Nightmares in Migraine: A Focused Review
Abstract
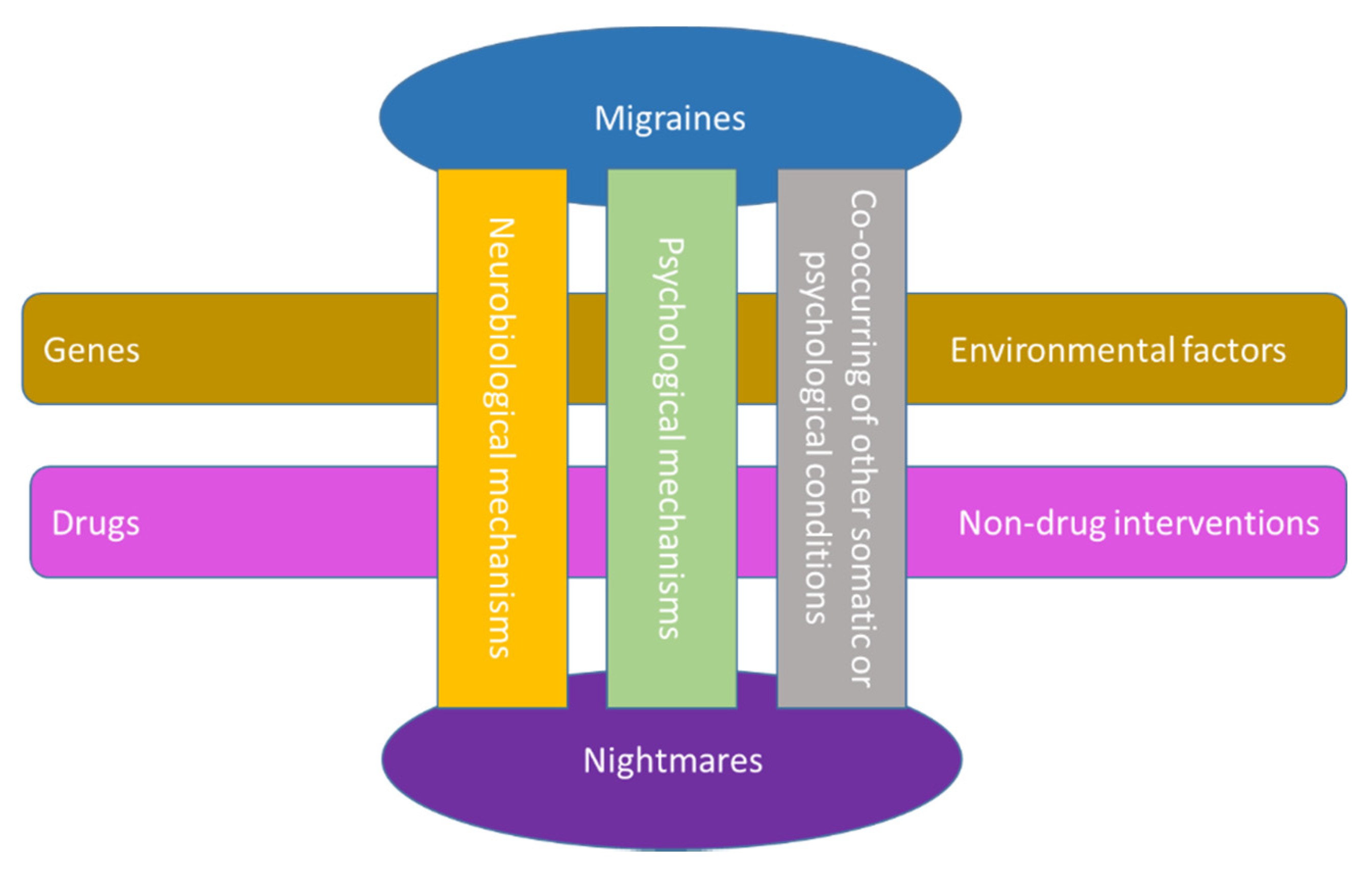
1. Introduction
1.1. Sleep Disorders and Pain
1.2. Sleep Disorders and Headaches
1.3. Sleep Disorders and Migraines
1.4. Nightmares among Sleep Disorders
2. Nightmares and Migraine
2.1. Dreams, Unpleasant Dreams, Nightmares, and Migraine
2.1.1. Dreams as Diagnostic Tools
2.1.2. Dreams as Provoking Factor of Nocturnal Migraine Attacks
2.1.3. Personality Traits, Nightmares, and Migraine
2.1.4. Content of Dreams in Migraine Compared with Nonmigraine or Other Headache Types
2.1.5. Miscellaneous
3. Interventions to Manage Nightmares in Migraine
4. Concluding Remarks and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cheatle, M.D.; Foster, S.; Pinkett, A.; Lesneski, M.; Qu, D.; Dhingra, L. Assessing and Managing Sleep Disturbance in 466 Patients with Chronic Pain. Anesthesiol. Clin. 2016, 34, 379–393. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Koffel, E.; Kroenke, K.; Bair, M.J.; Leverty, D.; Polusny, M.A.; Krebs, E.E. The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol. 2016, 35, 41–49. [Google Scholar] [CrossRef]
- Andersen, M.L.; Araujo, P.; Frange, C.; Tufik, S. Sleep Disturbance and Pain: A Tale of Two Common Problems. Chest 2018, 154, 1249–1259. [Google Scholar] [CrossRef]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.K. Insomnia Co-Occurring with Chronic Pain: Clinical Features, Interaction, Assessments and Possible Interventions. Rev. Pain 2008, 2, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Burns, J.W.; Buvanendran, A.; Gupta, R.; Chont, M.; Kennedy, M.; Bruehl, S. Associations Between Sleep Disturbance and Chronic Pain Intensity and Function: A Test of Direct and Indirect Pathways. Clin. J. Pain 2019, 35, 569–576. [Google Scholar] [CrossRef]
- Korabelnikova, E.A.; Danilov, A.B.; Danilov, A.B.; Vorobyeva, Y.D.; Latysheva, N.V.; Artemenko, A.R. Sleep Disorders and Headache: A Review of Correlation and Mutual Influence. Pain Ther. 2020, 9, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.P.; Martin, P.R.; Boschen, M.J. Psychological Sleep Interventions for Migraine and Tension-Type Headache: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 6411. [Google Scholar] [CrossRef] [PubMed]
- Lund, N.; Westergaard, M.L.; Barloese, M.; Glumer, C.; Jensen, R.H. Epidemiology of concurrent headache and sleep problems in Denmark. Cephalalgia 2014, 34, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.; Pavlovic, J.M. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache 2018, 58, 1030–1039. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Galbiati, A.; Combi, R. Sleep disorder-related headaches. Neurol. Sci. 2019, 40, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, C.; Vacca, A.; Felbush, A.; Filimonova, T.; Gai, A.; Glazyrina, T.; Hubalek, I.A.; Marchenko, Y.; Overeem, L.H.; Piroso, S.; et al. Migraine and sleep disorders: A systematic review. J. Headache Pain 2020, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosol, M.; Nowakowska-Kotas, M.; Chojdak-Lukasiewicz, J.; Budrewicz, S. Migraine and Sleep-An Unexplained Association? Int. J. Mol. Sci. 2021, 22, 5539. [Google Scholar] [CrossRef]
- Brennan, K.C.; Charles, A. Sleep and headache. Semin. Neurol. 2009, 29, 406–418. [Google Scholar] [CrossRef]
- Holland, P.R. Headache and sleep: Shared pathophysiological mechanisms. Cephalalgia 2014, 34, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, T.I.; Auerbach, S.; Casey, K.R.; Kristo, D.; Maganti, R.; Ramar, K.; Zak, R.; Kartje, R. Position Paper for the Treatment of Nightmare Disorder in Adults: An American Academy of Sleep Medicine Position Paper. J. Clin. Sleep Med. 2018, 14, 1041–1055. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- O’Hare, M.; Cowan, R.P. Sleep and Headache. In Sleep and Neurologic Disease; Miglis, M.G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 201–225. [Google Scholar] [CrossRef]
- May, A.; Burstein, R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019, 39, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Fried, N.T.; Elliott, M.B.; Oshinsky, M.L. The Role of Adenosine Signaling in Headache: A Review. Brain Sci. 2017, 7, 30. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Pasquini, S.; Borea, P.A.; Varani, K. Targeting Adenosine Receptors: A Potential Pharmacological Avenue for Acute and Chronic Pain. Int. J. Mol. Sci. 2020, 21, 8710. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Aulinas, A. Physiology of the Pineal Gland and Melatonin. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Brennan, R.; Jan, J.E.; Lyons, C.J. Light, dark, and melatonin: Emerging evidence for the importance of melatonin in ocular physiology. Eye 2007, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Zhang, X.; Huang, W.J. Pain control by melatonin: Physiological and pharmacological effects. Exp. Ther. Med. 2016, 12, 1963–1968. [Google Scholar] [CrossRef]
- Gelfand, A.A.; Goadsby, P.J. The Role of Melatonin in the Treatment of Primary Headache Disorders. Headache 2016, 56, 1257–1266. [Google Scholar] [CrossRef]
- Long, R.; Zhu, Y.; Zhou, S. Therapeutic role of melatonin in migraine prophylaxis: A systematic review. Medicine (Baltimore) 2019, 98, e14099. [Google Scholar] [CrossRef]
- Naegel, S.; Huhn, J.I.; Gaul, C.; Diener, H.C.; Obermann, M.; Holle, D. No Pattern Alteration in Single Nocturnal Melatonin Secretion in Patients with Hypnic Headache: A Case-Control Study. Headache 2017, 57, 648–653. [Google Scholar] [CrossRef]
- Peres, M.F. Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia 2005, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.; Masruha, M.R.; Zukerman, E.; Moreira-Filho, C.A.; Cavalheiro, E.A. Potential therapeutic use of melatonin in migraine and other headache disorders. Expert Opin. Investig. Drugs 2006, 15, 367–375. [Google Scholar] [CrossRef]
- Song, T.J.; Kim, B.S.; Chu, M.K. Therapeutic role of melatonin in migraine prophylaxis: Is there a link between sleep and migraine? Prog. Brain Res. 2020, 255, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Ji, B.; Pan, Y.; Xu, C.; Cheng, B.; Bai, B.; Chen, J. The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases. Front. Mol. Neurosci. 2018, 11, 220. [Google Scholar] [CrossRef]
- Rains, J.C.; Poceta, J.S. Headache and sleep disorders: Review and clinical implications for headache management. Headache 2006, 46, 1344–1363. [Google Scholar] [CrossRef]
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep 2013, 36, 1059–1068. [Google Scholar] [CrossRef]
- Hamel, E. Serotonin and migraine: Biology and clinical implications. Cephalalgia 2007, 27, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Ochandarena, N.E.; Philson, A.C.; Hyun, M.; Birnbaum, J.E.; Cicconet, M.; Sabatini, B.L. Molecular and anatomical organization of the dorsal raphe nucleus. Elife 2019, 8. [Google Scholar] [CrossRef]
- Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 1999, 21, 24S–27S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monti, J.M. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med. Rev. 2010, 14, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.P.; Nakai, Y. The dorsal raphe: An important nucleus in pain modulation. Brain Res. Bull. 1994, 34, 575–585. [Google Scholar] [CrossRef]
- Engstrom, M.; Hagen, K.; Bjork, M.; Sand, T. Answer to comment on “sleep quality, arousal and pain thresholds in migraineurs: A blinded controlled polysomnographic study”. J. Headache Pain 2013, 14, 56. [Google Scholar] [CrossRef]
- Engstrom, M.; Hagen, K.; Bjork, M.H.; Stovner, L.J.; Gravdahl, G.B.; Stjern, M.; Sand, T. Sleep quality, arousal and pain thresholds in migraineurs: A blinded controlled polysomnographic study. J. Headache Pain 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Vollono, C.; Testani, E.; Losurdo, A.; Mazza, S.; Della Marca, G. Migraine, arousal and sleep deprivation: Comment on: “sleep quality, arousal and pain thresholds in migraineurs: A blinded controlled polysomnographic study”. J. Headache Pain 2013, 14, 50. [Google Scholar] [CrossRef]
- Ong, J.C.; Park, M. Chronic headaches and insomnia: Working toward a biobehavioral model. Cephalalgia 2012, 32, 1059–1070. [Google Scholar] [CrossRef]
- Smith, M.T.; Haythornthwaite, J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med. Rev. 2004, 8, 119–132. [Google Scholar] [CrossRef]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Buenaver, L.F.; Quartana, P.J.; Grace, E.G.; Sarlani, E.; Simango, M.; Edwards, R.R.; Haythornthwaite, J.A.; Smith, M.T. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: The mediating role of sleep disturbance. Pain 2012, 153, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.P.; Spierings, E.L. Headache and insomnia: Their relation reviewed. Cranio 2013, 31, 165–170. [Google Scholar] [CrossRef]
- Odegard, S.S.; Engstrom, M.; Sand, T.; Stovner, L.J.; Zwart, J.A.; Hagen, K. Associations between sleep disturbance and primary headaches: The third Nord-Trondelag Health Study. J. Headache Pain 2010, 11, 197–206. [Google Scholar] [CrossRef]
- Rains, J.C. Sleep and Migraine: Assessment and Treatment of Comorbid Sleep Disorders. Headache 2018, 58, 1074–1091. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Sokolov, A.Y.; Lyubashina, O.A.; Amelin, A.V.; Panteleev, S.S. The role of gamma-aminobutyric acid in migraine pathogenesis. Neurochem. J. 2014, 8, 89–102. [Google Scholar] [CrossRef]
- Kilinc, E.; Guerrero-Toro, C.; Zakharov, A.; Vitale, C.; Gubert-Olive, M.; Koroleva, K.; Timonina, A.; Luz, L.L.; Shelukhina, I.; Giniatullina, R.; et al. Serotonergic mechanisms of trigeminal meningeal nociception: Implications for migraine pain. Neuropharmacology 2017, 116, 160–173. [Google Scholar] [CrossRef]
- Pilcher, J.J.; Ginter, D.R.; Sadowsky, B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. J. Psychosom. Res. 1997, 42, 583–596. [Google Scholar] [CrossRef]
- Bruni, O.; Russo, P.M.; Violani, C.; Guidetti, V. Sleep and migraine: An actigraphic study. Cephalalgia 2004, 24, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nayak, C.; Sinha, S.; Nagappa, M.; Nagaraj, K.; Kulkarni, G.B.; Thennarasu, K.; Taly, A.B. Study of sleep microstructure in patients of migraine without aura. Sleep Breath 2016, 20, 263–269. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lin, G.Y.; Lee, J.T.; Lee, M.S.; Tsai, C.K.; Hsu, Y.W.; Lin, Y.Z.; Tsai, Y.C.; Yang, F.C. Associations Between Sleep Quality and Migraine Frequency: A Cross-Sectional Case-Control Study. Medicine (Baltimore) 2016, 95, e3554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fan, X.; Li, X.; Tan, G.; Chen, L.; Zhou, J. Prevalence and predictive factors for poor sleep quality among migraineurs in a tertiary hospital headache clinic. Acta Neurol. Belg. 2013, 113, 229–235. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Li, S.X.; Zhang, B.; Li, A.M.; Wing, Y.K. Prevalence and correlates of frequent nightmares: A community-based 2-phase study. Sleep 2010, 33, 774–780. [Google Scholar] [CrossRef]
- Rek, S.; Sheaves, B.; Freeman, D. Nightmares in the general population: Identifying potential causal factors. Soc. Psychiatry Psychiatr. Epidemiol. 2017, 52, 1123–1133. [Google Scholar] [CrossRef]
- Woodward, S.H.; Arsenault, N.J.; Murray, C.; Bliwise, D.L. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol. Psychiatry 2000, 48, 1081–1087. [Google Scholar] [CrossRef]
- Semiz, U.B.; Basoglu, C.; Ebrinc, S.; Cetin, M. Nightmare disorder, dream anxiety, and subjective sleep quality in patients with borderline personality disorder. Psychiatry Clin. Neurosci. 2008, 62, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sheaves, B.; Onwumere, J.; Keen, N.; Stahl, D.; Kuipers, E. Nightmares in Patients with Psychosis: The Relation With Sleep, Psychotic, Affective, and Cognitive Symptoms. Can. J. Psychiatry 2015, 60, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gieselmann, A.; Ait Aoudia, M.; Carr, M.; Germain, A.; Gorzka, R.; Holzinger, B.; Kleim, B.; Krakow, B.; Kunze, A.E.; Lancee, J.; et al. Aetiology and treatment of nightmare disorder: State of the art and future perspectives. J. Sleep Res. 2019, 28, e12820. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, W.R.; Pinquart, M.; Conner, K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J. Clin. Psychiatry 2012, 73, e1160–e1167. [Google Scholar] [CrossRef] [PubMed]
- Phelps, A.J.; Forbes, D.; Creamer, M. Understanding posttraumatic nightmares: An empirical and conceptual review. Clin. Psychol. Rev. 2008, 28, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Zadra, A. Thematic and content analysis of idiopathic nightmares and bad dreams. Sleep 2014, 37, 409–417. [Google Scholar] [CrossRef]
- Jenkins, D. Nightmare resolution: Where to begin, where to end? A commentary on “The mechanisms of action underlying the efficacy of psychological nightmare treatments: A systematic review and thematic analysis of discussed hypotheses”. Sleep Med. Rev. 2019, 45, 129–130. [Google Scholar] [CrossRef]
- Rousseau, A.; Belleville, G. The mechanisms of action underlying the efficacy of psychological nightmare treatments: A systematic review and thematic analysis of discussed hypotheses. Sleep Med. Rev. 2018, 39, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.; Cui, L.; Merikangas, K.R. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache 2008, 48, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Levitan, H. Dreams which culminate in migraine headaches. Psychother. Psychosom. 1984, 41, 161–166. [Google Scholar] [CrossRef]
- Lippman, C.W. Recurrent dreams in migraine: An aid to diagnosis. J. Nerv. Ment. Dis. 1954, 120, 273–276. [Google Scholar] [CrossRef]
- Heather-Greener, G.Q.; Comstock, D.; Joyce, R. An investigation of the manifest dream content associated with migraine headaches: A study of the dreams that precede nocturnal migraines. Psychother. Psychosom. 1996, 65, 216–221. [Google Scholar] [CrossRef]
- Gallego-Mere, A. The manifest content of dreams. Am. J. Psychoanal. 1989, 49, 95–103. [Google Scholar] [CrossRef]
- Cartwright, R.D. The nature and function of repetitive dreams: A survey and speculation. Psychiatry 1979, 42, 131–137. [Google Scholar] [CrossRef]
- Kales, A.; Soldatos, C.R.; Caldwell, A.B.; Charney, D.S.; Kales, J.D.; Markel, D.; Cadieux, R. Nightmares: Clinical characteristics and personality patterns. Am. J. Psychiatry 1980, 137, 1197–1201. [Google Scholar] [CrossRef]
- Wright, J.; Koulack, D. Dreams and contemporary stress: A disruption-avoidance-adaptation model. Sleep 1987, 10, 172–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solms, M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav. Brain Sci. 2000, 23, 843–850, discussion 904–1121. [Google Scholar] [CrossRef]
- Domhoff, G.W. The Hall/Van de Castle System of Content Analysis. In Finding Meaning in Dreams. Emotions, Personality, and Psychotherapy; Springer: Boston, MA, USA, 1996; pp. 9–37. [Google Scholar] [CrossRef]
- Jonckheere, P. The chronic headache patient. A psychodynamic study of 30 cases, compared with cardio-vascular patients. Psychother. Psychosom. 1971, 19, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.L. A controlled epidemiological study of the role of psychological factors in migraine. Arch. Neurobiol. (Madr.) 1974, 37 SUPPL, 243–251. [Google Scholar]
- Greenberg, R.P.; O’Neill, R.M. The construct validity of the MMPI alexithymia scale with psychiatric inpatients. J. Pers. Assess. 1988, 52, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Pulver, S.E.; Renik, O. The clinical use of the manifest dream. Panel report. J. Am. Psychoanal Assoc. 1984, 32, 157–162. [Google Scholar] [CrossRef]
- Biondi, M.; Portuesi, G. Tension-type headache: Psychosomatic clinical assessment and treatment. Psychother. Psychosom. 1994, 61, 41–64. [Google Scholar] [CrossRef]
- Gottschalk, L.A. Uses of dreams. Psychother. Psychosom. 1995, 64, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Foral, P.; Knezevich, J.; Dewan, N.; Malesker, M. Medication-induced sleep disturbances. Consult. Pharm. 2011, 26, 414–425. [Google Scholar] [CrossRef]
- Ghaffarinejad, A.; Mehdizadeh Zareanari, A.; Pouya, F. Investigating the Dreaming Content of Migraineurs. Zahedan J. Res. Med. Sci. 2015, 17, e969. [Google Scholar] [CrossRef]
- De Angeli, F.; Lovati, C.; Giani, L.; Mariotti D’Alessandro, C.; Raimondi, E.; Scaglione, V.; Castoldi, D.; Capiluppi, E.; Mariani, C. Negative emotions in migraineurs dreams: The increased prevalence of oneiric fear and anguish, unrelated to 660 mood disorders. Behav. Neurol. 2014, 2014, 919627. [Google Scholar] [CrossRef]
- Lovati, C.; DeAngeli, F.; D’Amico, D.; Giani, L.; D’Alessandro, C.M.; Zardoni, M.; Scaglione, V.; Castoldi, D.; Capiluppi, E.; Curone, M.; et al. Is the brain of migraineurs “different” even in dreams? Neurol. Sci. 2014, 35 (Suppl. 1), 167–169. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Suzuki, S.; Watanabe, Y.; Takashima, R.; Hirata, K. Dream-enacting behaviour is associated with impaired sleep and severe headache-related disability in migraine patients. Cephalalgia 2013, 33, 868–878. [Google Scholar] [CrossRef]
- Nadorff, M.R.; Lambdin, K.K.; Germain, A. Pharmacological and non-pharmacological treatments for nightmare disorder. Int. Rev. Psychiatry 2014, 26, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, J.; Pham, C.; Nagappa, M.; Peng, P.W.H.; Englesakis, M.; Espie, C.A.; Morin, C.M.; Chung, F. Cognitive behavioral therapy for insomnia in patients with chronic pain - A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2021, 60, 101460. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.; Loveman, E.; Clegg, A.; Easton, S.; Berry, N. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults. Br. J. Pain 2015, 9, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Smitherman, T.A.; Kuka, A.J.; Calhoun, A.H.; Walters, A.B.P.; Davis-Martin, R.E.; Ambrose, C.E.; Rains, J.C.; Houle, T.T. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache 2018, 58, 1052–1059. [Google Scholar] [CrossRef]
- Crawford, M.R.; Luik, A.I.; Espie, C.A.; Taylor, H.L.; Burgess, H.J.; Jones, A.L.; Ong, J.C.; Team, R.U.S.R. Digital Cognitive Behavioral Therapy for Insomnia in Women With Chronic Migraines. Headache 2020, 60, 902–915. [Google Scholar] [CrossRef]
- Martin, P.R.; Reece, J.; Callan, M.; MacLeod, C.; Kaur, A.; Gregg, K.; Goadsby, P.J. Behavioral management of the triggers of recurrent headache: A randomized controlled trial. Behav. Res. Ther. 2014, 61, 1–11. [Google Scholar] [CrossRef]
- Ree, M.; Junge, M.; Cunnington, D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. 2017, 36 (Suppl. 1), S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Cervena, K.; Dauvilliers, Y.; Espa, F.; Touchon, J.; Matousek, M.; Billiard, M.; Besset, A. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. J. Sleep Res. 2004, 13, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.C. Chronic headache and potentially modifiable risk factors: Screening and behavioral management of sleep disorders. Headache 2008, 48, 32–39. [Google Scholar] [CrossRef]
- Yang, C.P.; Wang, S.J. Sleep in Patients with Chronic Migraine. Curr. Pain Headache Rep. 2017, 21, 39. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, S.; Kim, N.; Choi, J.W.; Park, J.; Kim, S.J.; Gwak, A.R.; Lee, Y.J. Changes in subcortical resting-state functional connectivity in patients with psychophysiological insomnia after cognitive-behavioral therapy: Changes in resting-state FC after CBT for insomnia patients. Neuroimage Clin. 2018, 17, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Perlis, M.L.; Chengazi, V.U.; Soeffing, J.; McCann, U. NREM sleep cerebral blood flow before and after behavior therapy for chronic primary insomnia: Preliminary single photon emission computed tomography (SPECT) data. Sleep Med. 2005, 6, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.J.; Saskin, P.; Thorpy, M.J. Treatment of chronic insomnia by restriction of time in bed. Sleep 1987, 10, 45–56. [Google Scholar]
- Zappaterra, M.; Jim, L.; Pangarkar, S. Chronic pain resolution after a lucid dream: A case for neural plasticity? Med. Hypotheses 2014, 82, 286–290. [Google Scholar] [CrossRef] [PubMed]
- van Schagen, A.; Lancee, J.; Swart, M.; Spoormaker, V.; van den Bout, J. Nightmare Disorder, Psychopathology Levels, and Coping in a Diverse Psychiatric Sample. J. Clin. Psychol. 2017, 73, 65–75. [Google Scholar] [CrossRef]
- Robblee, J.; Starling, A.J. SEEDS for success: Lifestyle management in migraine. Cleve Clin. J. Med. 2019, 86, 741–749. [Google Scholar] [CrossRef]
- Merrill, R.M.; Aldana, S.G.; Greenlaw, R.L.; Diehl, H.A.; Salberg, A. The effects of an intensive lifestyle modification program on sleep and stress disorders. J. Nutr. Health Aging 2007, 11, 242–248. [Google Scholar]
- Gazerani, P. A Bidirectional View of Migraine and Diet Relationship. Neuropsychiatr. Dis. Treat. 2021, 17, 435–451. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Amin, F.M.; Aristeidou, S.; Baraldi, C.; Czapinska-Ciepiela, E.K.; Ariadni, D.D.; Di Lenola, D.; Fenech, C.; Kampouris, K.; Karagiorgis, G.; Braschinsky, M.; et al. The association between migraine and physical exercise. J. Headache Pain 2018, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Migraine and Mood in Children. Behav. Sci. 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
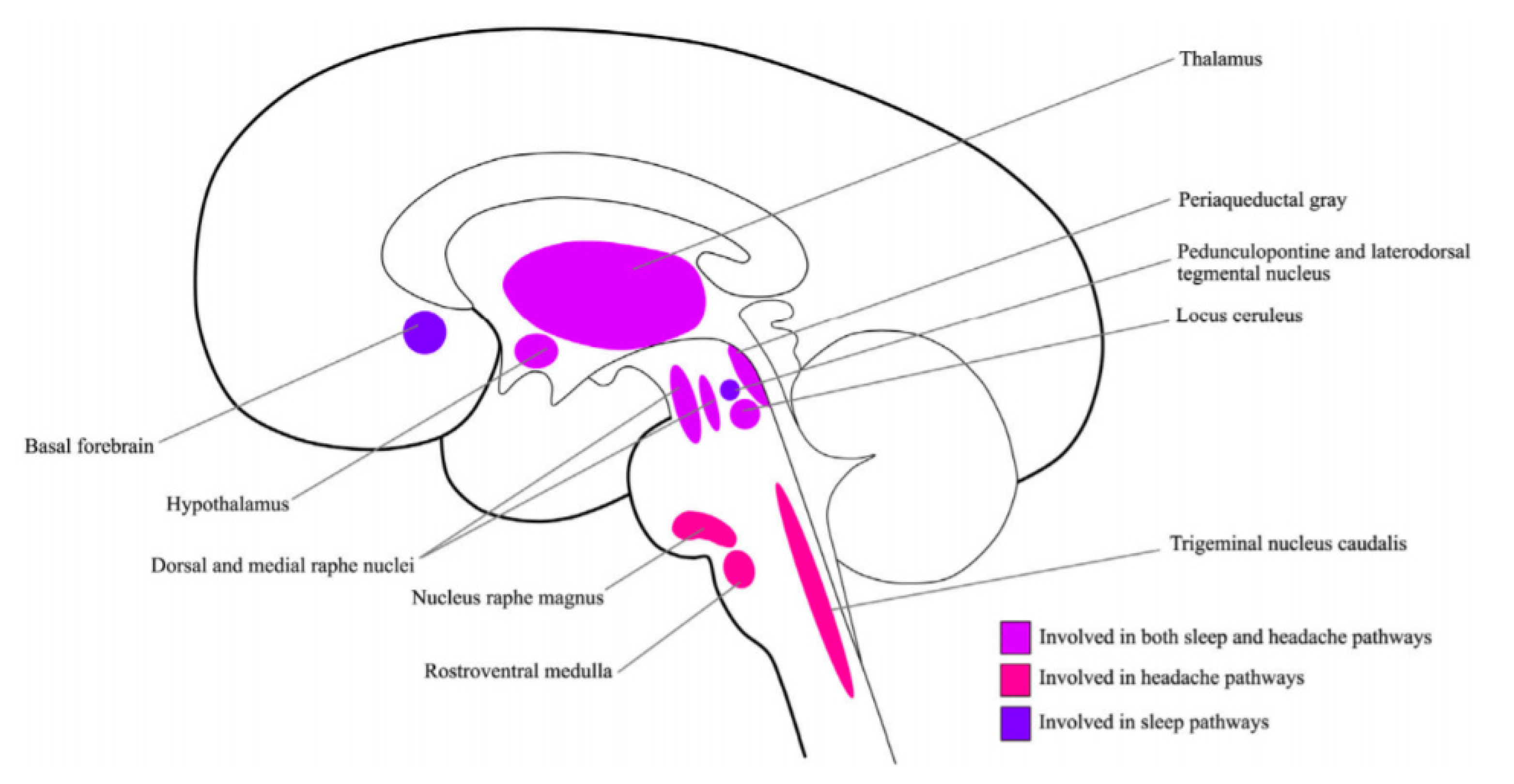
| Neurotransmitter/Neuromodulator | Sleep | Migraine |
|---|---|---|
| Adenosine | NREM and REM sleep induction (A1 or A2A receptor mediated) | Promotion of nociception (A2A receptor mediated) |
| Dopamine | Consolidation of wakefulness | Promotion of antinociception (D2 receptor mediated) |
| Melatonin | Promotion of REM sleep, and promotion of NREM sleep in some conditions | Promotion of antinociception (MT1/MT2 receptor mediated) |
| Orexin | Promotion of wakefulness | Promotion of antinociception (OXR1 mediated) |
| Serotonin | Inhibition of REM and initiation of sleep | Promotion of antinociception (most likely via central serotonergic antinociceptive system. * Please note the dual role of serotonin in migraine.) |
| Others: | ||
| GABA | Induction of deep NREM Stabilization of NREM sleep Reduction of REM sleep | Promotion of antinociception (most likely at the peripheral, spinal, and cortical levels. * Please note that the GABAA and GABAB receptors functionally complement each other, and each plays a role in control over trigemino thalamo cortical nociceptive transmission.) |
| Galanin | Promotion of NREM | Promotion of nociception at periphery, and antinociception centrally (* Please note the dual action.) |
| Histamine | Promotion of wakefulness | Promotion of nociception via H1; promotion of antinociception most likely via H3 (* Please note the dual action.) |
| Noradrenalin | Promotion of wakefulness | Promotion of nociception |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazerani, P. Nightmares in Migraine: A Focused Review. Behav. Sci. 2021, 11, 122. https://doi.org/10.3390/bs11090122
Gazerani P. Nightmares in Migraine: A Focused Review. Behavioral Sciences. 2021; 11(9):122. https://doi.org/10.3390/bs11090122
Chicago/Turabian StyleGazerani, Parisa. 2021. "Nightmares in Migraine: A Focused Review" Behavioral Sciences 11, no. 9: 122. https://doi.org/10.3390/bs11090122
APA StyleGazerani, P. (2021). Nightmares in Migraine: A Focused Review. Behavioral Sciences, 11(9), 122. https://doi.org/10.3390/bs11090122






