Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Housing
2.3. Dietary Supplementation
2.4. Measures and Behavioral Testing
2.4.1. Body Weights and Food Consumption Weights
2.4.2. Open Field Test (OFT)
2.4.3. Elevated Zero Maze (EZM)
2.4.4. Morris Water Maze (MWM)
2.4.5. Activities of Daily Living: Burrowing and Nesting
2.5. Statistical Analysis
3. Results
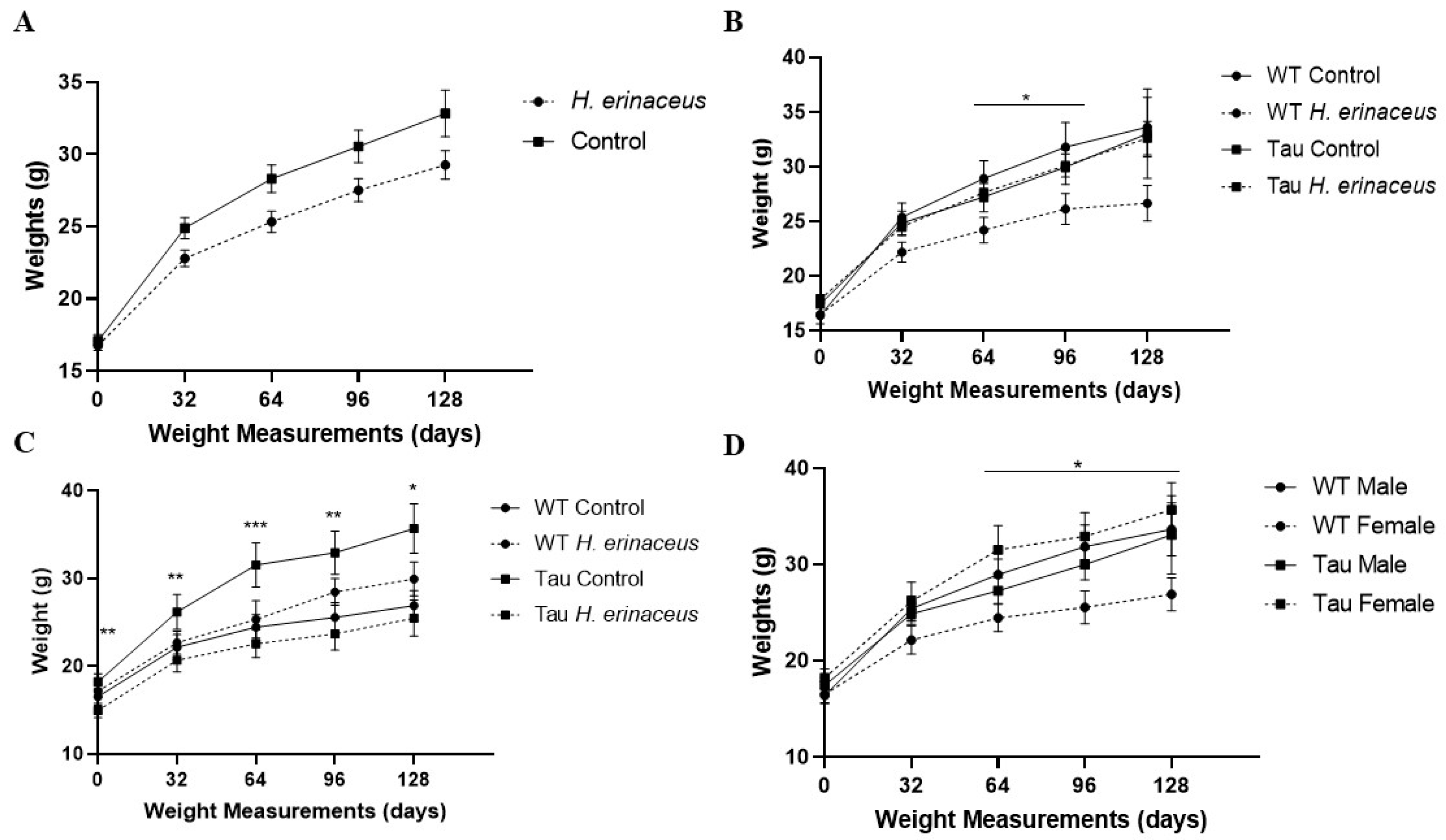
3.1. Animal Body Weights
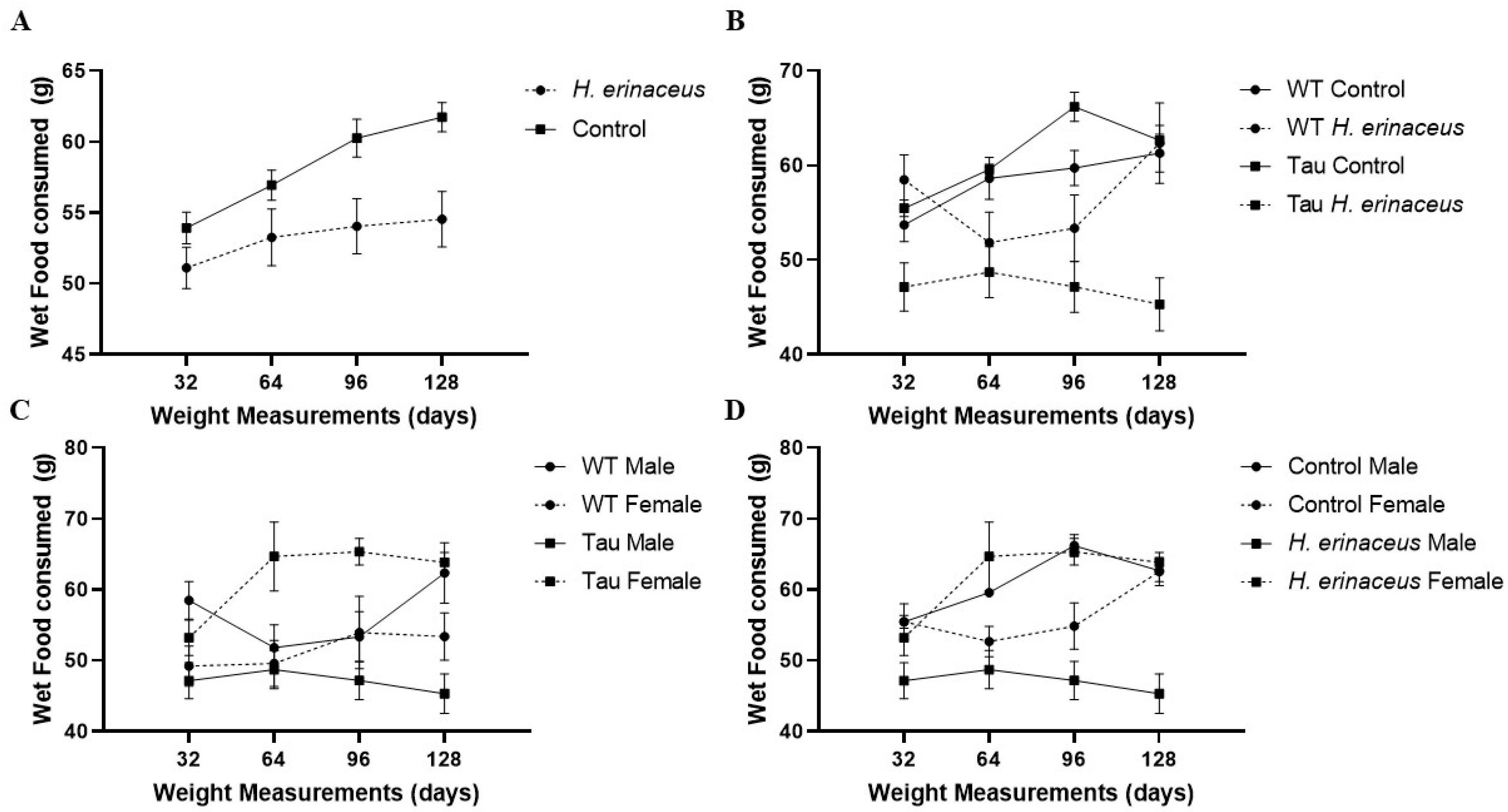
3.2. Wet Food Consumption
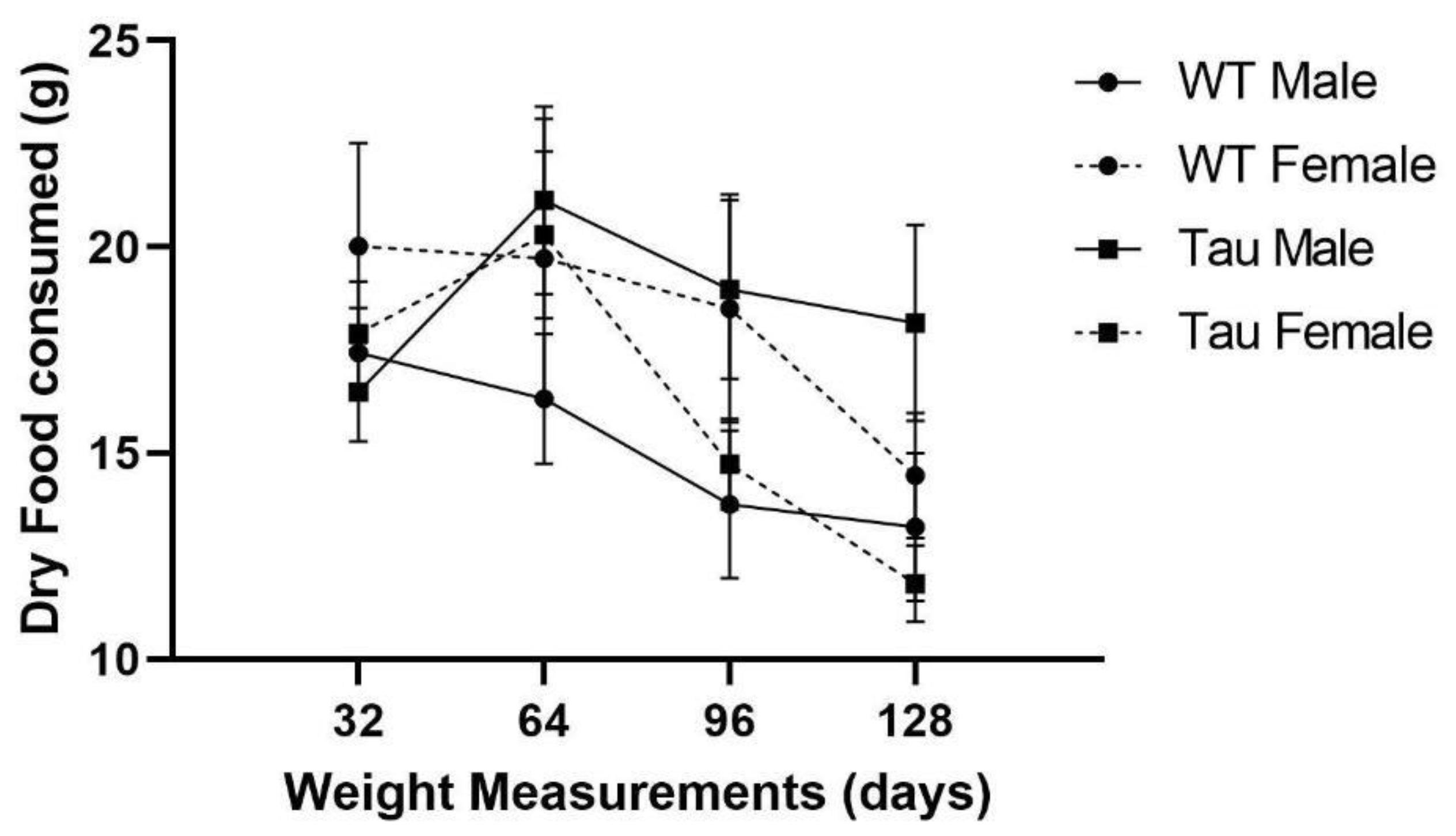
3.3. Dry Food Consumption
3.4. Open Field Test
3.4.1. Latency to Enter the Center
3.4.2. Total Distance in the Center (cm)
3.4.3. Percent Time Spent in the Center
3.4.4. Fecal Boli
3.5. Elevated Zero Maze
3.5.1. Head Dips
3.5.2. Total Transitions
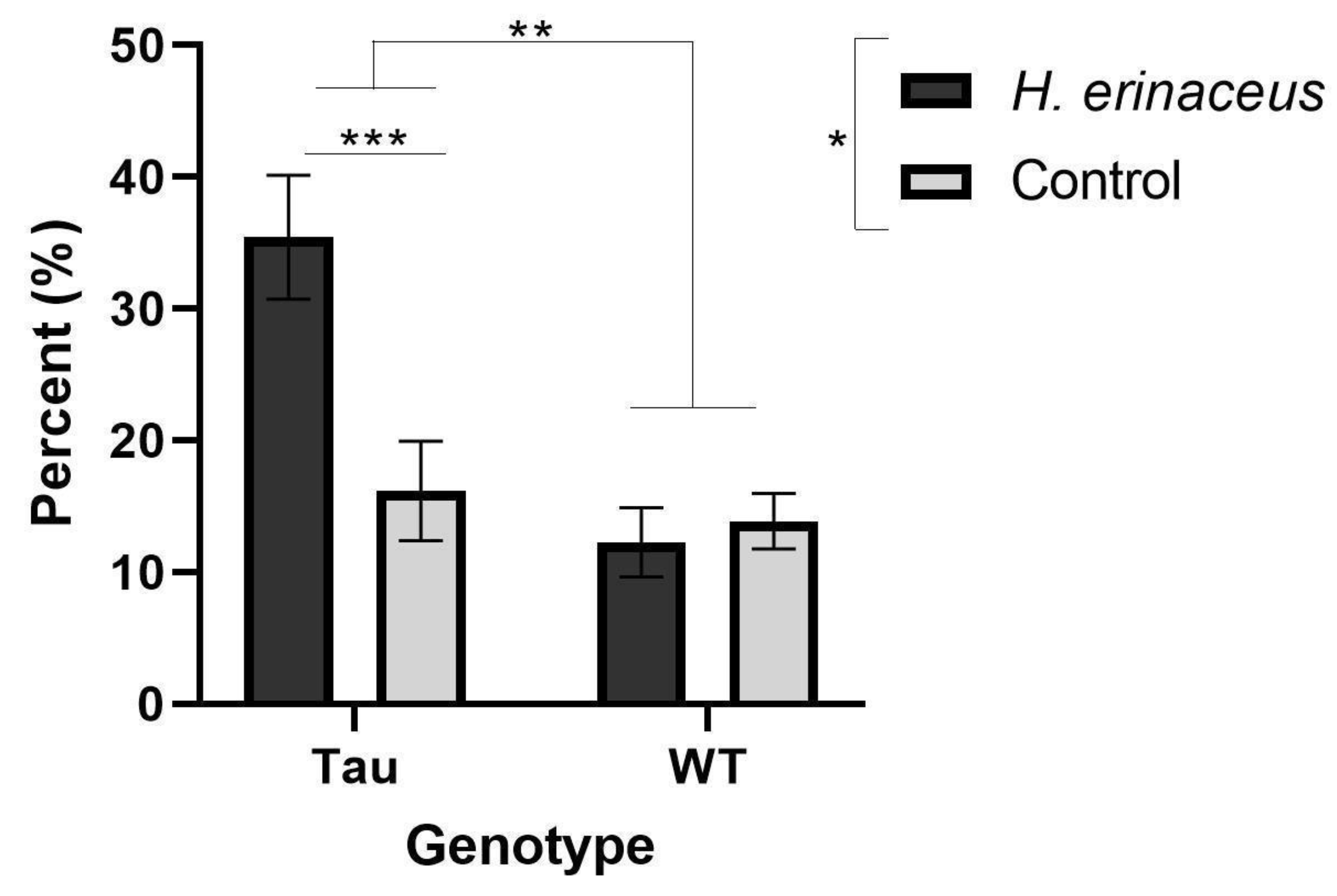
3.5.3. Percent Time Spent in the Open Arms
3.6. Morris Water Maze
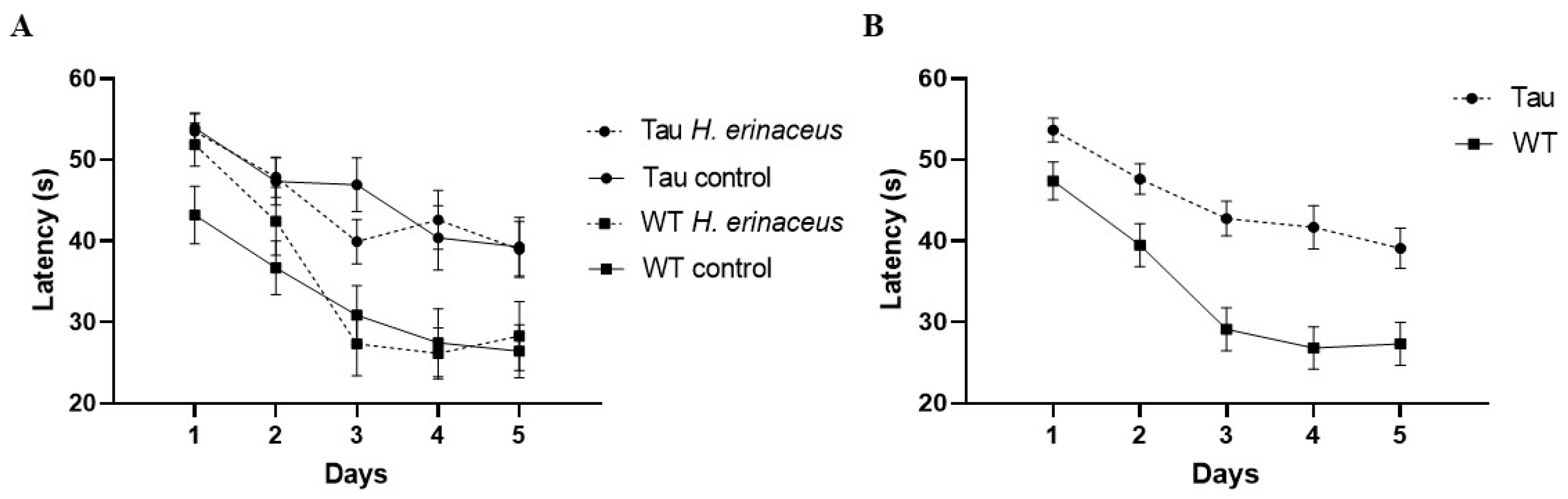
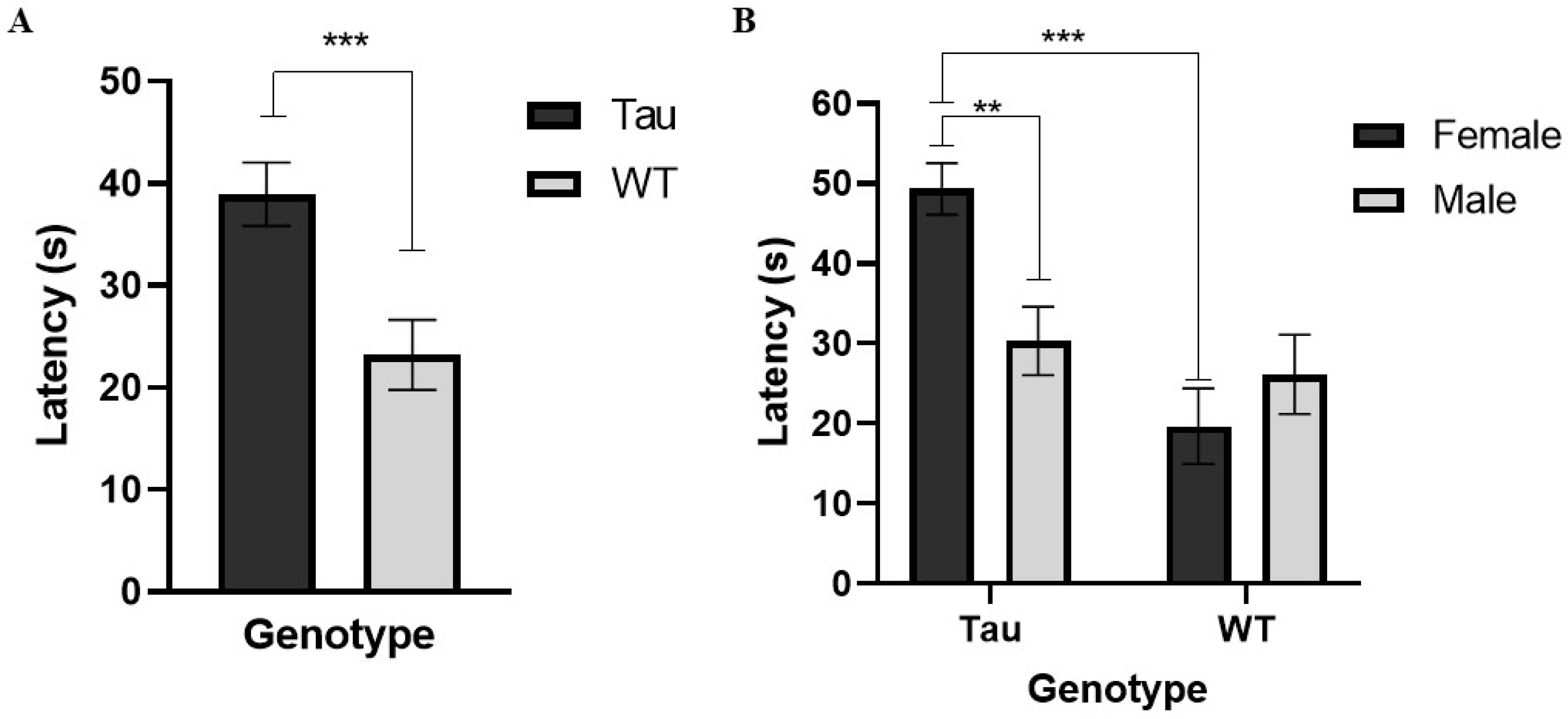
3.6.1. Latency to Platform
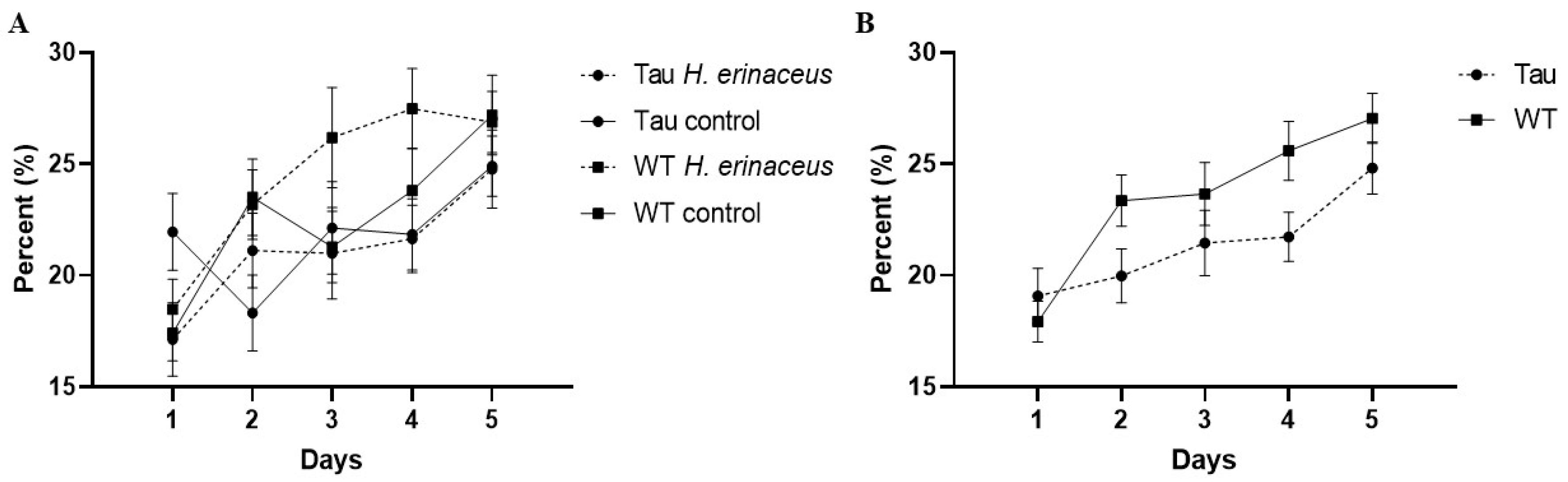
3.6.2. Percent Time Spent in the Target Quadrant
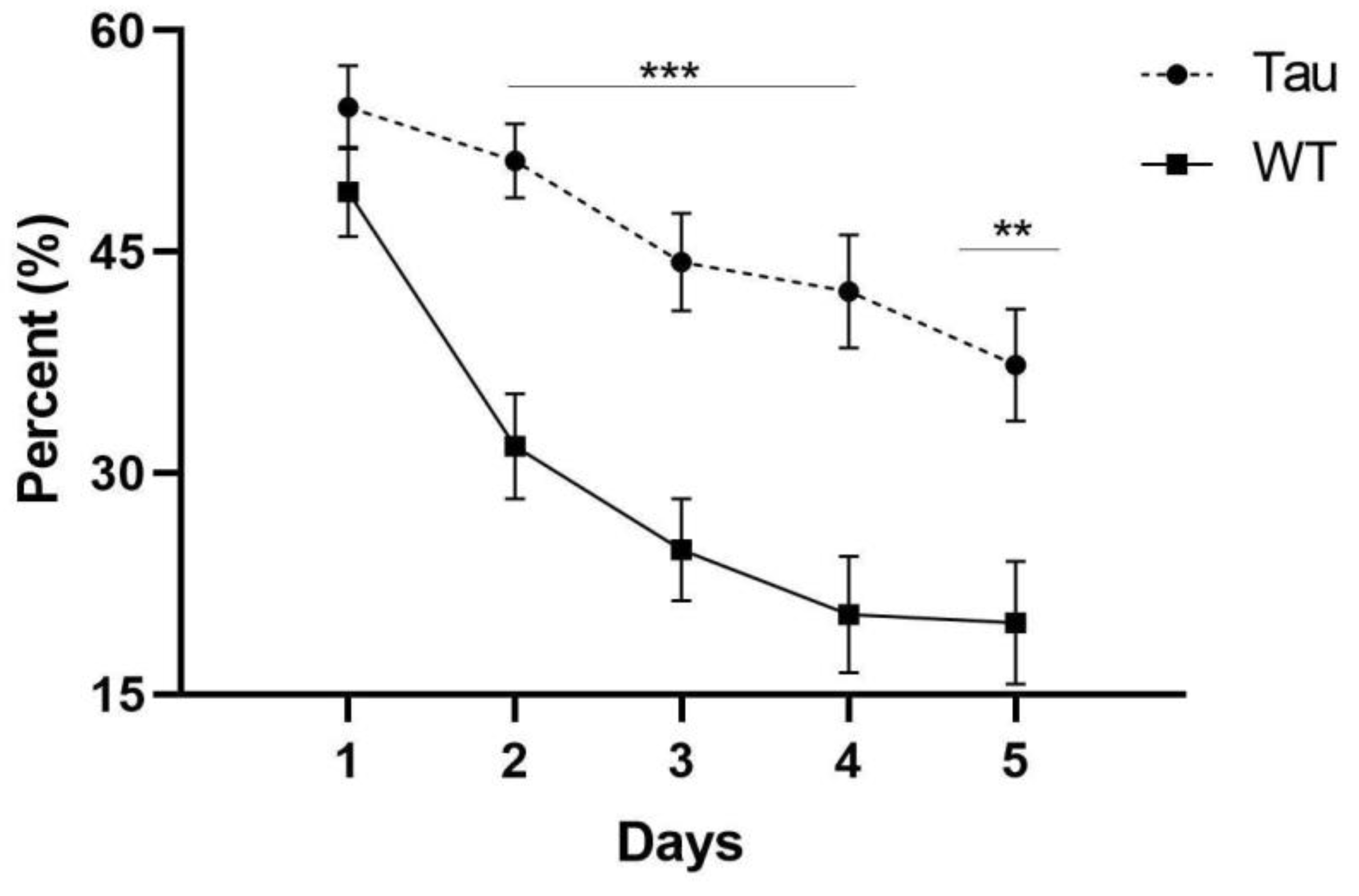
3.6.3. Thigmotaxicity
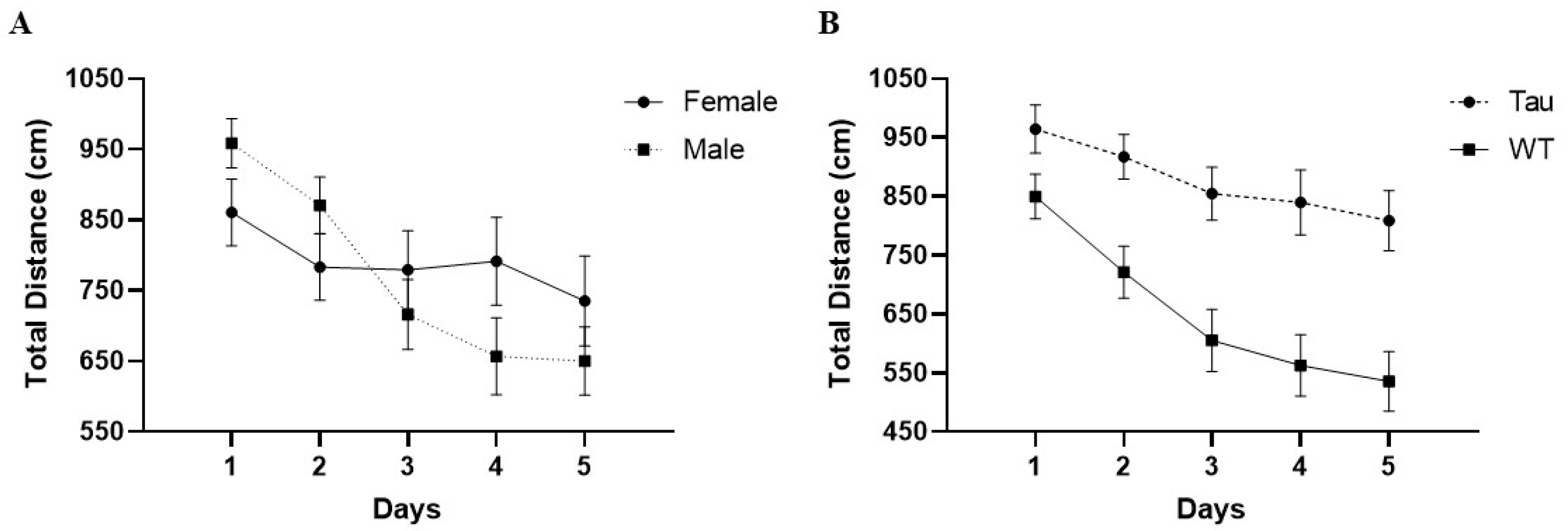
3.6.4. Total Distance
3.6.5. Latency to Platform (Probe Day)
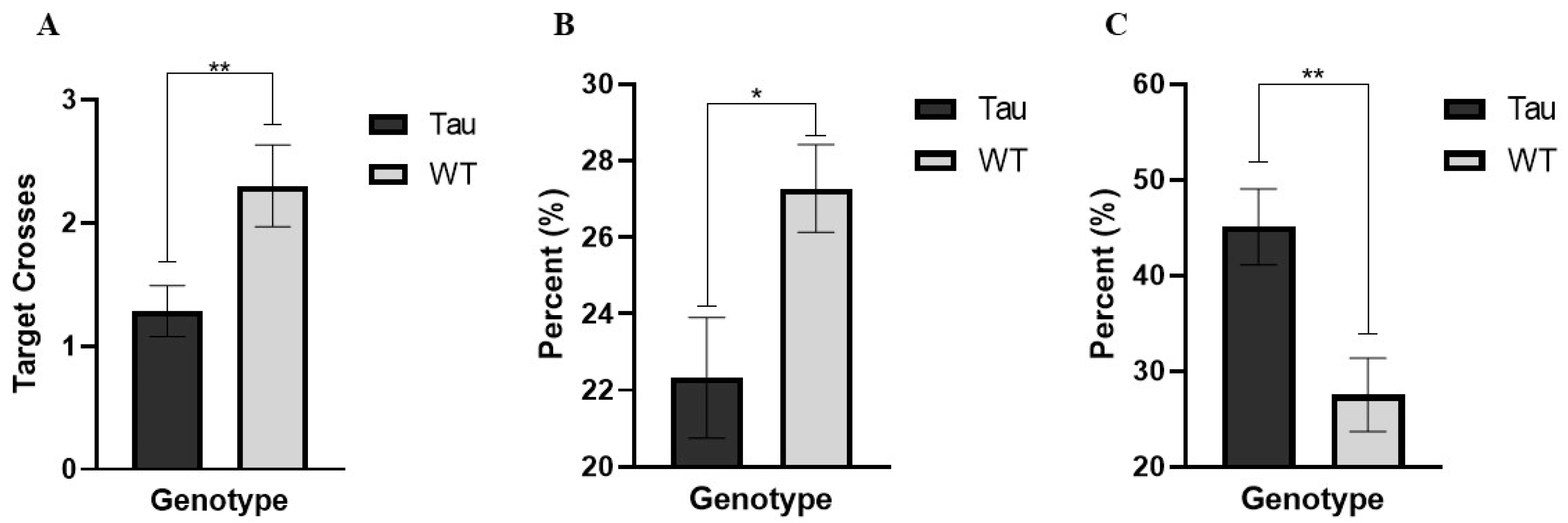
3.6.6. Target Crossings (Probe Day)
3.6.7. Percent Time Spent in the Target Quadrant (Probe Day)
3.6.8. Thigmotaxicity (Probe Day)
3.7. Activities of Daily Living
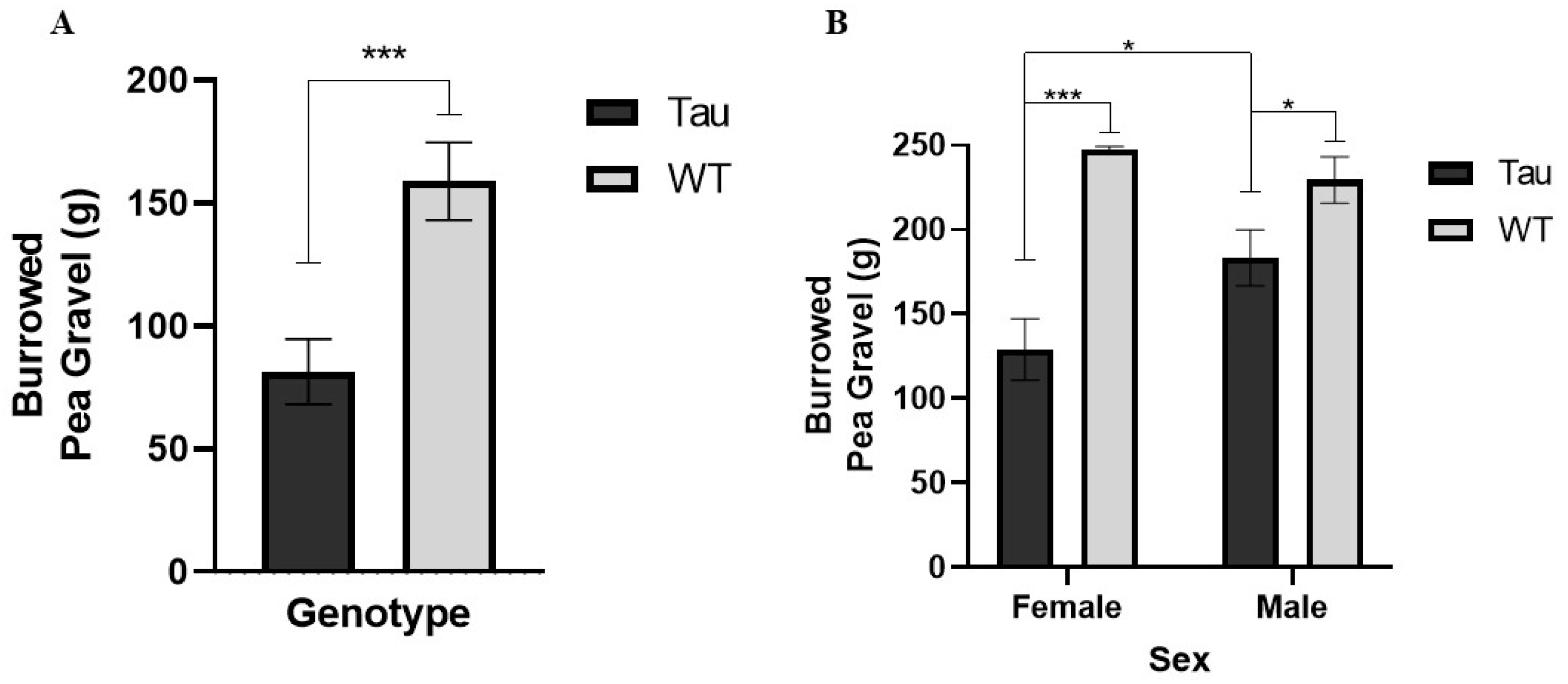
3.7.1. Burrowing
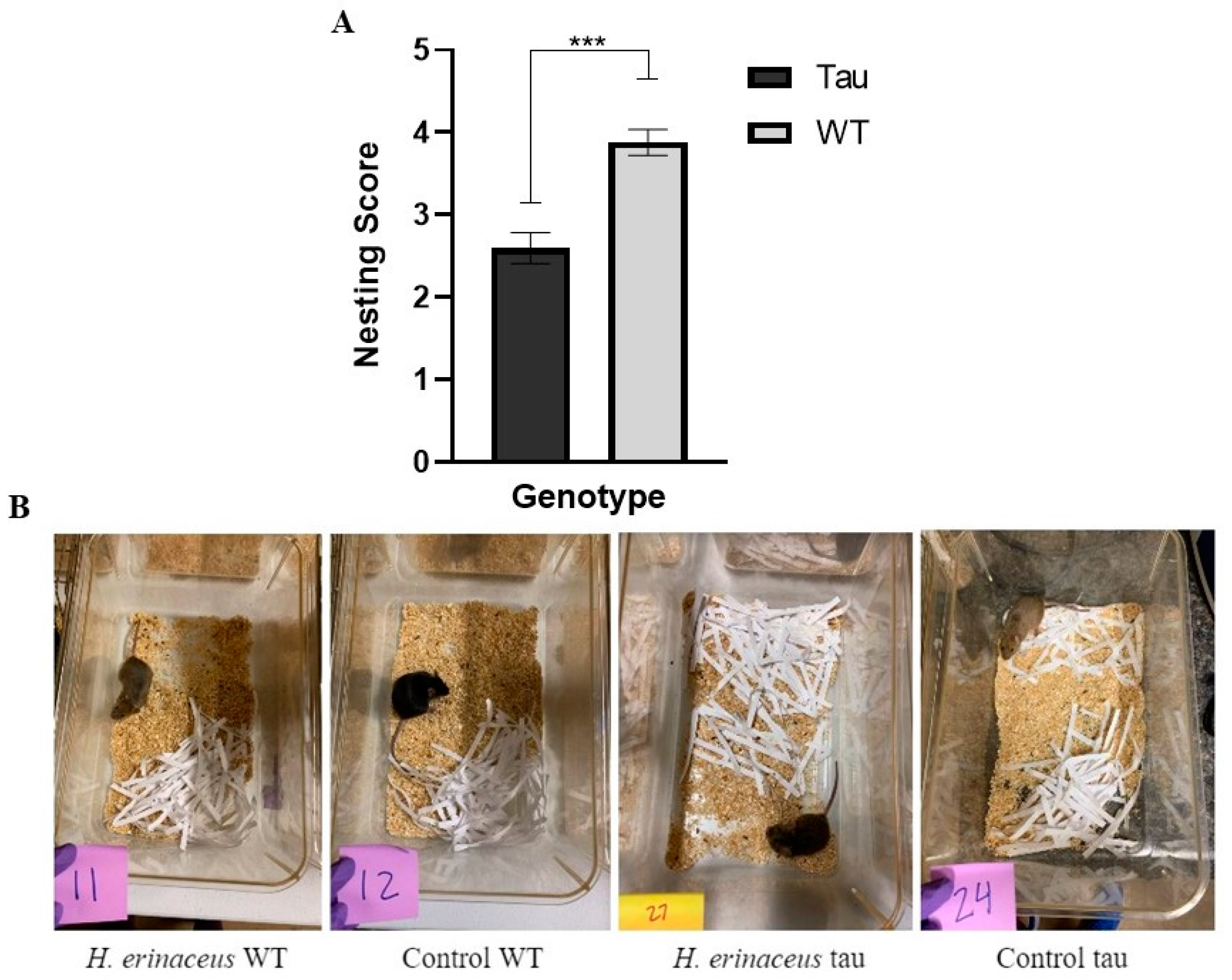
3.7.2. Nesting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, tangles, and Alzheimer’s disease. BBA—Mol. Basis Dis. 2005, 1739, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P. Prevention of early Alzheimer’s disease by erinacine a-enriched hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (accessed on 5 June 2022).
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline. Alzheimer’s Dement. 2021, 7, 12179. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.-H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Konno, S. Townsend Letter. Beneficial Neurological Effects of Amyloban 3399: A Product Made from Bioactive Extracts of Lion’s Mane (Hericium Erinaceum). 2014. Available online: https://www.townsendletter.com/Oct2014/beneneuro1014.html (accessed on 16 April 2022).
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary supplementation of hericium erinaceus increases mossy fiber-ca3 hippocampal neurotransmission and recognition memory in wild-type mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Ma, B.-J.; Shen, J.-W.; Yu, H.-Y.; Ruan, Y.; Wu, T.-T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (ngf) biosynthesis in hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-T.; Ho, C.-H.; Sung, H.-Y.; Lee, L.-Y.; Chen, W.-P.; Chen, Y.-W.; Chen, C.-C.; Yang, C.-S.; Tzeng, S.-F. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci. Rep. 2021, 11, 6551. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of hericium erinaceus on amyloid β (25–35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine a-enriched hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in appswe/ps1de9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef] [Green Version]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 171–177. [Google Scholar] [CrossRef]
- Li, I.C.; Lee, L.Y.; Tzeng, T.T.; Chen, W.P.; Shiao, Y.J.; Chen, C.C. Neurohealth properties of hericium erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018, 5802634. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, M.; Kotilinek, L.; Forster, C.; Paulson, J.; McGowan, E.; SantaCruz, K.; Guimaraes, A.; Yue, M.; Lewis, J.; Carlson, G.; et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 2005, 25, 10637–10647. [Google Scholar] [CrossRef]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; Slegtenhorst, M.V.; Gwinn-Hardy, K.; Murphy, M.P.; Baker, M.; Yu, X.; et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2001, 25, 402–405. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice—Neuromethods; Gould, T., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 42. [Google Scholar] [CrossRef]
- Tucker, L.B.; McCabe, J.T. Behavior of male and female c57bl/6j mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front. Behav. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.K.; Singh, K.; Bishnoi, M. Elevated zero maze: A paradigm to evaluate antianxiety effects of drugs. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.K.; Grewal, S.S.; Fletcher, A.; Bill, D.J.; Dourish, C.T. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology 1994, 116, 56–64. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Deacon, R. Assessing burrowing, nest construction, and hoarding in mice. J. Vis. Exp. JoVE 2012, 59, e2607. [Google Scholar] [CrossRef] [Green Version]
- Neely, C.L.C.; Pedemonte, K.A.; Boggs, K.N.; Flinn, J.M. Nest Building Behavior as an Early Indicator of Behavioral Deficits in Mice. J. Vis. Exp. 2019, 152, e60139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.-J.; Lee, T.-Y.; Lo, Y.; Lee, L.-Y.; Li, I.-C.; Chen, C.-C.; Chang, F.-C. Hericium erinaceus mycelium ameliorate anxiety induced by continuous sleep disturbance in vivo. BMC Complement. Med. Ther. 2021, 21, 295. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.-O. Hericium erinaceus extract reduces anxiety and depressive behaviors by promoting hippocampal neurogenesis in the adult mouse brain. J. Med. Food 2018, 21, 174–180. [Google Scholar] [CrossRef]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Dietary supplementation of lion’s mane medicinal mushroom, hericium erinaceus (agaricomycetes), and spatial memory in wild-type mice. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef]
- Gray, J.A. Emotionality in male and female rodents: A reply to Archer. British J. Psychol. 1979, 70, 425–440. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. JoVE 2015, 96, e52434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturman, O.; Germain, P.-L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bruell, J.H. Genetics and adaptive significance of emotional defecation in mice. Ann. N. Y. Acad. Sci. (3 Exp.) 1969, 159, 825–830. [Google Scholar] [CrossRef]
- Lovick, T.A.; Zangrossi, H. Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front. Psychiatry 2021, 12, 1492. [Google Scholar] [CrossRef]
- Vidal-Gómez, J. Consistent individual differences in some behaviors in mice of the c57bl/6j inbred strain. Anu. De Psicol. 2016, 46, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef]
- Braun, A.A.; Skelton, M.R.; Vorhees, C.V.; Williams, M.T. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: Effects of anxiolytic and anxiogenic agents. Pharmacol. Biochem. Behav. 2011, 97, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine a-enriched hericium erinaceus mycelium produces antidepressant-like effects through modulating bdnf/pi3k/akt/gsk-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Cho, J.; Kang, H. Protective effect of exercise training against the progression of Alzheimer’s disease in 3xtg-ad mice. Behav. Brain Res. 2019, 374, 112105. [Google Scholar] [CrossRef]
- Wang, C.; Gao, W.-R.; Yin, J.; Wang, Z.-J.; Qi, J.-S.; Cai, H.-Y.; Wu, M.-N. Chronic sleep deprivation exacerbates cognitive and synaptic plasticity impairments in app/ps1 transgenic mice. Behav. Brain Res. 2021, 412, 113400. [Google Scholar] [CrossRef]
- Diling, C.; Tianqiao, Y.; Jian, Y.; Chaoqun, Z.; Ou, S.; Yizhen, X. Docking Studies and Biological Evaluation of a Potential β-Secretase Inhibitor of 3-Hydroxyhericenone F from Hericium Erinaceus. Front. Pharmacol. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Hanna, A.; Wilson, J.; Roder, H.; Janus, C. Sex difference in pathology and memory decline in rtg4510 mouse model of tauopathy. Neurobiol. Aging 2011, 32, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Lippi, S.L.P.; Smith, M.L.; Flinn, J.M. A novel happ/htau mouse model of Alzheimer’s disease: Inclusion of APP with tau exacerbates behavioral deficits and zinc administration heightens tangle pathology. Front. Aging Neurosci. 2018, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The Neuroprotective Properties of Hericium erinaceus in Glutamate-Damaged Differentiated PC12 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2016, 17, 1810. [Google Scholar] [CrossRef] [PubMed]
- Joly-Amado, A.; Serraneau, K.S.; Brownlow, M.; Marín de Evsikova, C.; Speakman, J.R.; Gordon, M.N.; Morgan, D. Metabolic changes over the course of aging in a mouse model of tau deposition. Neurobiol. Aging 2016, 44, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of Metabolites in Italian Hericium erinaceus Mycelium, Primordium, and Sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [Green Version]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef] [Green Version]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Gamache, J.; Benzow, K.; Forster, C.; Kemper, L.; Hlynialuk, C.; Furrow, E.; Ashe, K.H.; Koob, M.D. Factors other than hTau overexpression that contribute to tauopathy-like phenotype in rTg4510 mice. Nat. Commun. 2019, 10, 2479. [Google Scholar] [CrossRef]
- Martinez-Marmol, R.; Chai, Y.; Gormal, R.S.; Meunier, F.A.; Khan, Z.; Hong, S.M.; Kim, S.Y.; Kim, S.B.; Lee, M.K.; Lee, D.H.; et al. Hericerin derivatives from hericium erinaceus exert bdnf-like neurotrophic activity in central hippocampal neurons and enhance memory. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Chou, W.; Chen, W.-P.; Wang, M.-F.; Chen, Y.-J.; Chen, C.-C.; Tung, K.-C. Erinacine a-enriched hericium erinaceus mycelium delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (samp8) mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Diet | Tau | Wildtype (WT) | |
|---|---|---|---|
| H. erinaceus | 25 12F, 13M | 16 8F, 8M | 41 20F, 21M |
| Control Diet | 17 7F, 10M | 17 7F, 10M | 34 14F, 20M |
| N = 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, M.N.; Lippi, S.L.P. Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model. Behav. Sci. 2022, 12, 235. https://doi.org/10.3390/bs12070235
Rodriguez MN, Lippi SLP. Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model. Behavioral Sciences. 2022; 12(7):235. https://doi.org/10.3390/bs12070235
Chicago/Turabian StyleRodriguez, Mya N., and Stephen L. P. Lippi. 2022. "Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model" Behavioral Sciences 12, no. 7: 235. https://doi.org/10.3390/bs12070235
APA StyleRodriguez, M. N., & Lippi, S. L. P. (2022). Lion’s Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model. Behavioral Sciences, 12(7), 235. https://doi.org/10.3390/bs12070235







