was used as the fragrance stimulus. The stimulus was presented to the subjects in a randomized sequence. The EEG recording room was maintained with a constant temperature (25 °C) and humidity (50%). The diluted A. rugosa essential oil (1%) was placed in the sample container and an EEG measurement was performed for a total of 120 s with 60 s of air with no odor and 60 s of air with A. rugosa essential oil. The odorless fresh air was pumped into the chamber at the rate of 3 L/min. The air outflow chamber was placed 5 cm in front of the subject’s nose (Figure 1B).
2.5.5. Data Analysis
The EEG power spectrum values [microvolt square (𝜇V2)] were calculated for 25 EEG analysis indicators (Table 1). To remove noise, a 1–1000 Hz band pass filter was employed with a 24 dB/octave roll-off, and a 60 Hz notch filter was applied. The t-mapping of EEG waves was constructed using the Telescan software package (LAXTHA Inc., Daejeon, Korea). Out of 60 s EEG data recorded, only 50 s EEG data were analyzed for each condition, such as air with no odor and air with A. rugosa essential oil. The SPSS statistical package 26 (IBM Inc.) was used to determine significant differences in EEG activity between air with no odor and air with A. rugosa essential oil, using a paired Student’s t-test. The p value < 0.05 was considered significant.

Table 1.
The abbreviations, full names, and wavelength ranges of the EEG power spectrum indices.
3. Results
3.1. Chemical Composition of the Essential Oil from the Aerial Parts of A. Rugosa
The essential oil extracted from the aerial parts of A. rugosa was transparent lemon in color and aromatic, herbal, oily, and spicy. The yield of steam distilled A. rugosa essential oil was 0.15 ± 0.02% (v/w). In the essential oil of A. rugosa, a total of 29 volatile components were identified based on the retention indices and mass spectra data, which accounted for 99.72 ± 0.13 of the total oil. The identified components are listed in order of their elution from a VF-5MS column. The essential oil of A. rugosa contains 10 sesquiterpenes, 9 monoterpenes, 3 phenylpropanoids, 2 esters, 1 alcohol, 1 ketone, 1 aldehyde, 1 hydrocarbon, and 1 phenol. The most abundant component in the essential oil of A. rugosa essential was estragole (89.49%), followed by D-limonene (3.40%), menthone (1.80%), and pulegone (1.86%). The concertation of the remaining components in the essential oil was less than 1%.
3.2. Effect of A. Rugosa Essential Oil on Human EEG Study
In this study, the essential oil of A. rugosa was used to stimulate the olfactory system. EEG power spectrum values were measured during the air with no odor and air with A. rugosa essential oil odor. The changes of 25 EEG indices were analyzed from 29 electrodes located on the scalp. As a result of EEG measurement in both genders, there were significant differences in six indices among the 25 EEG indices analyzed. Absolute theta (AT) and relative theta (RT) power spectra significantly decreased at different sites during exposure to A. rugosa essential oil. On the other hand, significant increases in relative alpha (RA) and relative slow alpha (RSA) spectral edge frequency 50% (SEF50), and spectral edge frequency 50% of alpha (ASEF) values were observed due to A. rugosa essential oil exposure when compared with no odor exposure (Table 2).

Table 2.
Overall significant decreases and increases in EEG activity during exposure to A. rugosa essential oil.
During exposure to A. rugosa essential oil, AT values significantly decreased in the frontal region (Fp1, Fp2, Af3, F7, F3, Fz, and Fc5), temporal region (T7, C3, and Cp5), parietal region (P7 and P3), and occipital region (Po5, Po3, and O1) (p < 0.05). In the case of RT spectrum, a significant decrease was observed in the frontal region (Af3, F7, and Fz), temporal region (C3, Cz, Cp5, and Cp6), parietal region (P7 and P3), and occipital region (Po5, Po3, Po4, O1, and O2) (Table 3 and Figure 2).

Table 3.
Significant changes of AT and RT power spectra between no odor exposure and A. rugosa essential oil odor exposure.
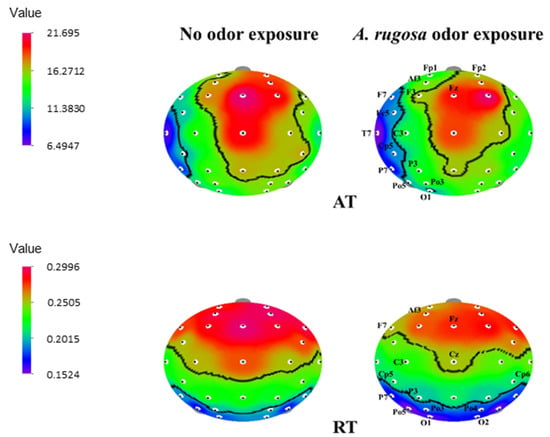
Figure 2.
The t-mapping of EEG power spectrum changes during no odor and A. rugosa essential oil odor conditions. AT, absolute theta; RT, relative beta. The marked sites in the t-mapping denote the significant changes during exposure to A. rugosa essential oil.
On the other hand, the RA power spectrum significantly increased (p < 0.05) in the temporal region (Cp5, and Cp6), parietal region (P8, P3, and P4), and overall occipital region (Po5, Po6, Po3, Po4, O1, and O2). Slow alpha waves increased significantly in the occipital lobe (Po3, O1, and O2) (p < 0.05). Furthermore, SEF50 values showed that the left frontal regions (Fp2 and Af3), frontal regions (F3 and Fz), and temporal region (Cp6) significantly increased. The ASEF index significantly increased at the right temporal region, Cp6 (p < 0.05) (Table 4 and Figure 3).

Table 4.
Significant changes of RA, RSA, SEF50 and ASEF power spectra between no odor exposure and A. rugosa essential oil odor exposure.
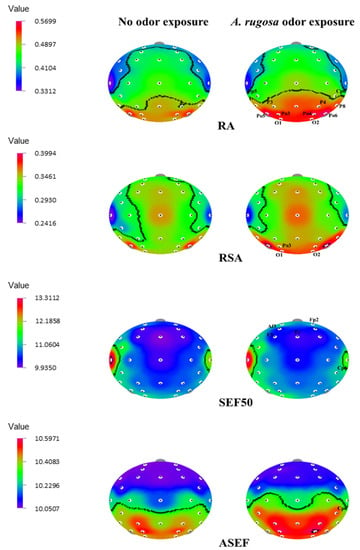
Figure 3.
The t-mapping of EEG power spectrum changes during no odor and A. rugosa essential oil odor conditions. ASEF, spectral edge frequency 50% of alpha; RA, relative alpha; RSA, relative slow alpha; SEF50, spectral edge frequency 50%. The marked sites in the t-mapping denote the significant changes during exposure to A. rugosa essential oil.
4. Discussion
Plants of the Lamiaceae family are extensively utilized for essential oils. Monoterpenes and sesquiterpenes are important essential oil constituents in a variety of aromatic plants. Among them, A. rugosa is an important traditional medicinal plant in Korea. In total, 29 chemical compounds have been identified in Korean-grown A. rugosa essential oil by GC–MS analysis. In the present study, estragole (89.49%) was the most abundant component in the essential oil, followed by D-limonene (3.40%), menthone (1.80%), and pulegone (1.86%). Similar to our findings, Lim et al. [38] found that estragole (84.25%) was the predominant compound in the essential oil of A. rugosa leaves. Yamani et al. [50] also reported that estragole was the main component in the essential oil of Australian-grown A. ruogsa. The major component estragole has analgesic properties [12]. In the essential oil from the leaves of A. rugosa collected in China, p-menthan-3-one (48.8%) was the main component, followed by estragole (20.8%) [31]. Another study demonstrated that methyl eugenol (50.51%), estragole (8.55%), eugenol (7.54%), thymol (3.62%), pulegone (2.56%), limonene (2.49%), and caryophyllene (2.38%) were major components in the essential oil of A. rugosa [51]. p-Menthan-3-one (48.8%), estragole (20.8%), monoterpenes (8.3%), and oxygenated terpenes (5.6%) were identified in A. rugosa leaf [52]. The variations in the essential oil yield and its composition could be influenced by various factors, including the cultivation techniques, geographical location, age of the plant, and climatic conditions, etc. [53].
Recently, several studies have been conducted on the psychophysiological properties of essential oil components using animal models. However, only a few studies have been investigated to determine the potential effectiveness of essential oils in humans [54]. Previous studies showed that aromatic components exhibit a positive change, such as improving alertness and concentration, increasing relaxation, and attenuating mental stress and tension via stimulation of the central nervous system [7,8]. EEG is widely used to evaluate the neurophysiological function of the human brain. With this background, we attempted to evaluate whether exposure to A. rugosa essential oil exhibits any effect on human EEG activity. In this study, changes in the 25 EEG power spectrum indices between air with no odor and air with A. rugosa essential oil odor were analyzed. During inhalation of A. rugosa essential oil, AT (4–8 Hz) and RT (4–8/4–50 Hz) activities were significantly decreased in different sites, whereas RA (8–13/4–50 Hz), RSA (8–11/4–50 Hz), SEF50 (4–50 Hz), and ASEF (8–13 Hz) were increased in different sites.
In the study, the AT value was significantly decreased in different brain regions, such as the frontal, temporal, parietal, and occipital regions during exposure to A. rugosa essential oil. Sowndhararajan et al. [9] also reported that absolute theta wave activity significantly decreased at the sites of FP1, FP2, F3, F4, T4, P3, and P4 during inhalation of the essential oil of Inula helenium root. In another study, AT wave activity significantly varied via left and right inhalation of aldehyde C10 odor when compared with inhalation via both nostrils [10]. During inhalation of the essential oil of A. koreana twigs, AT wave activity also significantly changed in the F3 and P4 regions [7]. The inhalation of Abies sibirica essential oil effectively reduced arousal levels by increasing theta waves [55]. The brain regions (lones) are classified into five major regions: frontal, temporal, parietal, and occipital regions, and each lobe is associated with different functions. Theta waves mainly appear during deep meditation and are also found in hippocampal and cortical regions. Theta waves are linked with the subconscious mind that controlles sleep, drowsiness, imaginative thinking, and creativity [16,56]. The reduction in theta wave activity is interrelated with memory formation. Further, during the implementation of a difficult task, theta waves have been believed to maintain attention [57]. The significant changes in AT due to the inhalation of A. rugosa essential oil may be associated with the drowsy or meditative state of the brain.
In the case of the RT power spectrum, significant decreases were noticed in frontal, temporal, parietal, and occipital regions during exposure to A. rugosa essential oil. RT wave activity also decreased at the site during inhalation of Inula helenium root essential oil (at FP1, FP2, F3, and F4 regions) and essential oil from the twigs of A. koreana (at F4 and P4 regions) [7,9]. Kim et al. [8] reported that the RT spectrum markedly decreased in the FP1 and P4 regions during inhalation of black pepper essential oil.
In the present study, RA, RSA, SEF50, and ASEF significantly increased in different brain regions during exposure to A. rugosa essential oil. Previous studies reported that the ASEF index significantly decreased during inhalation of A. koreana and black pepper essential oils [7,8]. However, ASEF spectrum activity increased in the Cp6 region due to A. rugosa essential oil exposure. Similarly, ASEF activity increased in the FC1, T8, AF4, and FPZ regions due to geosmin exposure [6]. Significant increases in the RA and RSA indices were observed during inhalation of essential oil of A. koreana twigs [7]. The SEF50 spectrum significantly decreased during inhalation of geosmin odor, black pepper essential oil, and supercritical carbon dioxide extract from the root of A. gigas [6,8,58]. The SEF50 is defined as the frequency below 50% of the overall EEG power and it specifies the spectral features of EEG data. Some reports indicated that the spectral edge frequency increased during light anesthesia conditions [49]. The significant changes of SEF50 due to A. rugosa essential oil inhalation may increase the concentration state of the brain function. In addition, previous studies found that gender and nostril variations play a major role in the EEG activity of different fragrances [7,8]. Another study indicated that men and women responded inversely during the exposure to fragrances [59]. Furthermore, gender variation occurred in the EEG activity of resting, stimulus, and non-stimulus conditions [8].
The data of this study clearly demonstrate that A. rugosa essential oil effectively stimulates brain wave activity in different regions of the brain. Furthermore, the major component, estragole, in the essential oil of A. rugosa may play a key role in the odor characteristics of this essential oil, thereby producing significant changes in EEG activity. Although exposure to the essential oil of A. rugosa produces significant changes in EEG activity, further studies are required in connection with different concentrations of an odor stimulus, and slightly longer EEG recording time with a placebo control and other commercial odor controls.
5. Conclusions
The GC–MS analysis revealed that the essential oil of A. rugosa mainly contains estragole. In the EEG study, exposure to A. rugosa essential oil exhibited significant decreases in absolute theta and relative theta power spectra and increases in relative alpha, relative slow alpha, spectral edge frequency 50%, and spectral edge frequency 50% of alpha spectra values. These EEG changes suggest that exposure to A. rugosa essential oil may be associated with the enhancement of the freshness and concentration states of the human brain. This essential oil can be used in aromatherapy for positive psychophysiological conditions.
Author Contributions
Conceptualization, S.K.; methodology, S.K., M.H. and M.K.; formal analysis, M.H., H.J., S.B. and M.K.; investigation, M.H.; resources, J.P.; data curation, M.H. and M.K.; writing—original draft preparation, P.D. and K.S.; writing—review and editing, S.K. and K.S.; supervision, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study followed the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the ethics committee from the Kangwon National University (KWNUIRB-2021–11-007–002), Chuncheon, Korea.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
This study was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014506), Rural Development Administration, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Cooke, B.; Ernst, E. Aromatherapy: A systematic review. Br. J. Gen. Pract. 2000, 50, 493–496. [Google Scholar] [PubMed]
- Yim, V.W.C.; Ng, A.K.Y.; Tsang, H.W.H.; Leung, A.Y. A review on the effects of aromatherapy for patients with depressive symptoms. J. Altern. Complement. Med. 2009, 15, 187–195. [Google Scholar] [CrossRef]
- Angelucci, F.L.; Silva, V.V.; Pizzol, C.D.; Spir, L.G.; Praes, C.E.; Maibach, H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Chen, M.C.; Fang, S.H.; Fang, L. The effects of aromatherapy in relieving symptoms related to job stress among nurses. Int. J. Nurs. Pract. 2015, 21, 87–93. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Kim, T.; Kim, J.E.; Yang, J.E.; Kim, S. Gender differences in electroencephalographic activity in response to the earthy odorants geosmin and 2-methylisoborneol. Appl. Sci. 2017, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Sowndhararajan, K.; Kim, S. Influence of binasal and uninasal inhalations of essential oil of Abies koreana twigs on electroencephalographic activity of human. Behav. Neurol. 2016, 2016, 9250935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Chhoeun, T.B.; Kim, T.; Sowndhararajan, K.; Kim, S. The gender variation on the electroencephalographic activity in response to the exposure of black pepper essential oil from Kampong Cham. Cambodia. Flavour Fragr. J. 2019, 35, 284–293. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Song, J.; Kim, S. Effect of inhalation of essential oil from Inula helenium L. root on electroencephalographic (EEG) activity of human. Eur. J. Integr. Med. 2016, 8, 453–457. [Google Scholar] [CrossRef]
- 10. Kim, M.; Sowndhararajan, K.; Choi, H.J.; Park, S.J.; Kim, S. Olfactory stimulation effect of aldehydes, nonanal, and decanal on the human electroencephalographic activity. According to nostril variation. Biomedicines 2019, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Heinbockel, T. The effects of essential oils and terpenes in relation to their routes of intake and application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandharakool, S.; Koomhin, P.; Sinlapasorn, J.; Suanjan, S.; Phungsai, J.; Suttipromma, N.; Songsamoe, S.; Matan, N.; Sattayakhom, A. Effects of tangerine essential oil on brain waves, moods, and sleep onset latency. Molecules 2020, 25, 4865. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.W.; Su, C.H.; Yang, M.H.; Liu, S.Y.; Su, T.P. A pilot study on essential oil aroma stimulation for enhancing slow-wave EEG in sleeping brain. Sci. Rep. 2021, 11, 1078. [Google Scholar] [CrossRef]
- Desai, R.; Tailor, A.; Bhat, T. Effects of yoga on brain waves and structural activation: A review. Complement. Ther. Clin. Pract. 2015, 21, 112–118. [Google Scholar] [CrossRef]
- Navarra, T. Encyclopedia of Vitamins, Minerals and Supplements, 2nd ed.; Facts On File: New York, NY, USA, 2004. [Google Scholar]
- Lee, Y.; Lim, H.W.; Ryu, I.W.; Huang, Y.H.; Park, M.; Chi, Y.M.; Lim, C.J. Anti-inflammatory, barrier-protective, and antiwrinkle properties of Agastache rugosa Kuntze in human epidermal keratinocytes. BioMed Res. Int. 2020, 2020, 1759067. [Google Scholar] [CrossRef]
- Seo, H.; Kim, C.; Kim, M.B.; Hwang, J.K. Anti-photoaging effect of Korean mint (Agastache rugosa Kuntze) extract on UVB-irradiated human dermal fibroblasts. Prev. Nutr. Food Sci. 2019, 24, 442–448. [Google Scholar] [CrossRef]
- Bae, K. The Medicinal Plants of Korea; Kyo-Hak Publishing: Seoul, Korea, 2000; p. 432. [Google Scholar]
- Lee, H.K.; Oh, S.R.; Kim, J.I.; Kim, J.W.; Lee, C.O.J. Agastaquinone, a new cytotoxic diterpenoid quinone from Agastache rugosa. J. Nat. Prod. 1995, 58, 1718–1721. [Google Scholar] [CrossRef]
- Cao, P.; Xie, P.; Wang, X.; Wang, J.; Wel, J.; Kang, W.Y. Chemical constituents and coagulation activity of Agastache rugosa. BMC Complement. Altern. Med. 2017, 17, 93. [Google Scholar] [CrossRef] [Green Version]
- Shin, S. Essential oil compounds from Agastache rugosa as antifungal agents against Trichophyton species. Arch. Pharm. Res. 2004, 27, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Lee, Y.; Huang, Y.H.; Yoon, J.Y.; Lee, S.H.; Kim, K.; Lim, C.J. Enhancement of skin antioxidant and anti-inflammatory potentials of Agastache rugosa leaf extract by probiotic bacterial fermentation in human epidermal keratinocytes. Microbiol. Biotechnol. Lett. 2017, 45, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Abreu, O.; Torres-Piera, M.; García-Jiménez, S.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Montes, S.; Rembao, D.; Estrada-Soto, S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013, 146, 187–191. [Google Scholar] [CrossRef]
- Guo, X.; Cao, W.; Yao, J.; Yuan, Y.; Hong, Y.; Wang, X.; Xing, J. Cardioprotective effects of tilianin in rat myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2015, 11, 2227–2233. [Google Scholar] [CrossRef] [Green Version]
- Desta, K.T.; Kim, G.S.; Kim, Y.H.; Lee, W.S.; Lee, S.J.; Jin, J.S.; Elaty, A.M.A.; Shin, H.C.; Shim, J.H.; Shin, S.C. The polyphenolic profiles and antioxidant effects of Agastache rugosa Kuntze (Banga) flower, leaf, stem and root. Biomed. Chromatogr. 2016, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.J.; Choi, J.H.; Oh, S.R.; Lee, H.K.; Park, J.H.; Lee, K.Y. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001, 495, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Lee, H.K.; Shin, C.G.; Huh, H. HIV integrase inhibitory activity of Agastache rugosa. Arch. Pharm. Res. 1999, 22, 520–523. [Google Scholar] [CrossRef]
- Lee, S.T.H.; Park, J.; Yoo, G. Demethyleugenol β-glucopyranoside isolated from Agastache rugosa decreases melanin synthesis via down-regulation of MITF and SOX9. J. Agric. Food Chem. 2016, 64, 7733–7742. [Google Scholar] [CrossRef]
- Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar] [CrossRef]
- Seo, Y.H.; Kang, S.Y.; Shin, J.S.; Ryu, S.M.; Lee, A.; Choi, G.; Moon, B.C.; Jang, D.S.; Shim, S.H.; Lee, D.; et al. Chemical constituents from the aerial parts of Agastache rugosa and their inhibitory activities on prostaglandin E2 production in lipopolysaccharide-treated RAW 264.7 macrophages. J. Nat. Prod. 2019, 82, 3379–3385. [Google Scholar] [CrossRef]
- Hwang, J.M.; Lee, M.H.; Lee, J.H.; Lee, J.H. Agastache rugosa extract and its bioactive compound tilianin suppress adipogenesis and lipogenesis on 3t3-l1 cells. Appl. Sci. 2021, 11, 7679. [Google Scholar] [CrossRef]
- Kim, J.M. Flavoral essential oil components in the stems of Agastache rugosa for aromatherapy. J. Korean Soc. Food Cult. 2021, 36, 317–324. [Google Scholar]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J. Agric. Food Chem. 2011, 60, 5945–5951. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Baek, S.C. Potent inhibitions of monoamine oxidase A and B by acacetin and its 7-O-(6-Omalonylglucoside) derivative from Agastache rugosa. Int. J. Biol. Macromol. 2017, 104, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, K.; Kang, M.A.; Kim, T.H.; Jang, H.J.; Ryu, H.W.; Oh, S.R.; Lee, H.J. Tilianin attenuates HDM-induced allergic asthma by suppressing Th2-immune responses via downregulation of IRF4 in dendritic cells. Phytomedicine 2021, 80, 153392. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Jang, J.M.; Park, W.T.; Uddin, M.R.; Chae, S.C.; Kim, H.H.; Park, S.U. Chemical composition of essential oils from flower and leaf of Korean mint, Agastache rugosa. Asian J. Chem. 2013, 25, 4361–4363. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by gas Chromatography/Mass Spectrometry; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kim, M.; Song, J.; Nishi, K.; Sowndhararajan, K.; Kim, S. Changes in the electroencephalographic activity in response to odors produced by organic compounds. J. Psychophysiol. 2020, 34, 35–49. [Google Scholar] [CrossRef]
- Fattinger, S.; Kurth, S.; Ringli, M.; Jenni, O.G.; Huber, R. Theta waves in children’s waking electroencephalogram resemble local aspects of sleep during wakefulness. Sci. Rep. 2017, 7, 11187. [Google Scholar] [CrossRef] [Green Version]
- Phneah, S.W.; Nisar, H. EEG-based alpha neurofeedback training for mood enhancement. Australas Phys. Eng. Sci. Med. 2017, 40, 325–336. [Google Scholar] [CrossRef]
- Xavier, G.; Ting, A.S.; Fauzan, N. Exploratory study of brain waves and corresponding brain regions of fatigue on-call doctors using quantitative electroencephalogram. J. Occup. Health. 2020, 62, e12121. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Reljin, N.; Bolkhovsky, J.B.; Orjuela-Cañón, A.D.; Chon, K.H. Brain Activity Correlates With Cognitive Performance Deterioration During Sleep Deprivation. Front. Neurosci. 2019, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D. Quantitative EEG, Event-Related Potentials and Neurotherapy; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Marlats, F.; Bao, G.; Chevallier, S.; Boubaya, M.; Djabelkhir-Jemmi, L.; Wu, Y.H.; Lenoir, H.; Rigaud, A.S.; Azabou, E. SMR/theta neurofeedback training improves cognitive performance and EEG activity in elderly with mild cognitive impairment: A pilot study. Front. Aging Neurosci. 2020, 12, 147. [Google Scholar] [CrossRef]
- Van Son, D.; de Rover, M.; De Blasio, F.M.; van der Does, W.; Barry, R.J.; Putman, P. Electroencephalography theta/beta ratio covaries with mind wandering and functional connectivity in the executive control network. Ann. N. Y. Acad. Sci. 2019, 1452, 52–64. [Google Scholar] [CrossRef]
- Tonner, P.H.; Bein, B. Classic electroencephalographic parameters: Median frequency, spectral edge frequency etc. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Yamani, H.; Mantri, N.; Morrison, P.D.; Pang, E. Analysis of volatile organic compounds from leaves, flower spikes, and nectar of Australian grown Agastache rugosa. BMC Complementary Altern Med. 2014, 14, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.Q.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and nematicidal activity of essential oil of Agastache rugosa against Meloidogyne incognita. Molecules 2013, 18, 4170–4180. [Google Scholar] [CrossRef]
- Gong, H.; Li, S.; He, L.; Kasimu, R. Microscopic identification and in vitro activity of Agastache rugosa (Fisch. et Mey) from Xinjiang, China. BMC Complement. Altern. Med. 2017, 17, 95. [Google Scholar] [CrossRef] [Green Version]
- Dhouioui, M.; Boulila, A.; Chaabane, H.; Zina, M.S.; Casabianca, H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Ind. Crops Prod. 2016, 83, 301–306. [Google Scholar]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Ohnuki, K.; Shimizu, K.; Kondo, R. The essential oil of Abies sibirica (Pinaceae) reduces arousal levels after visual display terminal work. Flavour Frag. J. 2011, 26, 204–210. [Google Scholar] [CrossRef]
- Lisman, J.E.; Idiart, M.A. Storage of 7 +/− 2 short–term memories in oscillatory subcycles. Science 1995, 267, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Burke, J.F.; Haque, R.; Kahana, M.J.; Zaghloul, K.A. Decreases in theta and increases in high frequency activity underlie associative memory encoding. NeuroImage 2015, 114, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowndhararajan, K.; Seo, M.; Kim, M.; Kim, H.; Kim, S. Effect of essential oil and supercritical carbon dioxide extract from the root of Angelica gigas on human EEG activity. Complement. Ther. Clin. Pract. 2017, 28, 161–168. [Google Scholar] [CrossRef]
- Haehner, A.; Maass, H.; Croy, I.; Hummel, T. Influence of room fragrance on attention, anxiety and mood. Flavour Frag. J. 2017, 32, 24–28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).